Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the South African Veterinary Association
versión On-line ISSN 2224-9435
versión impresa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.94 no.1 Pretoria 2023
http://dx.doi.org/10.36303/JSAVA.544
ORIGINAL RESEARCH
Chemical immobilisation of lions: weighing up drug effectiveness versus clinical effects
AC DonaldsonI, II, III, IV; A FullerI, II, IV; LCR MeyerI, II, IV; PE BussII, V, VI
IDepartment of Paraclinical Sciences, Faculty of Veterinary Science, University of Pretoria, South Africa
IICentre for Veterinary Wildlife Research, Faculty of Veterinary Science, University of Pretoria, South Africa
IIICenter for Zoo and Wild Animal Health, Copenhagen Zoo, Denmark
IVBrain Function Research Group, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, South Africa
VVeterinary Wildlife Services, South African National Parks, Kruger National Park, South Africa
VIDepartment of Production Animal Studies, Faculty of Veterinary Science, University of Pretoria, South Africa
ABSTRACT
Selection of an effective drug combination to immobilise African lions (Panthera leo) requires balancing immobilisation effectiveness with potential side effects. We compared the immobilisation effectiveness and changes to physiological variables induced by three drug combinations used for free-ranging African lions. The lions (12 animals per drug combination) were immobilised with tiletamine-zolazepam-medetomidine (TZM), ketamine-medetomidine (KM) or ketamine-butorphanol-medetomidine (KBM). Induction, immobilisation, and recovery were timed, evaluated using a scoring system, and physiological variables were monitored. The drugs used for immobilisation were antagonised with atipamezole and naltrexone. The quality of induction was rated as excellent for all drug combinations and induction times (mean ± SD) did not differ between the groups (10.54 ± 2.67 min for TZM, 10.49 ± 2.63 min for KM, and 11.11 ± 2.91 min for KBM). Immobilisation depth was similar over the immobilisation period in the TZM and KBM groups, and initially light, progressing to deeper in lions administered KM. Heart rate, respiratory rate and peripheral arterial haemoglobin saturation with oxygen were within the expected range for healthy, awake lions in all groups. All lions were severely hypertensive and hyperthermic throughout the immobilisation. Following antagonism of immobilising drugs, lions immobilised with KM and KBM recovered to walking sooner than those immobilised with TZM, at 15.29 ± 10.68 min, 10.88 ± 4.29 min and 29.73 ± 14.46 min, respectively. Only one lion in the KBM group exhibited ataxia during recovery compared to five and four lions in the TZM and KM groups, respectively. All three drug combinations provided smooth inductions and effective immobilisations but resulted in hypertension. KBM had an advantage of allowing for shorter, less ataxic recoveries.
Keywords: butorphanol, cardiorespiratory, induction, ketamine, medetomidine
Introduction
Free-ranging lions are routinely immobilised to collect tissue samples, conduct research, treat wounds, and investigate disease (Fahlman et al. 2005; Jacquier et al. 2006; Miller et al. 2015; Wenger et al. 2010). Immobilising drugs should result in a rapid induction, an adequate level of immobilisation with limited adverse cardiorespiratory effects, and be reversible to allow for rapid recovery (Burroughs et al. 2012).
Historically, cyclohexylamines such as phencyclidine (Quandt 1993), tiletamine (Stander & Morkel 1991) and ketamine (Smuts et al. 1973) have been used to immobilise lions. Tiletamine is available in combination with the benzodiazepine sedative zolazepam, which improves muscle relaxation and decreases the occurrence of convulsions (Lamont & Grimm 2014). Tiletamine-zolazepam (TZ), which can be reconstituted at high concentrations allowing less dart-volume compared to ketamine, causes rapid induction and has a wide safety margin with minor cardiorespiratory side effects (Buss & Miller 2019). However, a major disadvantage of this combination is a prolonged recovery, associated with the redistribution and metabolism of tiletamine. During this recovery period, a lion is vulnerable to attack from other animals and may injure itself with repeated attempts to stand (Buss & Miller 2019).
To reduce recovery times in immobilised lions, partially or fully reversible immobilising drug combinations have been explored and evaluated. Medetomidine, an α2-adrenoreceptor agonist, has been used extensively in the immobilisation of captive and free-ranging carnivores because its effects can be rapidly and completely antagonised by atipamezole (e.g. Blignaut 2020; Fahlman et al. 2005; Jacquier et al. 2006; Wenger et al. 2010). Doses of TZ usually used for lion immobilisation can be reduced by up to 75% if combined with medetomidine (Buss & Miller 2019). Tiletamine-zolazepam-medetomidine (TZM) is frequently used in immobilising lions (Buss & Miller 2019; Fahlman et al. 2005; Jacquier et al. 2006). Spontaneous recoveries can occur with the use of this drug combination and additional doses of both TZ and medetomidine are recommended in prolonged procedures (Fahlman et al. 2005; Johansson et al. 2013). Although easily calculated and administered, these additional doses may result in prolonged recovery times.
Ketamine hydrochloride is a relatively short-acting, dose-dependent, N-methyl-D-aspartate (NMDA) antagonist and dissociative anaesthetic. Limited potency and solubility prevent the administration of a sufficient dose (> 5 ml, 100 mg/ml concentration) in a single dart to result in the immobilisation of free-ranging lions (Ramsay 2014). However, the advent of concentrated medetomidine has enabled the reduction of ketamine doses and for an immobilising combination of ketamine and medetomidine (KM) to be administered by dart (Ramsay 2014). Antagonism of the medetomidine effects with atipamezole also allows for quicker recovery times because of a lower ketamine dose (Ramsay 2014). This drug combination has successfully been used in captive (Tomizawa et al. 1997) and free-ranging lions (Fyumagwa et al. 2012).
Butorphanol tartrate is a synthetically derived κ-opioid receptor agonist and μ-opioid receptor antagonist, or partial μ-opioid receptor agonist. Its effects can be rapidly and completely antagonised by naltrexone (Lamont & Grimm 2014). Butorphanol combined with an α2-adrenoreceptor agonist and/or a cyclohexylamine allows for reduced doses and side-effects of each drug as a result of their synergistic effects (Bush et al. 2012). Dissociative anaesthetics used in combination with α2-adrenoreceptor agonists result in anaesthesia, with the addition of opioids increasing the level of sedation and analgesia (Chabot-Doré et al. 2015). Spontaneous arousals can occur in large carnivores immobilised with butorphanol and medetomidine alone (Bush et al. 2012). Butorphanol in combination with medetomidine and midazolam (Wenger et al. 2010), and azaperone and medetomidine (Semjonov et al. 2017) has successfully been used for the immobilisation of lions.
The aim of this study was to evaluate and compare the effectiveness and effect on vital signs, of KM and ketamine-butorphanol-medetomidine (KBM) for the immobilisation of free-ranging lions to that of TZM. We hypothesised that KM or KBM would be effective in immobilising free-ranging lions and allow for increased control over immobilisation duration, while shortening recovery times and reducing adverse clinical effects, compared to TZM.
