Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.93 n.2 Pretoria 2022
http://dx.doi.org/10.36303/JSAVA.164
ORIGINAL RESEARCH
Knowledge, attitudes, and practices of veterinarians on antibiotic use and resistance and its containment in South Africa
SA Maruve; SY Essack
Antimicrobial Research Unit, College of Health Sciences, University of KwaZulu-Natal, South Africa
ABSTRACT
The inappropriate use of antibiotics in the veterinary sector has contributed to antibiotic resistance (ABR), which negatively impacts animal health and welfare. Understanding the knowledge, attitudes, and practices (KAP) on antibiotic use, ABR, and its containment amongst veterinarians is critical to optimise antibiotic use and contain resistance.
A quantitative questionnaire-based online survey was conducted amongst members of professional veterinary associations. The questionnaire consisted of four sections focusing on socio-demographic characteristics, KAP of participants on antibiotic use, ABR, and its containment in the South African veterinary sector. The Independent t-test, analysis of variance (ANOVA), and chi-square test were used to establish associations among selected socio-demographic variables and selected KAP parameters.
A total of 130 responses were received from 2 178 animal health professionals, yielding a response rate of six per cent, with 102 complete responses constituting the final sample size. Self-reported knowledge on antibiotic stewardship, ABR mechanisms, and pharmacology was good at 96 (94.1%), 91 (89.2%), and 70 (68.6%), respectively. Notably, most of the veterinarians (61; 59.8%) lacked an antibiotic stewardship programme at their practice. Place of practice was significantly associated (p = 0.004) with possession of knowledge about ABR. Veterinarians in urban practice were more knowledgeable about ABR than those in rural practice.
Antibiotic stewardship programmes need to be implemented in veterinary practice. Such programmes might encourage the frequent use of consensus guidelines for the appropriate use of antibiotics and microbiology-informed therapy.
Keywords: antibiotics, antibiotic resistance, antibiotic stewardship, knowledge, attitudes, practices
Introduction
Inappropriate antibiotic prescribing and increased antibiotic consumption in animals have contributed to antibiotic resistance (ABR) (Reygaert 2018). Human health is negatively impacted by ABR in animal health as observed in resistance to colistin due to its use in animal production. Colistin is an antibiotic of last resort in humans for the treatment of infections caused by multidrug-resistant Gram-negative bacteria (Rhouma et al. 2016). Loss of antibiotic efficacy compromises human therapy (Van Vuuren et al. 2007). Antibiotic use in animal health applies a selective pressure that favours ABR emergence and spread (Chantziaras et al. 2014; Pomba et al. 2017), limiting treatment options for infections in animals and resulting in treatment failure that negatively affects food production, livelihoods, and food security (Food and Agriculture Organisation [FAO] 2019). It is estimated that by 2050, an 11% drop in animal productivity is set to occur due to ABR (World Bank 2017). Loss of antibiotic efficacy due to resistance will also have a negative impact on animal health and welfare. Damage to the tissues and organs can occur as a result of bacterial infections, and there is a high risk of disease outbreaks and the dissemination of zoonotic bacteria. The problem is further exacerbated by a lack of new antibiotics that are specifically being produced for animal health (Vaarten 2012).
The Global Action Plan (GAP) on Antimicrobial Resistance (AMR) was adopted by the World Health Assembly in 2015 (World Health Organization [WHO] 2015). This was a joint initiative of the tripartite alliance of the WHO, World Organization for Animal Health (OIE) and the FAO of the United Nations (WHO 2015). In support of the GAP, the OIE aims to increase awareness and understanding and enhance knowledge via surveillance and research among its strategic objectives. Information materials targeting veterinarians and other stakeholders will be developed to understand ABR risks. A professional culture that ensures appropriate antibiotic use in animal health will be encouraged via veterinary institutions and veterinary governing bodies. OIE guidelines, scientific and educational materials used for reference in mitigating the development and dissemination of antibiotic-resistant organisms will be expanded while simultaneously encouraging efficient disease prevention measures to avoid unnecessary antibiotic use in animals (World Organization for Animal Health [OIE] 2016a). Also in support of GAP, FAO focuses on key areas that include improving the level of awareness on ABR and its associated threats and developing capacity for ABR and antibiotic use surveillance and monitoring in food and agriculture. FAO also focuses on promoting good practices in systems of food and agriculture and appropriate antibiotic use. Knowledge of ABR and antibiotic use will also be improved by developing educational materials, promoting research and contributing to continuing education, professional education, postgraduate training and certification (FAO 2016).
Veterinarians are responsible for prescribing and administering antibiotics to animal patients and are critical to appropriate antibiotic use in animal health. We therefore conducted an online questionnaire-based survey to determine the knowledge, attitudes and practices (KAP) of veterinarians on antibiotic use, resistance, and its containment in South Africa in order to inform interventions to optimise use and contain resistance.
