Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.94 n.1 Pretoria 2023
http://dx.doi.org/10.36303/jsava.575
ORIGINAL RESEARCH
Use of etorphine hydrochloride for immobilisation of Nubian giraffe for wire snare removal: a retrospective study
S BarnesI; J LubegaII; K MamaIII; M DriciruIV; S RaoIII; S FergusonII; MJ SadarIII
IJames L. Voss Veterinary Teaching Hospital, Colorado State University, United States of America
IIGiraffe Conservation Foundation, Namibia
IIIDepartment of Clinical Sciences, Colorado State University, United States of America
IVUganda Wildlife Authority, Uganda
ABSTRACT
The critically endangered Nubian giraffe (Giraffa camelopardalis camelopardalis) is distributed in small, fragmented populations across East Africa. Safe immobilisation to facilitate animal monitoring, care, and translocation is important for management directed at ensuring long term survival. Due to a high incidence of reported complications, including mortality during giraffe immobilisations, there is a need for developing and refining techniques and sharing information to facilitate widespread application. This retrospective study utilised immobilisation data acquired during wire snare removals from 80 Nubian giraffe induced with intramuscular etorphine hydrochloride. Recorded data included age (adult, subadult), sex, estimated weight, body condition score, induction and reversal drug dosage, induction time, quality of induction, duration of the procedure, time to reversal administration, and snare wound characteristics. There were no statistically significant differences between males and females for induction quality (p > 0.99), induction time (p = 0.72), and procedure time (p = 0.18). No significant differences were noted between adults and subadults for induction quality (p = 0.16) and procedure time (p = 0.35). There was a significant difference in induction time between adults (7.58 ± 0.42 minutes) and subadults (5.65 ± 0.56 minutes) (p < 0.01). On average, adults received 12.4 mg etorphine while subadults received 11.6 mg. Wound severity did not have a significant impact on induction quality. No mortality was observed. Based on these data, etorphine hydrochloride, followed by rapid reversal, was safe for induction of Nubian giraffe presenting for snare removal and should be considered in similar circumstances.
Keywords: giraffe, chemical immobilisation
Introduction
The Nubian giraffe (Giraffa camelopardalis camelopardalis) is a subspecies of the northern giraffe (G. camelopardalis), and in 2018 was added as critically endangered to the International Union for Conservation of Nature Red List (Fennessy et al. 2016; Wube et al. 2018; Coimbra et al. 2021). The ability to provide veterinary interventions, such as treatment of individuals affected by wire snares from poaching or translocation for conservation, is vitally important to ensuring survival. This often necessitates the use of chemical immobilisation. While information pertaining specifically to wild Nubian giraffe immobilisation is limited, it is broadly recognised that giraffe species have an increased risk of complications due to their unique anatomy and physiology (Kock & Burroughs 2021; Fennessy et al. 2022; Deacon et al. 2022). Mortality rates during giraffe immobilisation have been reported to be as high as 25-35% (Fowler et al. 1986). Causes of mortality are not always determined but capture myopathy and respiratory compromise are often suggested. Over the past two decades, wildlife veterinarians have had success in reducing mortality by altering the drug protocols and techniques for immobilisation of giraffe in their natural habitat (Bush et al. 2002; Kock & Burroughs 2021; Fennessy et al. 2022).
Both opioid- and non-opioid-based protocols have been reported for use in giraffe (Bush et al. 2002, Delk et al. 2019). Non-opioid-based protocols utilised for immobilisation have included succinylcholine (historic), and alpha-2 agonists (e.g. xylazine or medetomidine) combined with ketamine (Bush et al. 2001, Bush et al. 2002). An increased chance of regurgitation, potentially resulting in fatal aspiration pneumonia, longer induction times, an increased risk of capture-related myopathy or traumatic injury, and a higher incidence of re-sedation after reversal are reported with these drug combinations (Delk et al. 2019). Conversely, opioids may result in adverse effects due to associated excitatory behaviours, and possible severe respiratory depression, which can compound the respiratory compromise that is seen in giraffe due to their small intrathoracic volume (Mitchell & Skinner 2011). Combinations of potent mu opioids (e.g. thiafentanil) with mixed agonist-antagonist drugs (e.g. butorphanol) and tranquilisers or sedatives to alleviate some of these side-effects have been explored (Deacon et al. 2022). For example, azaperone, a butyrophenone tranquiliser, has been evaluated in combination with etorphine hydrochloride (Vitali et al. 2020), but the potential for delayed drug onset and lack of reversibility has limitation for animals being released to remote habitats (Kock & Burroughs 2021).