Materials and methods
Animals
Lions (23 three female, 13 male) were immobilised in the Kruger National Park, South Africa (24°23'52" S, 31°46'40" E) between April and July 2021. Body mass (mean ± SD) ranged from 74.0 to 225.5 kg (143.9 ± 31.6 kg) and age from 10 months to 12 years (5.5 ± 2.6 years).
The study was approved by the Animal Ethics Committees of the University of Pretoria (REC 102-20) and South African National Parks (SANParks) Animal Use and Care Committee (015-20). Procedures were implemented according to the SANParks standard operating procedure for the capture, transportation and maintenance in holding facilities of wildlife.
Experimental procedure
The immobilisation of lions for the study took place at night between 18:00 and 04:00. Lions were attracted to a capture site using a zebra carcass as bait and with recordings of a buffalo calf bellowing or hyaenas feeding at a kill (Buss & Miller 2019). Study animals were randomly allocated using a random number generator to three groups of 12 lions each and received either TZM, KM or KBM. Once a pride was feeding on the carcass, a suitable lion was selected and its body mass estimated. A 3 ml dart (Dan-Inject International, Pietermaritzburg, South Africa)
was prepared with either TZM - 0.6 mg/kg tiletamine-zolazepam (500 mg powder reconstituted in the supplied diluent to 100 mg/ml, Zoletil 100, Virbac RSA Pty Ltd, Halfway House, South Africa) plus 0.036 mg/kg medetomidine (Metonil 40 mg/ml, Wildlife Pharmaceuticals, White River, South Africa), KM - 3.0 mg/kg ketamine (Ketamine 1 g reconstituted with sterile water to 200 mg/ml, Kyron Laboratories, Johannesburg, South Africa) plus 0.036 mg/kg medetomidine, or KBM - 1.2 mg/kg ketamine plus 0.24 mg/kg butorphanol (Butonil 50 mg/ml, Wildlife Pharmaceuticals South Africa Pty Ltd., South Africa) plus 0.036 mg/kg medetomidine. The dart was fired using a carbon dioxide pressurised dart gun (Dan-Inject, International S.A., South Africa) and the drugs administered intramuscularly into the shoulder or upper hind leg. The times to initial effect (defined as first signs of ataxia if standing) and sternal recumbency following the administration of the immobilising drugs were recorded. A descriptive score ranging from 1 (excellent) to 4 (poor) was used to assess induction quality (Table I) (Wenger et al. 2010). Once a lion was recumbent and non-responsive to a stimulus (NRTS), applied by prodding with a three metre pole from the safety of a vehicle, it was considered sufficiently immobilised and safe to handle. The time from darting to NRTS was recorded. The immobilised lion was blind-folded and front limbs hobbled as a safety precaution, loaded onto a vehicle and transported away from the carcass to a processing site. At this site, the lion was placed in lateral recumbency on a steel table, instrumented and physiological variables measured starting at 15 minutes after lateral recumbency (T0) and every 10 minutes for 30 minutes (T10, T20 & T30).
Heart rate was evaluated by thoracic auscultation and respiratory rate was monitored by observing chest movements. Rectal temperature was determined using a rectal thermometer (HI 98509 Checktemp 1, Hanna Instruments, Woonsocket, USA; modified to include a protective sheath) inserted 9 cm into the rectum and placed up against the rectal wall. Peripheral arterial haemoglobin saturation with oxygen was measured using a transflectance probe placed under the third eyelid (Nonin PalmSAT 2500A, Kyron Laboratories, Johannesburg, South Africa).
A 22-gauge x 1" intravascular catheter (Introcan, BBraun Medical Inc., Bethlehem, Pennsylvania, USA) was inserted into a dorsal pedal artery. Systolic, diastolic and mean intra-arterial pressures were measured using a transducer (Deltran II, Utah Medical, Midvale, Utah, USA) placed at the level of the sternum and zeroed to atmospheric pressure before being connected to a field-ready intra-arterial blood pressure monitor (IntraTorr, IntraVitals, Coventry, England, UK).
Depth of immobilisation was assessed using a descriptive score ranging from 1 (limited effect) to 6 (excessive) (Table I) (Wenger et al. 2010). Lions were suspended on a stretcher of known mass below a scale (Crane Scale 500 kh, Miles Industrial Fasteners & Hardware CC, Benoni, South Africa) to measure body mass, aged (Smuts et al. 1978) and their sex was recorded. Body condition was subjectively scored from 1 (severely underweight) to 9 (obese) (Daigle et al. 2015), as was belly size, from 1 (extremely distended) to 5 (empty) (Bertram 1975). All scoring was conducted by a single observer. As part of ongoing SANParks monitoring, lions were branded and microchipped while immobilised.
At the end of the data collection, butorphanol and medetomidine effects were antagonised with naltrexone (2 mg/ mg butorphanol) (40 mg/ml, Kyron Laboratories, South Africa) and atipamezole (5 mg/mg medetomidine) (50 mg/ml, Vet Tech (Pty) Ltd), respectively, administered intramuscularly. Recovery times to sternal recumbency, standing and walking, following antagonist administration, were recorded. Quality of recovery was assessed using a descriptive score ranging from 1 (excellent) to 4 (poor) (Table I) (Wenger et al. 2010). All lions were monitored and protected from potential attack by other lions or hyaenas until they were fully recovered and able to rejoin the pride.
Statistical analyses
An a priori power analysis for an ANOVA with three groups was conducted in G*Power (Faul et al. 2007) to determine a sufficient sample size using an alpha of 0.05, a power of 0.80, and an effect size of 0.55. Statistical analyses were performed using RStudio version 3.6.1 (RStudio: Integrated Development for R. RStudio, PBC, Boston, MA). Normally distributed data are presented as mean ± SD. Non-normally distributed quantitative data are presented as median (range).
A one-way ANOVA was used to determine if there were differences in time from darting to initial effect, sternal recumbency and NRTS, and time from administration of antagonist to sternal recumbency, standing and walking between groups. A Kruskal-Wallis test was used to compare induction, immobilisation and recovery scores, as well as body condition and belly size, with between-group significance determined using a Dunn's test. After performing a Shapiro-Wilk test to confirm normality of the data, a one-way ANOVA was used to determine if there were differences between mean body mass and age of each group.
Physiological data collected over time were compared between groups using a linear mixed effects model. Heart rate, respiratory rate, rectal temperature, peripheral arterial haemoglobin saturation with oxygen, systolic, diastolic and mean arterial pressures were designated as response variables. Time, drug combination, sex, age, body mass and body condition were designated as fixed effects and lion ID was designated as the random effect. A temporal autocorrelation term was included in the model. For each variable, the residuals were calculated, and a Shapiro-Wilk test was used to confirm that the residuals were normally distributed. Residuals for peripheral arterial haemoglobin saturation with oxygen were not normally distributed; thus, the data for this variable was log-transformed, and residuals were re-tested to confirm normality. Significant values were compared using a Bonferroni correction for multiple pairwise comparisons.
Results
Mean TZM dose was 0.58 ± 0.04 mg/kg tiletamine-zolazepam and 0.034 ± 0.003 mg/kg medetomidine, KM was 2.93 ± 0.42 mg/kg ketamine and 0.035 ± 0.005 mg/kg medetomidine, and KBM was 1.15 ± 0.13 mg/kg ketamine, 0.23 ± 0.03 mg/kg butorphanol and 0.034 ± 0.004 mg/kg medetomidine (Table II). Median body condition and belly size score did not differ between treatment groups (Table II).