Materials and methods
Study design
A quantitative questionnaire-based online survey was conducted amongst members of the South African Veterinary Association (SAVA) and the South African Association for Laboratory Animal Science (SAALAS) whose members are veterinarians, veterinary nurses, animal health technicians and veterinary technologists. SAVA and SAALAS disseminated a link to the questionnaire to their membership via email. The survey was open from 1 October 2019 till 31 October 2019.
Survey instrument
Data was collected using an online self-administered questionnaire (supplementary material) compiled from four questionnaires used in previous similar studies (Ekakoro & Okafor 2019; Hardefeldt et al. 2018; Sadiq et al. 2018; University of Colorado Denver 2018). The questionnaire was divided into four sections consisting of closed-ended questions (yes or no) and Likert style (strongly agree/agree/neutral/disagree/strongly disagree) questions. Section 1 focused on the socio-demographic characteristics of participants and sections 2-4 dealt with KAP of participants on antibiotic use, ABR, and its containment in the South African veterinary sector. Two veterinarians were used in the pilot, and the questions were amended as appropriate.
Data analysis
Data was entered on the Microsoft Excel spreadsheet and analysed using commercial statistical software (SAS, version 9.4, SAS Institute Inc, Cary, NC). Variables were analysed using descriptive statistics and presented as percentages at 95% confidence intervals. Before analysis, responses to the questions were merged as follows: for knowledge, "very important" and "extremely important" responses were merged into one variable as were "strongly agree" and "agree", "strongly disagree" and "disagree", "not at all" and "a little", and, "quite a bit" and "very much"; while for attitudes, "not concerned" and "slightly concerned", "quite concerned" and "very concerned", "strongly disagree" and "disagree" and "agree" and "strongly agree" were merged as one variable. For practices, "very important" and "extremely important" were merged as one variable; this was also true for "never" and "rarely" and "very often" and "always". The merged variables were then analysed using the chi-square test. Knowledge-related statements on ABR carried a total score of 13 and a 50% score (6.5) was used to classify the respondent as knowledgeable. Correctly identifying contributors to antibiotic-resistant infections in people and factors in veterinary medicine leading to antibiotic-resistant infections carried a score of 5 and 7 respectively. The Independent t-test (comparing mean values of only two groups) and the analysis of variance (ANOVA) with Tukey multiple comparison (comparing mean values of more than two groups) were then used to establish associations between the socio-demographic variables and KAP parameter. The criterion for statistical significance was set at 0.05.
Results
In total, 130 responses were received from 2 178 animal health professionals (response rate 6%), all of whom were veterinarians. Among the 130 responses received, only 102 responses were fully complete and constituted the final sample. From the 102 responses that underwent analysis, 49 (48%) were female, and 53 (52%) were male. Seventy (68.6%) of the respondents were more than 40 years of age, while 32 (31.4%) were aged between 20 and 40. Fifty respondents (49%) had more than 20 years of veterinary experience, while 34 (33.3%) had less than 11 years of veterinary experience. Thirty-eight (37.3%) of the respondents were registered as specialists, 64 (62.7%) practised in an urban setting and 38 (37.3%) practised in a rural setting (Table I). The vast majority of respondents (74.5%) were in general small/ mixed animal practice and two-thirds spent 80-100% in clinical practice. Gauteng province had the highest number of respondents at 42 (41.2%) followed by Western Cape with 24 (23.5%) and KwaZulu-Natal with 15 (14.7%). Ninety-three (91.2%) of the respondents obtained their veterinary qualifications at the University of Pretoria, while nine (8.8%) attended other universities. Table I shows selected socio-demographic data used in statistical analysis with complete socio-demographic data contained in Supplementary Table SI.

Knowledge
Participants had good knowledge of ABR (Table II). (Based on a total score of 13, the average score for the respondents was 11.57 ± 1.33). Veterinarians in urban practice were more knowledgeable about ABR (11.86 ± 1.00) compared to those in rural practice (11.08 ± 1.67). The average score on contributors to antibiotic-resistant infections in people was low (Based on a total score of 5, the respondents' average score was 1.99 ± 1.13). The average score on selecting factors in veterinary medicine that may play a role in antibiotic-resistant infections in people was relatively good (Based on a total score of 7, the average score for the respondents was 3.95 ± 1.17). All the respondents were aware that drug-resistant infections could be difficult to treat and they also believed that antibiotics should be prescribed by veterinarians only when necessary. Almost all of the respondents recognised the danger antibiotic-resistant infections posed on surgical procedures. About 73% of the respondents recognised ABR as one of the biggest problems in livestock production. More than 60% of the respondents recognised that antibiotics should not be stored for later use in animals, i.e. bottles of antibiotic solutions for injection, once opened, need to be discarded after 28 days. Of note was that 6% of the participants believed that ABR is not an issue in South Africa.