When immobilising animals in their natural habitat, drug availability, concern for human safety, the terrain, and the need for rapid administration of reversal agents are additional considerations (Bush et al. 2002). Highly potent opioids, either alone or in combination, may be preferred due to small drugdart volumes, faster time to onset of immobilisation, and ease of reversibility, with recognition that respiratory depression must be managed (Kock & Burroughs 2021; Bush et al. 2002).
There is a lack of recent peer-reviewed publications regarding the efficacy and safety, including associated mortality rates, of such protocols. This retrospective study provides information on the time course and selected behavioural effects following administration of etorphine hydrochloride, followed by rapid reversal with either naltrexone or diprenorphine, in Nubian giraffe requiring wire snare removal in their natural habitat.
Materials and methods
The Uganda Wildlife Authority/Giraffe Conservation Foundation mobile veterinary team actively performs patrols in Murchison Falls National Park, Uganda, for giraffe entangled in wire snares from poaching. Patrols were scheduled three days per week (Monday, Wednesday, Friday) in areas (e.g. the northwestern portion of the park) with known high wire snaring densities. On days when not on patrol, the team was on standby to react to any reports of snared wildlife. Once an ensnared giraffe was sighted, the team prepared for the immobilisation procedure. After assessing the size/estimating body weight, a 1.5-3 ml dart fitted with a non-barbed needle (60 mm non-barbed needles: Danlnject Kolding, Denmark) was prepared for intramuscular administration of etorphine hydrochloride (Captivon 9.8 mg/ml; Wildlife Pharmaceuticals, South Africa) with a carbon dioxide projectile rifle (Danlnject JM Special; Danlnject Kolding, Denmark). The veterinarian determined the dosage based on the animal's size/ estimated body weight, terrain, and perceived excitability; higher doses were administered to animals demonstrating excitability prior to darting in an attempt to reduce complications, such as capture myopathy, resulting from longer durations of running. Subadults were differentiated from adults based on a number of factors, including height at the withers, neck length, size of eyes, height of median horn, and appearance of face and coat (Strauss 2015). Animal size/weight was determined by estimations before or during preparations for darting. The veterinarian used their experience in visual examination and referenced known weights for a given age and sex (young, subadult and adult, male and female). Prior to darting, either naltrexone (50 mg/ml; Wildlife Pharmaceuticals, White River, South Africa) or diprenorphine (12 mg/ml; Wildlife Pharmaceuticals, White River, South Africa) was prepared for subsequent administration along with any other necessary treatments, such as antibiotics and antiseptic solutions. Naltrexone was the preferred reversal agent; however, during periods of drug unavailability, diprenorphine, a partial antagonist, was substituted.
Each snared giraffe was approached by vehicle and darted into the musculature of the hindquarter or shoulder at a range of 10-40 meters. Dart placement was evaluated and there were set cut off points for when a second dart would be warranted. Once signs of drug effect, such as a high stepping gait, ear flicking, stargazing, and/or a loping gait, were observed, the capture team was transported close to the animal, and they facilitated the capture and transition of the animal to lateral recumbency with the assistance of ropes. Induction time was defined as time from darting to when the giraffe was in lateral recumbency.
Induction quality was evaluated by the veterinarian as poor, fair, or good based on time and quality. lnduction was considered good if the giraffe showed signs of drug effect within three minutes and drug administration resulted in a smooth assisted transition to lateral recumbency within eight minutes of dart contact. Induction was considered fair if first effects were not seen until three to five minutes post darting with a generally smooth transition to lateral recumbency achieved by 11 minutes. An induction was rated poor when a giraffe did not show drug effect and was unable to be successfully transitioned to lateral recumbency or the time exceeded 11 minutes. The primary darting sites were the hind quarters and shoulder which bear thick muscle. If the dart was on the abdomen or thoracic areas and it had fully discharged, veterinarians would wait for up to 10 minutes. If giraffe were not induced within this time, re-darting was considered. When other darting failures like partial discharge, full discharge in the subcutaneous or tendons occurred, a second dart was considered. A poor initial darting was not cause for exclusion in this study.