In all treatment groups, median induction was scored as excellent (Supplementary Table I). Time from darting to sternal recumbency (p = 0.15) and NRTS (p = 0.84) did not differ significantly between combinations, although there was some variation in times to initial effects (p = 0.03) (Figure 1; Supplementary Table I). Immobilisation scores of lions immobilised with KM were initially lower (p = 0.04) than those immobilised with TZM or KBM but by T10 immobilisation scores did not differ between groups for the rest of the immobilisation period (Supplementary Table I). One lion immobilised with TZM, one with KM and two with KBM vomited or displayed signs of nausea.
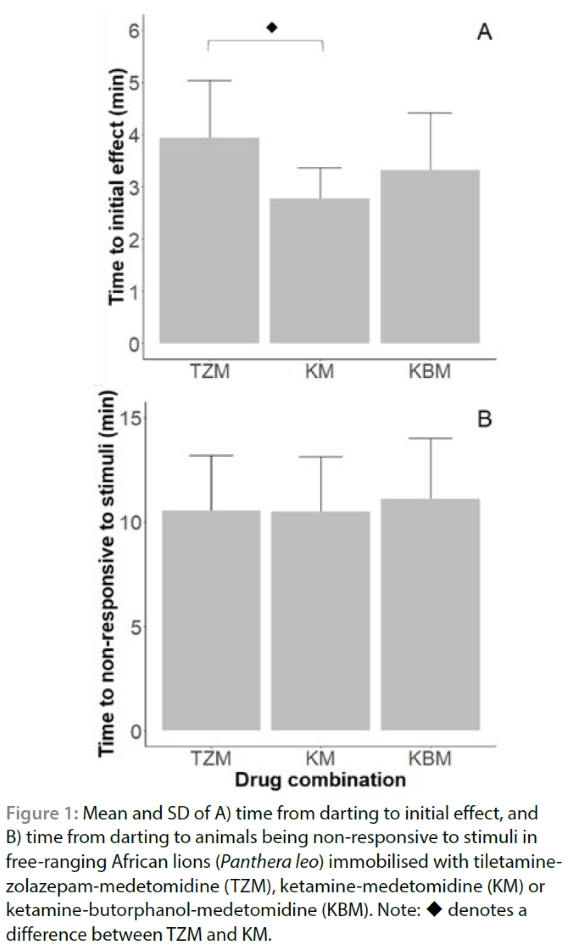
Figure 2 shows the vital signs for lions immobilised with the three drug combinations. Heart rates at T0 did not differ between drug combinations (p = 0.27). Mean heart rate in all three groups had decreased significantly at T30 (p < 0.01). Mean respiratory rate did not differ significantly between drug combinations (p = 0.34), and remained constant over the 30-minute immobilisation period (p = 0.31) (Supplementary Table II). SpO2 at T0 did not differ between drug combinations (p = 0.48) or between T0 and T30 (p = 0.82) (Supplementary Table II). Mean rectal temperature decreased between T0 and T30 in the lions in all three groups (p < 0.01). Rectal temperature at all time points did not differ between lions immobilised with the different drug combinations (p = 1.00) (Supplementary Table II).
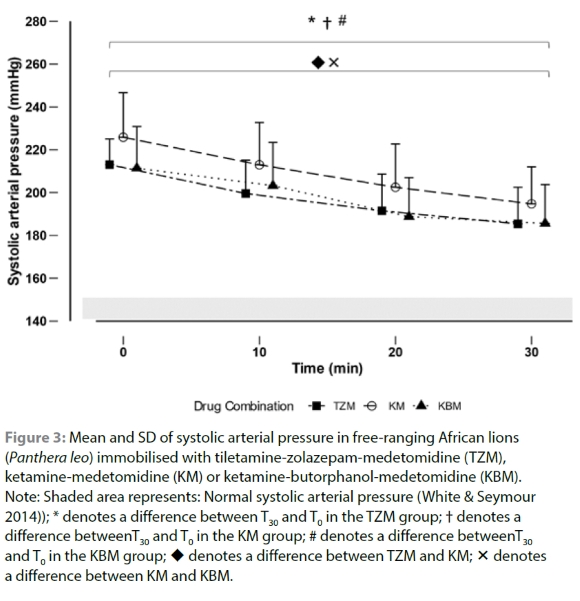
Lions immobilised with all three drug combinations were hypertensive for the entire 30-minute immobilisation period. Mean MAP at T0 did not differ between lions immobilised with each drug combination (p = 0.06) and decreased by T30 (p < 0.01) (Supplementary Table II). Mean SAP was significantly higher in lions immobilised with KM than in those immobilised with ZM (p = 0.05) and KBM (p = 0.01) at T0. Mean SAP decreased significantly by T30 (p < 0.01) (Figure 3 and Supplementary Table II). Mean DAP at T0 did not differ between lions immobilised with each drug combination (p = 1.00) and decreased by T30 (p < 0.01) (Supplementary Table II).
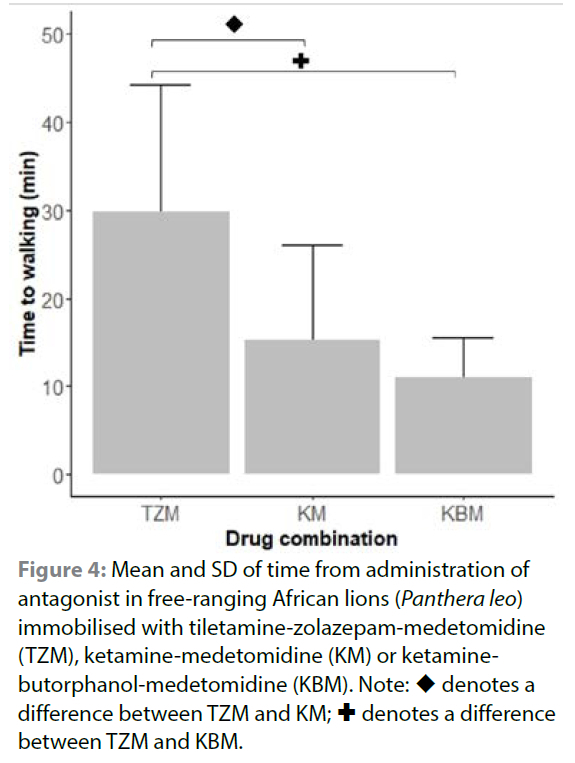
Two lions immobilised with TZM (at 67 and 62 minutes after NRTS), one lion immobilised with KM (at 66 minutes after NRTS) and one lion immobilised with KBM (at 60 minutes after NRTS) showed signs of arousal when moved to be weighed but became immobile again when left undisturbed. Two lions immobilised with KM (at 56 and 59 minutes after NRTS) and one immobilised with KBM (at 66 minutes after NRTS) spontaneously recovered before antagonist drugs were administered. Immobilisation drugs were antagonised with atipamezole and naltrexone (Table II). Following antagonist administration, 28 lions recovered smoothly while five lions in the TZM group, four in the KM group and one in the KBM group exhibited moderate ataxia. Recovery scores did not differ between lions immobilised with each of the three drug combinations (p = 0.37) (Supplementary Table I). Lions immobilised with KM and KBM recovered to walking significantly faster than those immobilised with TZM (p = 0.01 and p < 0.01 respectively) (Figure 4; Supplementary Table I). Recovery to walking in lions immobilised with KM did not differ from those immobilised with KBM (p = 0.82).