None of the veterinarians thought antibiotics were warranted in ectoparasite infestation, while 80 (78.4%) and 98 (96.1%) thought they were necessary for skin infection and respiratory tract infection, respectively. Thirty-one (30.4%) thought they were necessary in diarrhoea, and 32 (31.4%) thought the same for fever (Supplementary Table SII). The narrow-spectrum antibiotics amoxicillin and procaine penicillin were identified as first-line treatment by 79 (77.5 %) and 50 (49%) respondents, respectively. Most respondents identified other broad-spectrum antibiotics such as amoxicillin-clavulanate (62; 60.8%) and metronidazole (64; 62.7%) as first-line, while a few identified rifampicin (1; 1%), amikacin (2; 2%), and marbofloxacin (5; 4.9%) as first-line (Figure 1A). Penicillins were prescribed most often by most respondents (72; 70.6%), while aminoglycosides were less frequently prescribed (46; 45.1%) (Figure 1B). Notably, a majority never administered or prescribed the antibiotics linezolid (102; 100%), vancomycin (99; 97.1%), and imipenem (96; 94.1%). However, most respondents (72; 70.6%) had prescribed polymyxin B (Terra-cortril® and or Surolan®) at some point in time.
Generally, the respondents asserted that they had good knowledge of the pharmacology of antibiotics (70; 68.6%) and appropriate antibiotic use that minimises the development of ABR (83; 81.4%). This was also articulated in relation to understanding antibiotic stewardship (96; 94.1%) and ABR mechanisms (91; 89.2%) (Supplementary Figure 1). More than half the respondents were confident that their veterinary training equipped them quite a bit/very much (60; 58.8%) on knowledge on rational antibiotic use. A majority of the respondents (91; 89.2%) were interested in obtaining continuing professional education on antibiotic use, resistance, or stewardship while a few were not sure (8; 7.8%). Only three (2.9%) were not interested in obtaining continuing professional education. The veterinary formulary, peer-reviewed scientific literature and textbooks or drug handbooks were considered to be the most important sources of information on antibiotics by 83 (81.4%), 82 (80.4%) and 80 (78.5%) of the participants, respectively (Supplementary Figure 2).
Attitudes
A majority of the respondents (81; 79.4%) believed that antibiotics were sometimes prescribed for suspected but not confirmed infections. Fifteen (14.7%) believed antibiotics were prescribed based on confirmed infection, while six (5.9%) believed antibiotics were sometimes prescribed based on no documented evidence of infection. A little more than half (56; 54.9%) the respondents believed that antibiotics were optimally prescribed, 45 (44.1%) believed that antibiotics were overprescribed and one respondent (1%) believed antibiotics were under-prescribed at their practice (Supplementary Figure 3). The concern related to antibiotic-resistant infections amongst veterinarians was inverted amongst their clients (Figure 2) with the majority of the respondents (77; 75.5%) being quite concerned/very concerned about antibiotic-resistant infections compared to 19 (18.7%) of clients expressing the same concern (Figure 2).

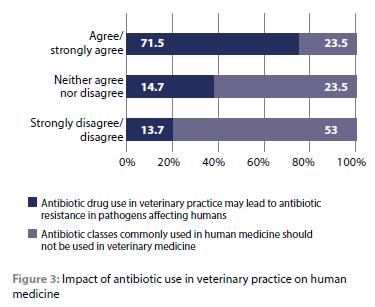
Only 24 (23.5%) respondents agreed/strongly agreed, while 54 (53%) disagreed/strongly disagreed that the antibiotic classes commonly used in human medicine should not be used in veterinary medicine as they select for antibiotic-resistant bacteria. A majority of the respondents (73; 71.5%) agreed that antibiotic drug use in veterinary practice might lead to ABR in pathogens affecting humans (Figure 3).
The cost of diagnostics (90; 88.2%), time to results of diagnostics (83; 81.4%), and client expectations of receiving antibiotics (74; 72.5%) were the most prominent barriers to implementing an antibiotic stewardship plan in their veterinary practices.
Practices and experiences
Persistent infection (89; 87.3%), recurring infections (73; 71.6%), and client finances (48; 47.1%) were the most important factors in influencing the decisions of veterinarians to submit samples for microbial culture and susceptibility testing. Results of bacteriological culture and antibiotic susceptibility (86; 84.3%), animal owner compliance with the prescription (77; 75.5%), clinical signs (75; 73.5%) and appropriate use guidelines (73; 71.5%) were considered to be very important in determining the need for and choice of antibiotics while pressure from clients (76; 74.5%), fear of litigation by the animal owner in the event of an undesirable outcome (72; 70.5%) and concern about antibiotic-resistant infections in humans (33; 32.4%) were slightly important (Figure 4).