Once the giraffe was recumbent, naltrexone or diprenorphine was administered within 30 seconds via intravenous injection into the jugular vein, and manual restraint was used to maintain the giraffe in lateral recumbency. Immediate reversal was necessary to reverse the respiratory depression associated with the doses of etorphine hydrochloride used. This strategy has been previously utilised when immobilising giraffe with potent opioids for short procedures to minimise occurrence of hypoventilation and hypoxaemia (Kock & Burroughs 2021; Deacon et al. 2022). Depending on the animal's reaction to reversal and restraint, the limbs would need to be fixated. Some animals were calm with limited struggling and did not require limb fixation. With kicking and struggling, limbs were tied with ropes. The wire snares were then removed by elevating the wire from the skin with a metal hook and cutting the wire using bolt cutters. Wound severity was assessed by the veterinarian and rated as severe, moderate, mild, or none (Table I). Once the wire was removed, wounds were manually debrided and giraffe were treated with long-acting injectable antibiotics, as needed. Oxytetracycline (200 mg/mL; Hebei Yuanzheng Pharmaceutical CO, Hebei, China) was administered once due to difficulties with finding the same animal twice after the initial procedure. In addition, due to limited immobilising drugs being available in Uganda, only giraffe with snares were permitted to be captured. Giraffe that had lacerations caused by wire snares received antibiotics. Those that had snares, but no wound, did not receive antibiotics. Upon completion of all procedures, the giraffe were allowed to stand and return to their natural environment. Procedure time was defined as the time from administration of the reversal agent to the time the giraffe was released.
Statistical analysis was performed using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, California USA). Induction quality category was compared between adults and subadults using Fisher's exact test. The categories of fair and poor immobilisation were combined to fit the parameters of the test. Fisher's exact test was also used to compare induction quality between males and females. Again, fair and poor induction ratings were combined for the purposes of this test. Induction time was compared between males versus females and adults versus subadults. The data for males and females, and adults and subadults, were assessed for normality using a Shapiro-Wilk test.
Data that did not pass the normality test were evaluated using a Mann-Whitney U test and included induction time for males versus females, and adults versus subadults. Data for procedure time for males and females, and adults and subadults, were assessed for normality. All data for procedure time lacked a normal distribution and were therefore assessed with a Mann-Whitney U test. Lastly, wound severity was compared to induction quality. None-to-mild and moderate-to-severe wounds were combined into two respective categories. These categories were analysed using a Chi square test. Significance for all tests was set with a p value < 0.05.
Results
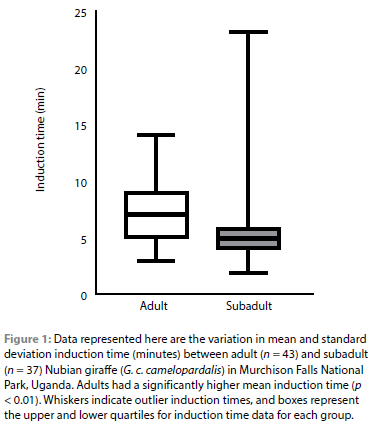
Data from 80 Nubian giraffe immobilisations, 57 males and 23 females, were included in this study. Of these giraffe, 43 were classified as adults and 37 as subadults. Etorphine hydrochloride doses ranged from 5-13 mg per giraffe. On average, adults received 12.4 mg (estimated at 0.017 mg/kg based on mean weight and mg dose) etorphine hydrochloride, and subadults received an average dose of 11.6 mg (estimated at 0.02 mg/kg based on mean weight and mg dose). The mean ± SD induction time varied significantly (p < 0.01) between adults (7.58 ± 0.42 minutes) and subadults (5.65 ± 0.56 minutes) (Figure 1). In contrast, the mean induction time between males (6.23 ± 0.57 minutes) and females (5.7 ± 0.38 minutes) was not significantly different (p = 0.72). The mean procedure times were also not significantly different (p = 0.18) between males (6.23 ± 0.57 minutes) and females (4.5 ± 0.43 minutes) or for adults (5.65 ± 0.62 minutes) and subadults (5.95 ± 0.61 minutes) (p = 0.35). The naltrexone dose was approximately 10 times the etorphine hydrochloride dose and ranged from 50-200 mg per giraffe. The diprenorphine dose was approximately two times the etorphine hydrochloride dose and ranged from 14-26 mg per giraffe. Thirty-five giraffe received naltrexone and 54 received diprenorphine. No significant differences in recovery were observed between the two reversals during the immediate post-reversal period. The giraffe were not monitored after release, therefore no observations were made on the long-term recovery period. No mortalities occurred during the immobilisation events.