Discussion
We found that the TZM, KM and KBM combinations resulted in effective immobilisation in free-ranging lions. Mild ataxia and central nervous system (CNS) excitement were observed during induction, and lions immobilised with all three drug combinations could be safely handled within 12 minutes of darting. Immobilisation in lions administered KM initially appeared to be shallower when compared to that of lions receiving either TZM or KBM. Heart and respiratory rates in lions immobilised with all three drug combinations were within the range for conscious lions at rest. Even though heart rates were normal, lions immobilised with all three drug combinations exhibited hypertension, which decreased over the study period but remained above normal by the end of the immobilisation. Lions immobilised with all three drug combinations were hyperthermic. Spontaneous recoveries occurred in two lions immobilised with KM and one with KBM before 60 minutes from NRTS. Following antagonist administration, most lions recovered from all drug combinations with mild ataxia. Lions immobilised with TZM were slower to recover than those immobilised with KM, with lions that received KM being the most ataxic during recovery.
The target doses of TZM, KM and KBM in our study (Table II) were based on previous lion immobilisation studies (Fyumagwa et al. 2012; Jacquier et al. 2006) and are on the lower end of the recommended range for lions (Ramsay 2014), and lower than what has been used previously (Quandt 1993; Fahlman et al. 2005; Wenger et al. 2010). We selected lower doses in order to allow for quicker recovery times. An advantage of using lower doses is that in future studies the doses could be increased for use in longer procedures or to reduce the likelihood of spontaneous recoveries. The lions in this study were free-ranging and therefore their body mass had to be visually estimated before darting. Because of this estimation, lions immobilised with all three drug combinations received, on average, a 2-4% lower drug dose (mg/kg) than intended (Table II). Despite the under-dosing, all lions became sufficiently immobilised to ensure the safety of people working with them, and no lion spontaneously aroused until at least 56 min after NRTS. Induction times ranged between about five and seven minutes, on average, until sternal recumbency (Figure 1), similar to induction times reported previously in felids for the drug combinations we used (Blignaut 2020; Fahlman et al. 2005; Fyumagwa et al. 2012).
Inductions with mild ataxia and CNS stimulation, similar to those in our study (median score of excellent), are a well-documented benefit of using TZM in lions (Fahlman et al. 2005; Jacquier et al. 2006). Medetomidine induces muscle relaxation and sedation (Sinclair 2003) and its inclusion in drug combinations is beneficial as it potentiates the effect of other drugs (Sinclair 2003). In lions immobilised with TZ alone, substantial excitation was observed (Quandt 1993). The KM combination used in our study also produced inductions with mild CNS stimulation (median score of excellent), in contrast to lions immobilised with ketamine plus xylazine, in which ataxia and excitation was observed (Herbst et al. 1985), possibly due to medetomidine being more potent and selective to α2-adrenoreceptors than xylazine (Sinclair 2003). Induction in lions in our study immobilised with KBM also occurred with minimal ataxia, with a median score of excellent. Serval (Leptailurus serval) immobilised with this combination experienced prolonged inductions despite drug doses being higher than in this study (Blignaut 2020); however the serval were transported in a cage to a secondary processing location prior to darting, so increased stress may have affected their inductions. Although the inductions in our study were excellent, there were episodes of vomiting. Free-ranging lions are normally captured using a bait station (Buss & Miller 2019) and are therefore most likely to have eaten and have full stomachs, which may predispose them to vomiting. One lion immobilised with TZM, one with KM and two with KBM vomited during induction. Vomiting is a common side effect of medetomidine (Sinclair 2003) in various species. Vomiting, or signs of nausea, have been reported in free-ranging lions anaesthetised with TZM (Fahlman et al. 2005) and KM (Quandt 1993).
Despite excellent inductions, immobilisation scores of lions immobilised with the three drug combinations differed. During immobilisation with α2-adrenoreceptor agonists, domestic cats that appear sedated may suddenly become aroused if disturbed and may exhibit an increased sensitivity to initial tactile stimulation (Sinclair 2003). Lions immobilised with KM initially had a lower immobilisation score, and thus a lighter depth of immobilisation, than lions in the other two groups and, although unresponsive when prodded or their tails were manipulated, they tended to become aroused when they were blindfolded. The drug doses used in this study may have influenced the initial immobilisation scores in lions immobilised with KM. The ketamine dose used in the KM combination is similar to the dose reported by Fyumagwa et al. (2012). However, the medetomidine dose used in our study was 50% less than that used by Fyumagwa et al. (2012). We recommend that lions administered lower medetomidine doses should be left undisturbed for a longer period to allow sufficient sedation to occur before they can be safely handled. Lions immobilised with KBM did not exhibit the initial lighter depth of immobilisation compared to those immobilised with KM. Butorphanol is combined with ketamine-medetomidine in domestic cats to increase sedation and muscle relaxation (Clarke et al. 2014), and the addition of butorphanol to the KM combination may explain the higher immobilisation score, and thus a deeper depth of immobilisation, in lions immobilised with KBM at T0 compared to those immobilised with KM. Deeper levels of anaesthesia have been observed in other species when immobilised with KBM compared to KM (Muller et al. 2007) which is likely due to the potentiating effect of butorphanol on the immobilising and sedative effects of the ketamine-medetomidine combination (Tomizawa et al. 1997). Despite some differences in induction times and depth, all three drug combinations we used allowed for placement of arterial catheters, blood sampling, weighing, branding and placement of microchips. More invasive procedures in lions may need the administration of additional anaesthetic drugs and, or the use of local anaesthesia.
Even though induction times and immobilisation scores differed between lions immobilised with each drug combination, cardiorespiratory effects in each treatment group were similar. Systolic, mean and diastolic arterial blood pressures for awake lions are expected to be between 140-150 mmHg, 112-118 mmHg and 92-98 mmHg, respectively (White & Seymour 2014). Lions in this study exhibited clinically severe hypertension (Taylor et al. 2017) throughout the immobilisation procedure with no differences between animals immobilised with each drug combination. The hypertension observed in all three groups decreased over the immobilisation period, but blood pressures remained elevated at the end. Medetomidine's α2-adrenoreceptor agonist effects cause vasoconstriction leading to hypertension (Sinclair 2003), while ketamine and tiletamine cause centrally mediated sympathomimetic effects (White & Ryan 1996). Hypertension has also been observed in other felids immobilised with TZM (Buck et al. 2022; Deem et al. 1998; Stegmann & Jago 2006) and KM (Stegmann & Jago 2006; Buck et al. 2022). The initial hypertension in the lions could also be as a consequence of an excitement-induced stress response due to stimulation and the resultant increase in sympathetic drive caused by feeding on the carcass (Ulrich-Lai & Herman 2009) and the impact of the dart.