Veterinary guidelines for appropriate use of antibiotics was sometimes read by 45 (44.1%) of the respondents, 43 (42.2%) did it very often/always while 13 (12.8%) never/rarely did so. Most veterinarians (41; 40.2%) prescribed antibiotics for therapeutic purposes three to five times a day, while 13 (12.8%) prescribed antibiotics twice a day. There was minimal difference in both preoperative prophylaxis of infections and postoperative prevention of infections where veterinarians prescribed antibiotics in one to two of every 10 surgical cases
(Supplementary Figure 3). Twelve (11.7%) of the veterinarians never/rarely discussed ABR with their clients while 54 (52.9%) often/always discussed ABR with their clients. A majority of the respondents (61; 59.8%) did not have an antibiotic stewardship programme at their practice, clinic or institution, only 20 (19.6%) had an antibiotic stewardship programme, while 15 (14.7%) were not sure.
Knowledge on ABR was significantly higher amongst veterinarians in urban practice (p = 0.004) (Supplementary Table III). Rating client concerns on ABR was significantly associated with gender (p = 0.000) (Table III). The frequency of discussing ABR with clients was significantly associated with place of practice (p = 0.035) (Table III). The extent of agreeing on antibiotic drug use in veterinary practice leading to ABR in pathogens affecting humans was significantly associated with veterinary experience (p = 0.006) (Table III). Client finances as an influence on the decision to submit samples for culture and sensitivity was significantly associated with gender (p = 0.002), age (p = 0.035) and experience (p = 0.003) (Table III).
Discussion
A quantitative questionnaire-based online survey was conducted among veterinarians in South Africa. Average knowledge scores on antibiotic use, antibiotic resistance, and its containment in animal health in South Africa were good. The veterinary training the respondents received appeared to equip the vast majority of respondents with adequate knowledge on the rational use of antibiotics. There was a high degree of concern about antibiotic-resistant infections. The veterinarians sometimes consulted veterinary guidelines for appropriate use of antibiotics, and penicillins (procaine penicillin, amoxicillin, amoxicillin-clavulanate) were the most preferred antibiotic class.
Veterinarians in urban practice were more knowledgeable about ABR than those in rural practice (p = 0.004). This may be due to fewer ABR awareness campaigns targeting the rural areas as compared to urban areas. From the results, a few of the respondents believed that ABR is not an issue in South Africa. ABR is not only a threat in South Africa but worldwide. This is evident in efforts to address ABR in the form of the South African National Strategy Framework (locally) (Department of Health [DoH] 2018) and the FAO and OIE action plans (internationally) (FAO 2016; OIE 2016a). Having a belief that ABR is not a threat might hinder the implementation of antibiotic stewardship programmes in animal health, thus worsening ABR, as was evident in this study where few respondents had antimicrobial stewardship programmes in their practices. Antibiotic stewardship refers to approaches that encompass correct antibiotic usage, dosage, and duration whilst preventing ABR. It involves the use of guidelines for appropriate use of antibiotics, culture and sensitivity testing before antibiotic usage, surveillance and monitoring of antibiotic usage, client education, and the implementation of effective infection prevention and control programmes (Guardabassi & Prescott 2015).
Generally, the respondents asserted that they had good knowledge of antibiotics' pharmacology, appropriate antibiotic use that minimises the development of ABR, antibiotic stewardship and ABR mechanisms. This is in contrast to a study done in 2017 in Nigeria to assess the perceptions, knowledge, and practices of antibiotic stewardship among 280 veterinarians in Enugu State. In the study, few respondents had heard about antibiotic stewardship (17.1%), and overall knowledge on the matter was poor (21.4%) (Anyanwu & Kolade 2017). Such differences in the two studies could be attributed to recent antibiotic stewardship implementation efforts in animal health. Instituting antibiotic stewardship programmes at curricular and practice level will enable appropriate use of antibiotics, thus aiding in minimising ABR emergence and spread. However, effective implementation might be hindered by poor use of microbial culture and susceptibility tests, absence of well-trained veterinary professionals on antibiotic stewardship, gaps in knowledge on current resistance patterns and the lack of evidence-based guidelines (Guardabassi & Prescott 2015).