There were nine poor/fair inductions (eight males, one female) and 71 good (49 males, 22 females). The quality of induction was assessed as good in 86% of males and 96% of females, and this difference was not significant (p > 0.99). In contrast, there was a slightly larger difference between the induction quality between adults and subadults. Of the nine poor/fair inductions, seven were adults and two were subadults, and there were 36 adults and 25 subadults with good inductions. Overall, 84% of adult inductions were scored as good, versus 95% for subadults; this difference was not statistically significant (p = 0.16).
Wire snares were predominately located on the lower portion of the limbs. There were a total of 43 giraffe with none-to-mild wounds and 37 with wounds classified as moderate-to-severe. Of the none-to-mild wounds, six had poor/fair inductions and 37 had good inductions. Of the moderate-to-severe wounds, three had poor/fair inductions and 34 had good inductions. Animals that had none-to-mild wounds experienced good inductions 86% of the time, and similarly, moderate-to-severe wounds had good inductions 92% of the time. This difference was not statistically significant (p = 0.84). Of the 61 male immobilisation events, 20 had no wounds, whereas 13 were classified as mild, 15 as moderate, and 13 as severe. Of the 28 females, eight had no wounds despite a snare being present, five were classified as mild, four as moderate, and 11 as severe.
Discussion
The results of this retrospective study found that the use of etorphine hydrochloride, followed by reversal with either naltrexone or diprenorphine, provided safe and effective induction for ensnared wild Nubian giraffe across different ages, sexes, and wound severities. The only statistically significant difference observed was the induction time between adults and subadults. This is likely explained by subadults receiving a larger dose of etorphine hydrochloride. Subadults in this study received an average dose of 11.6 mg. Current recommendations for etorphine hydrochloride doses for free ranging giraffe are a total dose of 7-9 mg for subadults, 10-17 mg for cows, and 12-24 mg for adult bulls (Kock & Burroughs 2021; Fennessy et al. 2022). Higher opioid doses have been shown to result in a more rapid induction time (Fennessy et al. 2022). In this report, higher doses were selected more frequently for subadult giraffe, based on veterinary assessment of the animals' temperament.
Once recumbent, the reversal agent was administered immediately to minimise known opioid complications, such as respiratory depression. While the intent was to use naltrexone due to its complete antagonism of etorphine hydrochloride, drug availability did not allow for this. However, it allowed for a subjective comparison of reversal between naltrexone and diprenorphine in the immediate post-immobilisation period; no clinically relevant differences in giraffe recovery were observed. This information is useful as there may be circumstances when one drug is not available and the other may have to be utilised. As animals were not followed post-release, longer term effects, including potential re-narcotisation, could not be characterised. Although not statistically significant, the mean procedure time between males and females differed by 1.7 minutes. On average, males tended to have longer procedure times. This difference is likely attributable to their larger body size. Males on average across all age classes had an estimated weight of 778 kg and females were estimated at 667 kg. The larger body size served as an additional challenge during the manual restraint needed for wire snare removal, which may have resulted in longer procedure times. Most giraffe in this study had good quality inductions, regardless of the extent of their injuries related to the presence of a snare. Based on this, it appears wound severity did not have an impact on induction quality.
At dosages reported, etorphine hydrochloride, followed by rapid reversal with either naltrexone or diprenorphine, was safe for induction of Nubian giraffe presenting for snare removal, and provides another option for chemical immobilisation of these animals in their natural habitat, as an alternative to the drug protocols that are currently being used or studied.
Acknowledgements
We thank the Uganda Wildlife Authority for granting the permits for research and data used in the manuscript under the Memorandum of Understanding with the Giraffe Conservation Foundation. Additionally, we thank the Giraffe Conservation Foundation and their partners and donors for the financial support.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
All funding for the field component was provided by the Giraffe Conservation Foundation. The Giraffe Conservation Foundation was involved in the study design, data collection and manuscript writing.