Despite the persistent hypertension in all lions, mean heart rates for lions immobilised with all three drug combinations were within the range expected for healthy, awake lions (55-65 beats/ minute; Al-Naji et al. 2019) over the 30-minute immobilisation period, and similar to heart rates of lions in previous studies using TZM and KM (Fahlman et al. 2005; Fyumagwa et al. 2012; Jacquier et al. 2006). Heart rates in our lions were similar to those in lions immobilised with higher doses of medetomidine and ketamine, but lower than those recorded in lions administered TZ alone (Stander & Morkel 1991; Quandt 1993). A normal heart rate in the TZM, KM and KBM immobilised lions is an unexpected finding. Bradycardia is usually expected following the administration of a2-adrenoreceptor agonists such as medetomidine due to a baroreceptor reflex to α2-adrenoreceptor agonists-induced vasoconstriction and hypertension, and usually results in a lowering of blood pressure (Sinclair 2003). The results of our study suggest that the positive chronotropic effects of ketamine (White & Ryan 1996) and tiletamine (Wilson et al. 1993) counteract decreases in heart rate and blood pressure associated with medetomidine, as has been previously noted (Larsen et al. 2002). Although within normal limits, heart rates of lions immobilised with all three drug combinations did decrease over the immobilisation period, accounted for by decreased drug effects due to metabolism and redistribution of immobilising drugs.
As with heart rates, respiratory rates of lions immobilised with all three drug combinations were within expected range for healthy, awake lions at rest (Al-Naji et al. 2019). Similar respiratory rates have been recorded in lions immobilised with TZM (Fahlman et al. 2005; Jacquier et al. 2006) and KM (Fyumagwa et al. 2012). In addition to normal respiratory rates, mean SpO2 of lions immobilised with all three drug combinations was above 95%, indicating that oxygen haemoglobin saturation was adequate; a pulse oximetry reading of 95-98% is considered normal for a cat breathing room air at sea level (Ayres 2012). However, the readings obtained in this study should be interpreted with caution. Complete SpO2 data were collected for six lions immobilised with TZM, eleven immobilised with KM and six immobilised with KBM. SpO2 readings that were not recorded at sampling points was due to failure of the pulse oximeter to obtain a reading. The poor performance of the pulse oximeter may be explained by reduced peripheral blood flow, partly resulting from medetomidine-induced vasoconstriction (Sinclair 2003). It appears as if cold ambient conditions also played a role as the device particularly struggled to provide readings on the colder nights. Since the third eyelid is part of the peripheral tissues, this could be related to cold-induced vasoconstriction. Further, the site of placement of pulse oximeter probes in different species is important for obtaining accurate readings (Mtetwa et al. 2022). The pulse oximeter was placed under the third eyelid in this study as lions were instrumented with a face mask as part of another in depth study on ventilation and metabolism and the tongue was inaccessible.
The most appropriate site for pulse oximeter probe placement differs between species (Mathews et al. 2003), and further validation for the use of pulse oximetry in lions, both at this site and others, and under different environmental and lighting conditions, is required.
Respiratory variables may also have been affected by increased body temperatures. A rise in body temperature may result in increased cellular oxygen consumption (Jessen 2001). Rectal temperatures of lions in this study were elevated regardless of the drug combination used. Body temperature of healthy, awake lions is between 37.3 and 37.8 °C (Trethowan et al. 2017). High body temperatures have also been observed in lions and other carnivores when immobilised with different drug combinations (Fahlman et al. 2005; Jacquier et al. 2006; King et al. 2008), and hyperthermia has been reported to occur during immobilisation with TZM (Fahlman et al. 2005) and KM (Arnemo et al. 2013). Most drugs used for immobilisation influence the ability of animals to thermoregulate adequately. α2-adrenoreceptor agonists depress the hypothalamus and disrupt central noradrenergic mechanisms, decreasing the animal's ability to thermoregulate (Caulkett & Arnemo 2015). Reduced heat loss due to vasoconstriction may also be relevant when α2-adrenoreceptor agonists are used (Sinclair 2003). Under normal conditions, NMDA receptors play a role in thermoregulation by modulating noradrenergic and serotonergic neurons in the locus coeruleus (Singewald et al. 1998; Van Gaalen et al. 1997). Similar to α2-adrenoreceptor agonists, cyclohexylamine drugs cause hypothalamic depression and disruption of thermoregulation by preventing the binding of excitatory neurotransmitters to NMDA receptors (De Witte & Sessler 2002). The resultant hypo-or hyperthermia is likely dependent on the ambient conditions in which the animal is placed, as well as factors such as agitation during capture. Domestic cats anaesthetised with TZ (Clarke et al. 2014), and those anaesthetised with ketamine and low doses of butorphanol developed hyperthermia (Posner et al. 2010). Opioids alter thermoregulation by resetting the control threshold of the hypothalamus (Kurz 2008), resulting in an elevated heat response threshold and reduced cold response threshold, and may possibly affect an animal's ability to limit a thermogenic response. Some opioids also depress overall sympathetic outflow, further affecting an animal's ability to thermoregulate (Diaz & Becker 2010). Although the immobilising drug combinations likely played a role in the development of the observed hyperthermia, other factors probably also played an important role. As the lions fed on the bait prior to being darted, the increase in body temperatures may have been caused by elevated metabolism (Trethowan et al. 2017). The metabolic response to the ingestion of food is termed the "specific dynamic action" and refers to the increased heat production which follows ingestion of food (Feher 2017). A protein-rich diet exerts a larger thermogenic effect than a diet rich in starch or fats (Feher 2017). Based on the stomach scores given to animals in this study, each lion was estimated to have eaten between 5 kg and 10 kg of the carcass before immobilisation. In addition, an excitement-induced stress response (Hetem et al. 2013) caused by the feeding frenzy at the carcass, and possibly also from the impact of the dart, may have also contributed to the development of hyperthermia.
Since rectal temperature was elevated in all lions, it is important to monitor body temperature throughout the immobilisation to detect changes and possibly implement cooling treatments to prevent severe (> 40 °C) hyperthermia from occurring and causing pathophysiological effects such as increased cellular metabolism and requirements for oxygen (Q10 effect) (Kiyatkin 2005), and heat cytotoxicity causing disruption to metabolic pathways and cell membrane function, resulting in cellular damage or death (Lepock 2003). In hot climates lions pant to cool themselves, but this response is depressed during anaesthesia, which increases the risk of hyperthermia (Van Wyk 1986). Lions in our study were immobilised at night during cooler months of the year, which may have to some degree mitigated body temperature increases and explain why body temperature decreased over time while the lion was immobilised.
The results of this study suggest a working time of approximately one hour before lions start becoming unsafe to handle. Spontaneous recoveries occurred in three animals immobilised with KM and KBM, between 56 and 66 minutes after animals became NRTS, but the animals became immobilised again when left unstimulated. Such arousals occurred near the end of the immobilisation period when there was more movement and sound from the team around the animals, which, combined with waning drug effects, may have accelerated arousals. Redistribution and metabolism of the immobilising drugs account for the waning drug effects. Towards the end of immobilisation lipid-soluble immobilising drugs move from the brain and other fatty tissues into the blood where they are metabolised in the liver to water-soluble components which are excreted via the kidneys. In some cases, instead of being metabolised, drugs in the blood may be redistributed from fatty tissues to the brain, which may account for the animals becoming immobilised again after arousal (Burroughs et al. 2012).