To a greater extent, the respondents from our study believed the veterinary training they received adequately equipped them with knowledge on rational antibiotic use. One would have expected the recent prioritisation of ABR on the public health agenda to cause disparities between recently graduated veterinarians and their counterparts with more veterinary experience. However, this was not the case in this study. Adequate knowledge of rational antibiotic use combined with the right attitude will enable best practice and effective ABR containment strategies. However, adequate training alone on rational antibiotic use might not translate to appropriate antibiotic use. Gaps might exist in the application of theoretical aspects in practice (Smith et al. 2019). This might have been the case in our study, where respondents identified some broad-spectrum antibiotics as first-line. Knowledge of rational antibiotic use should be among the priority areas in continuing professional development and postgraduate veterinary studies (Smith et al. 2019).
In our study, most respondents were concerned about ABR. It is worth noting that the GAP on AMR was published in 2015 and followed by the OIE and FAO action plans in 2016. Such initiatives could have managed to raise awareness on the implications of antibiotic-resistant infections in animal health, thus leading to a high level of concern among veterinarians, concern that is necessary for the effective implementation of antibiotic stewardship programmes and effective ABR containment strategies. Combined with adequate knowledge, it will also favour practices that help preserve the existing finite antibiotic arsenal. Generally, the veterinarians viewed their clients as being slightly concerned over antibiotic-resistant infections. This may be due to a low frequency of discussions with clients over the ABR matter. It could have also been due to the lack of clarity when discussions on the matter were held with veterinarians. In our study, veterinarians sometimes discussed ABR with their clients. A more significant percentage of veterinarians in rural practice frequently carried out such discussions than their urban counterparts (p = 0.035). Such disparities might be influenced by an over-reliance on antibiotics in clients from rural areas compared to those in urban areas, hence the need for frequent discussions in those areas. Busy schedules due to high patient loads might hinder such discussions from taking place. Discussing ABR with the clients will assist in raising awareness on the subject. This will help ensure that clients adhere to antibiotic dosages stipulated by veterinarians for their animals, thus, averting client practices that favour ABR emergence and spread.
In our study, most respondents believed that antibiotics were sometimes prescribed for suspected but not confirmed infections regardless of bacteriological culture and antibiotic susceptibility importance. This suggests that an empiric approach to treatment could be preferred compared to a microbiology-guided approach in some instances. Microbiology forms an integral part of antibiotic stewardship with microbial culture and susceptibility testing as part of the diagnostic testing (Guardabassi & Prescott 2015). Notably, most respondents indicated the cost of diagnostic testing, time to results of diagnostics and client expectations of receiving antibiotics as barriers to the implementation of antibiotic stewardship programmes. As expected, client finances were among the important factors that could influence the submission of samples for microbiological investigations. All these factors combined could be responsible for such an empiric approach to antibiotic treatment. Microbiology-guided treatment would be preferable to empiric treatment. This is because it aids the veterinarian in choosing an ideal antibiotic in an infection, thus preserving the currently available antibiotic arsenal and maintaining animal welfare in the process (Watts et al. 2018).
Most respondents read guidelines for appropriate use of antibiotics. The use of any veterinary formulary, peer-reviewed scientific literature, textbooks or drug handbooks were chosen as the most important sources of information in determining antibiotic choice for clinical use. The results are almost similar to a study done at a veterinary teaching facility in the USA among veterinary clinicians. In that study, the respondents chose peer-reviewed scientific literature (56.5%) and textbook or drug handbook (24.2%) as the most important sources of information (Ekakoro & Okafor 2019). Conversely, in a study done in Australia among veterinarians in companion animal medicine, only 36% of them used peer-reviewed literature as an information source (Hardefeldt et al. 2017). The use of peer-reviewed literature, drug handbooks, and formularies assists in ensuring appropriate antibiotic use. It can also control the use of antibiotics designated to be critically important for both human and veterinary medicines (De Briyne et al. 2014). Consequently, this may assist in curbing the further emergence and dissemination of ABR. One major drawback is that the use of information from such sources may not be based on local resistance surveillance data. Currently, a national antimicrobial surveillance programme is yet to be reintroduced in the South African animal health sector (DoH 2018). The use of local resistance surveillance data is imperative in order to guide in antibiotic prescribing in a manner that minimises ABR dissemination while optimising on antibiotic choice. Notably, antibiotic classes and their respective antibiotic agents which are of clinical importance both in human and veterinary medicine must be included in monitoring programmes to help preserve their efficacy. This entails using the WHO list of critically important antibiotics and the OIE list of antibiotics of veterinary importance (OIE 2015).
Most respondents prescribed antibiotics for preoperative prophylaxis and the prevention of postoperative infections.