Ethics
For the IRB, all animal immobilisations were interventions necessary for animal rescue from illegal wire snares as part of the Uganda Wildlife Authority mandate for species conservation. The Giraffe Conservation Foundation has an internal IACUC that includes the methods of immobilisation used for this retrospective study. Immobilisations were part of routine/rescue operations.
The author/s declare that this submission is in accordance with the principles laid down by the Responsible Research Publication Position Statements as developed at the 2nd World Conference on Research Integrity in Singapore, 2010.
ORCID
S Barnes https://orcid.org/0009-0006-7632-6295
J Lubeqa https://orcid.org/0009-0007-1081-025X
KMama https://orcid.org/0000-0001-5165-9233
M Dirchiu https://orcid.org/0009-0001-1747-9623
S Rao https://orcid.org/0000-0002-9234-0082
S Ferguson https://orcid.org/0000-0002-8086-6706
References
Bush, M., Grobler, D.G., Raath, J.P., 2002, The art and science of giraffe (Giraffa camilopardalis) immobilization/anesthesia, In: Heard D., editor. Zoological Restraint and Anesthesia. International Veterinary Information Service; Ithica, NY, USA: 2002. [ Links ]
Coimbra, R.T.F., Winter, S., Kumar, V., et al., 2021, Whole-genome analysis of giraffe supports four distinct species, Curr Biol 31(13): 2929-2938. https://doi.org/10.1016/j.cub.2021.04.033. [ Links ]
Deacon, F., Daffue, W., Nel, P., et al., 2022, Effective field immobilisation and capture of giraffe (Giraffa camelopardalis), Animals 12(10), 1290. https://doi.org/10.3390/ani12101290. [ Links ]
Delk, K.W., Mama, K.R., Rao, S., et al., 2019, Comparison of adult giraffe (Giraffa camelopardalis) using medetomidine-ketamine with and without a potent opioid, J Zoo Wildl Med 50, 457-460. https://doi.org/10.1638/2018-0119. [ Links ]
Fennessy, J., Bidon, T., Reuss, F., et al. 2016, Multi-locus analysis reveal four giraffe species instead of one, Curr Biol 26, 2543-2549. https://doi.org/10.1016/j.cub.2016.07.036. [ Links ]
Fennessy, J., Bower, V., Castles, M., et al., 2022, A Journey of Giraffe - A practical guide to wild giraffe translocations, Giraffe Conservation Foundation, Windhoek, Namibia. [ Links ]
Fowler, M., Boever, WJ., 1986, Giraffidae (giraffe and okapi): Restraint and anesthesia. P.987 in Zoo and Wild Animal Medicine, 2nd ed. M/ Fowler, ed., Philadelphia, W.B. Saunders Co., 1986. [ Links ]
Kock, M., Burroughs, R., 2021, Chemical and Physical Restraint of African Wild Animals, 3rd ed.; International Wildlife Veterinary Services: Greyton, South Africa, 2021; pp. 184-189. [ Links ]
Mitchell, G., Skinner, J.D., 2011, Lung volumes in giraffes, Giraffa camelopardalis, Comp Biochem Physiol A Mol Integr Physiol 158, 72-78. https://doi.org/10.1016/j.cbpa.2010.09.003. [ Links ]
Strauss, M.K.L., 2015, A guide to estimating the age of Masai giraffes (Giraffa camelopardalis tippelskirchi), Giraffe Conservation Foundation, Windhoek, Namibia. [ Links ]
Wube, T., Doherty, J.B., Fennessy, J., et al., 2018, Giraffa camelopardalis ssp. Camelopardalis, The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T88420707A88420710.en. [ Links ]
Vitali, F., Kariuki, E.K., Mijele, D., et al., 2020, Etorphine-azaperone immobilisation for translocation of free-ranging Masai Giraffes (Giraffa camelopardalis tippelskirchi): A pilot study, Animals 10, 322. https://doi.org/10.3390/ani10020322. [ Links ]
 Correspondence:
Correspondence:
MJ Sadar
Email: miranda.sadar@gmail.com