The lions that spontaneously recovered had been darted with appropriate doses of drugs for their body mass, with optimal dart placement, so it is unlikely they received a lower drug dose (Isaza 2014). Mean duration of immobilisation with KM in free-ranging lions has previously been reported to be about 1.3 hours, with spontaneous recovery occurrences preceded only by subtle signs, such as deeper breathing (Quandt 1993). Spontaneous arousals at approximately one hour have been documented in lions immobilised with TZM at similar doses to our study (Fahlman et al. 2005), indicating that procedures should be complete by this point or top-up doses, or inhalational anaesthetics may need to be administered, especially if painful procedures still need to be done.
Ideally, once all procedures are completed, immobilising drugs should be antagonised. Medetomidine was antagonised with atipamezole in all three drug combinations, and butorphanol was antagonised with naltrexone in KBM lions. Recovery times in both the KM and KBM groups were significantly shorter than in the TZM group. The difference in these recovery times can firstly be attributed to the shorter duration of action of ketamine compared to tiletamine (Lamont & Grimm 2014). Secondly, the addition of a third drug to the KM combination allowed a decrease in the dose of the non-antagonisable ketamine, which resulted in quicker recoveries. Lower doses of other drugs can be used when butorphanol is added to drug combinations due to the synergistic effects of the drugs used in combination (Bush et al. 2012), allowing for a reduction in dose while still being effective. Recovery time of lions immobilised with TZM was similar to that previously reported (Fahlman et al. 2005); however, recovery from TZM appears to be dose-dependent, with lions administered higher doses taking longer to recover (Jacquier et al. 2006). Atipamezole was administered in previous studies which suggests that prolonged recovery time is associated with increased residual tiletamine and zolazepam effects when higher drug doses are administered (Fahlman et al. 2005; Jacquier et al. 2006).
Median recovery scores did not differ between groups in this study, but five lions immobilised with TZM, four immobilised with KM, and only one immobilised with KBM had recovery scores of 2 (moderate ataxia and longer recovery times). The increased ataxia that was observed in the five lions in the TZM group may have been due to residual tiletamine and zolazepam effects. While the half-life is unknown for lions, in domestic cats the plasma half-life of zolazepam is 4.5 hours, longer than that of tiletamine which has a half-life of two to four hours (Lin et al. 1993). Recovery from TZ immobilisation in free-ranging lions is calmer and shorter if a benzodiazepine antagonist such as flumazenil is used (Stander & Morkel 1991). In lions anaesthetised with TZM, the addition of a benzodiazepine antagonist to the reversal protocol would probably reduce ataxia during recovery by antagonising the effects of zolazepam but would increase the cost of immobilisation procedures. In addition, its effectiveness may be dependent on how soon after the start of anaesthesia the flumazenil is administered. Less ataxia observed during recovery in the KBM group compared to the KM group may be attributed to lower doses of ketamine. As with TZ, the half-life in lions is unknown, but in domestic cats, ketamine has a half-life of 81 minutes (Hanna et al. 1988), suggesting that once the medetomidine and butorphanol were antagonised at the end of the study period, the active concentration of ketamine in the lions' systems would still be present but significantly reduced.
Conclusion
We found that TZM, KM and KBM were effective in immobilising free-ranging lions. Inductions were smooth and immobilisation depth was sufficient for minor procedures in all three groups. Lions immobilised with KM had an initially shallower depth of immobilisation than those immobilised with TZM or KBM. With all three drug combinations lions could be safely handled for up to an hour following induction into recumbency. Heart rate and respiratory rate were within expected ranges for lions during immobilisation with these drug combinations, but hyperthermia and severe and persistent hypertension occurred. Our study has shown that further research is needed to determine if severe cardiovascular side effects of commonly used drug combinations that may unknowingly increase morbidity risk in lions can be prevented or reduced during immobilisation. Peripheral arterial haemoglobin saturations with oxygen measured from the third eyelid were above 95%, suggesting that the animals were not hypoxaemic, but these results need to be properly validated. Although all the drug combinations were effective at immobilising free-ranging lions, and had similar effects on their vital signs, KBM had an advantage of allowing for shorter recoveries with less ataxia during recovery.
Acknowledgements
The authors would like to thank the Copenhagen Zoo for financial support. We thank the many members of staff of SANParks Veterinary Wildlife Services that helped with the capture of the lions and gave logistical support during the study.
Conflict of interest
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this article.
Ethical approval
The study was approved by the Animal Ethics Committees of the University of Pretoria (REC 102-20) and South African National Parks (SANParks) Animal Use and Care Committee (015-20).
ORCID
AC Donaldson https://orcid.org/0000-0003-1586-4744
A Fuller https://orcid.org/0000-0003-3014-3427
LCR Meyer https://orcid.org/0000-0002-5122-2469
PE Buss https://orcid.org/0000-0001-5614-0975
References
Al-Naji, A., Tao, Y., Smith, I. et al., 2019, A pilot study for estimating the cardiopulmonary signals of diverse exotic animals using a digital camera, Sensors 19, 5445. https://doi.org/10.3390/s19245445. [ Links ]
Arnemo, J.M., Evans, A.L., Ahlqvist, P., et al., 2013, Evaluation of medetomidine-ketamine and atipamezole for reversible anesthesia of free-ranging gray wolves (Canis lupus), J Wildl Dis 49, 403-407. https://doi.org/10.7589/2011-12-366. [ Links ]