Avoiding prophylactic antibiotic use is necessary to minimise the selection of resistant bacteria and possible transfer. However, prophylactic use of antibiotics can be implemented in high infection risk cases and where the infection consequences are grave. Such cases include surgical procedures with a duration of more than one and a half hours and surgery involving a break in asepsis. Prophylactic antibiotic use is also indicated in surgery of the central nervous system, gastrointestinal tract, and contaminated wounds (Ramsey 2017). Although the cases in which antibiotics were indicated for preoperative prophylaxis and the prevention of postoperative infections were not assessed in our study, appropriate prophylactic antibiotic use should still be encouraged among veterinarians.
A number of the respondents believed antibiotics were neces-sary in both diarrhoea and fever cases from our study. Diarrhoea cases do not generally require antibiotic therapy unless the identification of a specific bacterial causative agent has taken place or haemorrhage due to intestinal ulceration is observed (Battersby & Harvey 2006). Antimicrobial stewardship programme implementation might help ensure that proper diagnostic approaches are followed to prevent unnecessary antibiotic use.
Antibiotics that were regarded as the first-line by many respondents were generally the frequently prescribed antibiotics classes. A majority of these antibiotics fall under the OIE list of antibiotics of veterinary importance. These drugs are important in specific infections and have to be preserved because of the few alternatives available (OIE 2019). Impressively, most respondents never prescribed linezolid, vancomycin, and imipenem. These drugs fall under the WHO list of critically important antibiotics. Most respondents had however prescribed polymyxin B at some point in time (WHO 2019).
It should be noted that polymyxin B is structurally similar to colistin and both fall under the polymyxin class of antibiotics. In 2016, the WHO list of critically important antibiotics underwent revision, and polymyxins were re-classified into critically important antimicrobials of the highest priority. The re-classification came after an increase in colistin use in the treatment of severe human infections the world over, the discovery of genes that confer resistance to colistin on plasmids (mcr genes), and the dissemination of colistin-resistant bacteria through the food chain (WHO 2019). OIE thus set the following recommendations on the use of colistin: (i) it should not be used to prevent infection nor be administered through water or feed without clinical signs in the animal(s) proposed to receive treatment, (ii) use as a first-line antibiotic should be justified and use as a second-line antibiotic should be based on bacteriological test results, (iii) its off-label use must be restricted, and, in such instances, it should be used in the absence of alternatives and should be in line with the current national legislation, and (iv) use in growth promotion is prohibited (OIE 2019). In our study, many respondents considered the broad-spectrum antibiotics amoxicillin-clavulanate and oxytetracycline to be first-line. Narrow-spectrum antibiotics should be preferred to broad-spectrum antibiotics in first-line treatment because broad-spectrum antibiotics are more likely to promote ABR emergence (Da Silva et al 2013).
Our results are similar to those observed among veterinarians at a USA veterinary teaching facility in a 2017 study where penicillins were frequently prescribed and preferred to other antibiotic classes of medical importance (Ekakoro & Okafor 2019). The OIE has also provided some useful insight in its annual reports on antibiotic usage in animals that have been published since 2016. The reports are intended to create a clear understanding of antibiotic use in animals from a global perspective, which can assist in providing effective containment strategies (OIE 2016b). In these reports, data was collected from OIE and non-OIE members through the use of templates and guidance documents developed by OIE and tested among member countries. The templates determined antibiotic classes sold or used for growth promotion and therapy for all animal species in kg of active ingredient (OIE 2016b; OIE 2017; OIE 2018). According to data collected between 2012 and 2015 from 34 European OIE member states, penicillins accounted for a third (34%) of all antibiotics used in animals (OIE 2016b). Penicillins constituted almost 30% of all antibiotic classes used in animals from data collected from 107 countries between 2013 and 2016 (OIE 2017). Furthermore, data collected in 116 countries between 2015 and 2017 showed tetracyclines (34.5%) to be the most commonly reported antibiotic class followed by penicillins making up 15.2% of the total proportion of antibiotic classes used in animals (OIE 2018). Penicillins are currently classified under critically important antibiotics by the OIE. They are important in the management of potential life-threatening animal conditions such as urinary tract infections and septicaemias, where alternative cost-effective antibiotics are few (OIE 2019). Penicillins are also currently classified under medically important antibiotics in human medicine by the WHO (WHO 2019). Frequent prescribing of penicillin and all other antibiotics exerts selection pressure for resistance development which poses a serious human and animal health risk the world over. It is thus vital that veterinary prescribers exercise due diligence when prescribing all antibiotics.