Ayres, D.A., 2012, Pulse oximetry and CO-oximetry, in J.M. Burkitt Creedon & H. Davis (eds), Advanced monitoring and procedures for small animal emergency and critical care. pp. 274-285, Wiley Blackwell, Ames. https://doi.org/10.1002/9781118997246.ch21. [ Links ]
Bertram, B.C.R., 1975, Weights and measures of lions, Afr J Ecol 13, 141-143. https://doi.org/10.1111/j.1365-2028.1975.tb00128.x. [ Links ]
Blignaut, C.J., 2020, Ketamine-butorphanol-medetomidine versus butorphanol-midazolam-medetomidine immobilisation of serval (Leptailurus serval), MSc Dissertation, Faculty of Veterinary Science, University of Pretoria. Available from: https://repository.up.ac.za/bitstream/handle/2263/77438/Blignaut_Ketamine_2019.pdf?sequence=1. [ Links ]
Buck, R.K., Tordiffe, A.S.W., Zeiler G.E., 2022, Ketamine-medetomidine compared to tiletamine-zolazepam-medetomidine for immobilisation of semi-captive cheetahs (Acinonyx jubatus), J S Afr Vet Assoc 93, 25-30. https://doi.org/10.36303/JSAVA.2022.93.1.489. [ Links ]
Burroughs, R., Meltzer, D., Morkel P., 2012, Applied pharmacology, in S. Kathleen (ed.), Chemical and Physical Restraint of Wild Animals, pp. 53-80, IWVS, Greyton. [ Links ]
Bush, M., Citino, S.B., Lance, W.R., 2012, The use of butorphanol in anesthesia protocols for zoo and wild mammals, in R.E. Miller & M. Fowler (eds), Fowler's Zoo and Wild Animal medicine, pp. 596-603, Elsevier, St. Louis. https://doi.org/10.1016/B978-1-4377-1986-4.00077-9. [ Links ]
Buss, P., Miller, M., 2019, Update on field anesthesia protocols for free-ranging African lions, in E. Miller, N. Lamberski & P. Calle, (eds), Fowler's Zoo and Wild Animal Medicine Current Therapy, pp. 536-538, WB Saunders, Edinburgh. https://doi.org/10.1016/B978-0-323-55228-8.00076-X. [ Links ]
Caulkett, N., Arnemo, J., 2015, Chemical immobilization of free-ranging terrestrial mammals, in W.J. Tranquilli, J.C. Thurmon & K.A. Grimm, (eds) Lumb & Jones Veterinary Anesthesia and Analgesia, pp. 807-831, Blackwell Publishing, Ames. [ Links ]
Chabot-Doré, A.J., Schuster, D.J., Stone, L.S., et al., 2015, Analgesic synergy between opioid and a2-adrenoceptors, Br J Pharmacol 172, 388-402. https://doi.org/10.1111/bph.12695. [ Links ]
Clarke, K.W., Trim, C.M., Hall, L.W. (eds), 2014, Veterinary Anaesthesia. 11th edn. pp. 499-530, W.B. Saunders, Oxford. [ Links ]
Daigle, C., Brown, J.L., Carlstead, K., et al., 2015, Multi-institutional survey of social, management, husbandry and environmental factors for the SSP African lion Panthera leo population: examining the effects of a breeding moratorium in relation to reproductive success, Int Zoo Yearb 49, 198-213. https://doi.org/10.1111/izy.12073. [ Links ]
Deem, S.L., Ko, J.C., Citino, S.B., 1998, Anesthetic and cardiorespiratory effects of tiletamine-zolazepam-medetomidine in cheetahs, J Am Vet Med Assoc 213, 1022-1026. [ Links ]
De Witte, J., Sessler, D.I., 2002, Perioperative shivering: physiology and pharmacology, Anaesthesiology 96, 467-484. https://doi.org/10.1097/00000542-200202000-00036. [ Links ]
Díaz, M., Becker, D.E., 2010, Thermoregulation: physiological and clinical considerations during sedation and general anesthesia, Anesth Prog 57, 25-33. https://doi.org/10.2344/0003-3006-57.L25. [ Links ]
Fahlman, A., Loveridge, A., Wenham, C., et al., 2005, Reversible anaesthesia of free-ranging lions (Panthera leo) in Zimbabwe, J S Afr Vet Assoc 76, 187-192. https://doi.org/10.4102/jsava.v76i4.424. [ Links ]
Faul, F., Erdfelder, E., Lang, A.G. et al., 2007, G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences, Behav Res Methods 39,175-191. https://doi.org/10.3758/BF03193146. [ Links ]
Feher, J., 2017, Energy balance and regulation of food intake, in J. Feher (ed.), Quantitative Human Physiology, 2nd edn, pp. 834-846, Academic Press, Boston. https://doi.org/10.1016/B978-0-12-800883-6.00082-3. [ Links ]
Fyumagwa, R.D., Bugwesa, Z.K., Mdaki, M.L., et al., 2012, Comparison of anaesthesia and cost of two immobilization protocols in free-ranging lions, South African Journal of Wildlife Research 42, 67-70. https://doi.org/10.3957/056.042.0102. [ Links ]
Hanna, R.M., Borchard, R.E., Schmidt, S.L., 1988, Pharmacokinetics of ketamine HC1 and metabolite I in the cat: a comparison of i.v., i.m., and rectal administration, J Vet Pharmacol Ther 11, 84-93. https://doi.org/10.1111/j.1365-2885.1988.tb00125.x. [ Links ]
Herbst, L.H., Packer, C., Seal, U.S., 1985, Immobilization of free-ranging African lions (Panthera leo) with a combination of xylazine hydrochloride and ketamine hydrochloride, J Wildl Dis 21, 401-404. https://doi.org/10.7589/0090-3558-21.4.401. [ Links ]
Hetem, R.S., Mitchell, D., De Witt, B.A., et al., 2013, Cheetah do not abandon hunts because they overheat, Biol Lett 9, 20130472. https://doi.org/10.1098/rsbl.2013.0472. [ Links ]
Isaza R., 2014, Remote drug delivery, in G. West, D. Heard & N. Caulkett (eds), Zoo animal and wildlife immobilization and anesthesia, pp. 155-169, Wiley Blackwell, Ames. https://doi.org/10.1002/9781118792919.ch11. [ Links ]
Jacquier, M., Aarhaug, P., Arnemo, J.M., et al., 2006, Reversible immobilization of free-ranging African lions (Panthera leo) with medetomidine-tiletamine-zolazepam and atipamezole, J Wildl Dis 42, 432-436. https://doi.org/10.7589/0090-3558-42.2.432. [ Links ]
Jessen C., 2001, Temperature regulation in humans and other mammals, Springer-Verlag, Berlin, pp. 193. https://doi.org/10.1007/978-3-642-59461-8. [ Links ]
Johansson, Ö., Malmsten, J., Mishra, C., et al., 2013, Reversible immobilization of free-ranging Snow Leopards (Panthera uncia) with a combination of medetomidine and tiletamine-zolazepam, J Wildl Dis 49, 338-346. https://doi.org/10.7589/2012-02-049. [ Links ]
King, R., Lapid, R., Epstein, A., et al., 2008, Field anesthesia of golden jackals (Canis aureus) with the use of medetomidine-ketamine or medetomidine-midazolam with atipamezole reversal, J Zoo Wildl Med 39, 576-581. https://doi.org/10.1638/2008-0010.1. [ Links ]
Kiyatkin, E.A., 2005, Brain hyperthermia as physiological and pathological phenomena, Brain Res Rev 50, 27-56. https://doi.org/10.1016/j.brainresrev.2005.04.001. [ Links ]
Kurz, A., 2008. Physiology of thermoregulation, Best Pract Res Clin Anaesthesiol 22, 627-644. https://doi.org/10.1016/j.bpa.2008.06.004. [ Links ]
Lamont, L.A., Grimm, K.A., 2014, Clinical Pharmacology, in G. West, D. Heard & N. Caulkett (eds), Zoo animal and wildlife immobilization and anesthesia, Wiley Blackwell, Ames, pp. 4-42. https://doi.org/10.1002/9781118792919.ch1. [ Links ]