Conclusion
From our study, gaps in the KAP on antibiotic use, ABR, and its containment were identified. Knowledge of rational antibiotic use and ABR should be included in continuing professional development programmes to address such gaps in different geographical locations. Antibiotic stewardship programmes need to be implemented in veterinary practice, cost-effective diagnostic tests with shorter turnaround time might assist in achieving such. Antibiotic stewardship programmes might encourage the frequent use of guidelines for the appropriate use of antibiotics and microbiology-informed therapy. Client education on ABR is imperative; more discussions between veterinarians and their clients over ABR are necessary to help change client attitudes. There is a need for veterinarians to use narrow-spectrum antibiotics as first-line drugs where possible. Use of the OIE list of veterinary important antibiotics and the WHO list of medically important antibiotics should be encouraged to conserve the antibiotics of critical importance. Veterinarians need to be proactive in reporting adverse antimicrobial drug events together with suspected lack of efficacy antibiotics which may be attributed to ABR to the respective pharmaceutical companies. Such reporting will help the pharmaceutical industry to investigate increasing trends in ABR to their products and act accordingly.
Acknowledgements
The authors are grateful to the veterinarians who participated in the study, Prof. M van Vuuren, Dr S Mangera, and Dr K McIver for piloting the questionnaire, Mr K Ngwenya for programming the questionnaire online, Dr ZG Dessie for undertaking the statistical analysis, and SAALAS and SAVA for circulating the questionnaire amongst its membership.
Conflict of interest
Professor Essack is the chairperson of the Global Respiratory Infection Partnership and member of the Global Hygiene Council, both sponsored by unrestricted educational grants from Reckitt and Benckiser (Ltd.) UK. The other author does not have any competing interest.
Authors contributions
SYE and SA co-conceptualised the project. SA applied for ethical approval, did the fieldwork and wrote the draft manuscript. SYE facilitated ethical approval, guided and vetted the data analysis and undertook critical revision of the manuscript.
Funding source
This work was supported by the South African Medical Research Council (SAMRC) and Swedish FORTE as well as the University of KwaZulu-Natal College Of Health Sciences Masters Scholarship. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the organisations or agencies that provided support for the project. The funders had no role in the study design, nor the decision to submit the work for publication.
Data availability statement
Data supporting the study findings are available in its supplementary material.
Disclaimer
Views and opinions presented in this article are those of the authors. They do not reflect the official policy or position of any of the author affiliated agencies.
Ethical approval
Ethical approval was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (Reference No: BE033/19). Informed voluntary consent was obtained from all the participants in the preamble to the questionnaire.
References
Anyanwu, M.U., Kolade, O.A., 2017, Veterinarians' perception, knowledge and practices of antibiotic stewardship in Enugu State Southeast, Nigeria, Notulae Scientia Biologicae 9(3), 321-31. https://doi.org/10.15835/nsb9310061. [ Links ]
Battersby, I., Harvey, A., 2006, Differential diagnosis and treatment of acute diarrhoea in the dog and cat, In Practice 28(8), 480-488. https://doi.org/10.1136/inpract.28.8.480. [ Links ]
Chantziaras, I., Boyen, F., Callens, B., Dewulf, J., 2014, Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries, J Antimicrob Chemother 69(3), 827-834. https://doi.org/10.1093/jac/dkt443. [ Links ]
Da Silva, K.C., Knobl, T., Moreno, A.M., 2013, Antimicrobial resistance in veterinary medicine, Brazilian Journal of Veterinary Research and Animal Science 50(3), 171-183. [ Links ]
De Briyne, N., Atkinson, J., Pokludova, L., Borriello, S.P., 2014, Antibiotics used most commonly to treat animals in Europe, Vet Rec 175(13), 325. https://doi.org/10.1136/vr.102462. [ Links ]
Department of Health (DoH), 2018, South African Antimicrobial Resistance National Strategy Framework: A One Health Approach, 2018-2024. [Online] Available from: https://www.health.gov.za/index.php/antimicrobial-resistance?download=3308:amr-national-action-plan-2018-2024. Accessed 15 Jul 2020. [ Links ]
Ekakoro, J.E., Okafor, C.C., 2019, Antimicrobial use practices of veterinary clinicians at a veterinary teaching hospital in the United States, Vet Anim Sci 7(1), 100038. https://doi.org/10.1016Zj.vas.2018.09.002. [ Links ]