Larsen, R.S., Loomis, M.R., Kelly, B.T., et al., 2002, Cardiorespiratory effects of medetomidine-butorphanol, medetomidine-butorphanol-diazepam, and medetomidine-butorphanol-ketamine in captive red wolves (Canis rufus), J Zoo Wildl Med 33, 101-107. https://doi.org/10.1638/1042-7260(2002)033[0101:CEOMBM]2.0.CO;2. [ Links ]
Lepock, J.R., 2003, Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage, Int J Hyperthermia 19, 252-266. https://doi.org/10.1080/0265673031000065042. [ Links ]
Lin, H.C., Thurmon, J.C., Benson, G.J. et al., 1993, Telazol-a review of its pharmacology and use in veterinary medicine, J Vet Pharmacol Ther 16, 383-418. https://doi.org/10.1111/j.1365-2885.1993.tb00206.x. [ Links ]
Mathews, N.S., Hartke, S., Allen, J.C., 2003, An evaluation of pulse oximeters in dogs, cats and horses, Vet Anaesth Analg 30, 3-14. https://doi.org/10.1046/j.1467-2995.2003.00121.x. [ Links ]
Miller, M., Buss, P., Hofmeyr, J., et al., 2015, Antemortem diagnosis of Mycobacterium bovis infection in free-ranging African lions (Panthera leo) and implications for transmission, J Wildl Dis 51, 493-497. https://doi.org/10.7589/2014-07-170. [ Links ]
Mtetwa, T.K., Snelling, E.P., Buss, P.E., et al., 2022, Reliability of pulse oximetry, at four different attachment sites, in immobilized white rhinoceros (Ceratotherium simum), Vet Anaesth Analg, 49(6), 650-655. https://doi.org/10.1016/j.vaa.2022.08.006. [ Links ]
Muller, L.I., Osborn, D.A., Ramsay, E.C., et al., 2007, Use of xylazine/ketamine or medetomidine combined with either ketamine, ketamine/butorphanol, or ketamine/telazol for immobilization of White-tailed deer (Odocoileus virginianus), Journal of Animal Veterinary Advances 6, 435-440. [ Links ]
Posner, L.P., Pavuk, A.A., Rokshar, J.L., et al., 2010, Effects of opioids and anesthetic drugs on body temperature in cats, Vet Anaesth Analg 37, 35-43. https://doi.org/10.1111/j.1467-2995.2009.00508.x. [ Links ]
Quandt, S.K.F., 1993, Immobilization of African lions (Panthera leo) with medetomidine/ketamine, in comparison with tiletamine/zolazepam and phencyclidine, in Proceedings of an International Symposium on the Capture, Care and Management of Threatened Mammals. Skukuza, Kruger National Park, South Africa, 14-18 September, 1993, pp. 64-75. [ Links ]
Ramsay, E.C., 2014, Felids, in G. West, D. Heard & N. Caulkett (eds), Zoo animal and wildlife immobilization and anesthesia, Wiley Blackwell, Ames, pp. 635-647. https://doi.org/10.1002/9781118792919.ch45. [ Links ]
Semjonov, A., Adrianov, V., Raath, J.P., et al., 2017, Evaluation of BAM (butorphanol-azaperone-medetomidine) in captive African lion (Panthera leo) immobilization, Vet Anaesth Analg 44, 883-889. https://doi.org/10.1016/j.vaa.2017.02.001. [ Links ]
Sinclair, M.D., 2003, A review of the physiological effects of a2-agonists related to the clinical use of medetomidine in small animal practice, Can Vet J 44, 885-897. [ Links ]
Singewald, N., Kaehler, S.T., Hemeida, R., et al., 1998, Influence of excitatory amino acids on basal and sensory stimuli-induced release of 5-HT in the locus coeruleus, Br J Pharmacol 123, 746-752. https://doi.org/10.1038/sj.bjp.0701656. [ Links ]
Smuts, G., Anderson, J., Austin, J., 1978, Age determination of the African lion (Panthera leo), J Zool 185, 115-146. https://doi.org/10.1111/j.1469-7998.1978.tb03317.x. [ Links ]
Smuts, G.L., Bryden, B.R., De Vos, V. et al., 1973, Some practical advantages of CI-581 (ketamine) for the field immobilization of larger wild felines, with comparative notes on baboons and impala, The Lammergeyer 18, 1-14. [ Links ]
Stander, P.E., Morkel, P., 1991, Field immobilization of lions using dissociative anaesthetics in combination with sedatives, Afr J Ecol 29, 137-148. https://doi.org/10.1111/j.1365-2028.1991.tb00994.x. [ Links ]
Stegmann, G.F., Jago, M., 2006, Cardiopulmonary effects of medetomidine or midazolam in combination with ketamine or tiletamine/zolazepam for the immobilisation of captive cheetahs (Acinonyx jubatus), J S Afr Vet Assoc 77, 205-209. https://doi.org/10.4102/jsava.v77i4.378. [ Links ]
Taylor, S.S., Sparkes, A.H., Briscoe, K., et al., 2017, ISFM consensus guidelines on the diagnosis and management of hypertension in cats, J Feline Med Surg 19, 288-303. https://doi.org/10.1177/1098612X17693500. [ Links ]
Tomizawa, N., Tsujimoto, T., Itoh, K., et al., 1997, Chemical restraint of African lions (Panthera leo) with medetomidine-ketamine, J Vet Med Sci 59, 307-310. https://doi.org/10.1292/jvms.59.307. [ Links ]
Trethowan, P., Fuller, A., Haw, A., et al., 2017, Getting to the core: Internal body temperatures help reveal the ecological function and thermal implications of the lions' mane, Ecol Evol 7, 253-262. https://doi.org/10.1002/ece3.2556. [ Links ]
Ulrich-Lai, Y.M., Herman, J.P., 2009, Neural regulation of endocrine and autonomic stress responses, Nat Rev Neurosci 10, 397-409. https://doi.org/10.1038/nrn2647. [ Links ]
Van Gaalen, M., Kawahara, H., Kawahara, Y, et al., 1997, The locus coeruleus noradrenergic system in the rat brain studied by dual-probe microdialysis, Brain Res 763, 56-62. https://doi.org/10.1016/S0006-8993(97)00416-2. [ Links ]
Van Wyk, H.H., Berry, T.C., 1986, Tolazoline as an antagonist in free-living lions immobilised with a ketamine-xylazine combination, J S Afr Vet Assoc 57, 221-224. [ Links ]
Wenger, S., Buss, P., Joubert, J., et al., 2010, Evaluation of butorphanol, medetomidine and midazolam as a reversible narcotic combination in free-ranging African lions (Panthera leo), Vet Anaesth Analg 37, 491-500. https://doi.org/10.1111/j.1467-2995.2010.00569.x. [ Links ]
White, C.R., Seymour, R.S., 2014, The role of gravity in the evolution of mammalian blood pressure, Evolution 68, 901-908. https://doi.org/10.1111/evo.12298. [ Links ]
White, J.M., Ryan, C.F., 1996, Pharmacological properties of ketamine, Drug Alcohol Rev 15, 145-155. https://doi.org/10.1080/09595239600185801. [ Links ]
Wilson, R.P., Zagon, I.S., Larach, D.R., et al., 1993, Cardiovascular and respiratory effects of tiletamine-zolazepam, Pharmacol Biochem Behav 44, 1-8. https://doi.org/10.1016/0091-3057(93)90274-W. [ Links ]
 Corresponding author
Corresponding author
email: acdonaldson@gmail.com
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