Food and Agriculture Organisation (United Nations) (FAO), 2016, The FAO Action Plan on Antimicrobial Resistance 2016-2020. Paris, France: FAO. [Online] Available from: http://www.fao.org/3AH5996e. Accessed 31 Mar 2020. [ Links ]
Food and Agriculture Organisation (United Nations) (FAO), 2019, FAO taking a bottom-up approach to understanding antimicrobial use. [Online]. Available from: http://www.fao.org/antimicrobial-resistance/news-and-events/news/news-details/en/c/1252384/. Accessed 6 Apr 2020. [ Links ]
Guardabassi, L., Prescott, J.F., 2015, Antimicrobial stewardship in small animal veterinary practice: From theory to practice, Vet Clin North Am Small Anim Pract 45(2), 361-376. https://doi.org/10.1016/j.cvsm.2014.11.005. [ Links ]
Hardefeldt, L.Y., Browning, G.F., Thursky, K., et al., 2017, Antimicrobials used for surgical prophylaxis by companion animal veterinarians in Australia, Vet Microbiol 203(1), 301-307. https://doi.org/10.1016/j.vetmic.2017.03.027. [ Links ]
Hardefeldt, L., Nielsen, T., Crabb, H., et al., 2018, Veterinary students' knowledge and perceptions about antimicrobial stewardship and biosecurity - A national survey, Antibiotics 7(2), 1-15. https://doi.org/10.3390/antibiotics7020034. [ Links ]
Pomba, C., Rantala, M., Greko, C., et al., 2017, Public health risk of antimicrobial resistance transfer from companion animals, J Antimicrob Chemother 72(4), 957-968. https://doi.org/10.1093/jac/dkw481. [ Links ]
Ramsey, I., 2017, Guidelines for responsible antibacterial use. In: Ramsey, I., Argyle, S.A., Batchelor, D., Bexfield, N., Chan, D.L., Featherstone, H., Frowde, P., Helm, J., Hodgkiss-Geere, H., Jackson, H., Maddox, T., Mills, D.S., Murrell, J., Stalin, C. & von Heimendahl, A. (eds). BSAVA Small Animal Formulary, 9th edn - Part A: Canine and Feline, pp. 1-492. Gloucester: British Small Animal Veterinary Association. [ Links ]
Reygaert, W.C., 2018, An overview of the antimicrobial resistance mechanisms of bacteria, AIMS Microbiol 4(3), 482-501. https://doi.org/10.3934/microbiol.2018.3.482. [ Links ]
Rhouma, M., Beaudry, F., Letellier, A., 2016, Resistance to colistin: what is the fate for this antibiotic in pig production?, Int J Antimicrob Agents 48(2), 119-126. https://doi.org/10.1016/j.ijantimicag.2016.04.008. [ Links ]
Sadiq, M.B., Syed-Hussein, S.S., Ramanoon, S.Z., et al., 2018, Knowledge, Attitude and Perception Regarding Antimicrobial Resistance and Usage Among Ruminant Farmers in Selangor, Malaysia, Prev Vet Med 156(1), 76-83. https://doi.org/10.1016/j.prevetmed.2018.04.013. [ Links ]
Smith, P.W., Agbaje, M., Le Roux-Pullen, L,. et al., 2019, Implication of the knowledge and perceptions of veterinary students of antimicrobial resistance for future prescription of antimicrobials in animal health, South Africa, J S Afr Vet Assoc 90(1), 1-8. https://doi.org/10.4102/jsava.v90i0.1765. [ Links ]
University of Colorado Denver., 2018, Small animal prescription practices survey. Available from: https://ucdenver.co1.qualtrics.com/jfe/form/SV_1BqwUotCHPLRgAl. Accessed 23 Jun 2020. [ Links ]
Vaarten, J., 2012, Clinical impact of antimicrobial resistance in animals, Rev Sci Tech 31(1), 221-229. https://doi.org/10.20506/rst.31.L2110. [ Links ]
Van Vuuren, M., Picard, J., Greyling, J., 2007, SANVAD 2007: South African National Veterinary Surveillance and Monitoring Programme for Resistance to Antimicrobial Drugs. Pretoria: University of Pretoria, Faculty of Veterinary Science, Department of Veterinary Tropical Diseases. [ Links ]
Watts, J.L., Sweeney, M.T., Lubbers, B.V., 2018, Antimicrobial susceptibility testing of bacteria of veterinary origin, Microbiol Spectr 6(2), 1-16. https://doi.org/10.1128/microbiolspec.ARBA-0001-2017. [ Links ]
World Bank, 2017, Drug-Resistant Infections: A Threat to Our Economic Future. Washington DC, USA: World Bank. [Online] Available from: http://documents1.worldbank.org/curated/en/323311493396993758/pdf/final-report.pdf. Accessed 10 Apr 2020. [ Links ]
World Health Organization (WHO), 2015, Global Action Plan on Antimicrobial Resistance. Geneva, Switzerland: WHO Press. [Online] Available from: https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1. Accessed 3 Jul 2020. [ Links ]
World Health Organization (WHO), 2019, Critically important antimicrobials for human medicine - 6th revision. Geneva: WHO. [Online] Available from: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf?ua=1. Accessed 9 May 2020. [ Links ]
World Organization for Animal Health (OIE), 2015, OIE Standards, Guidelines and Resolution on antimicrobial resistance and the use of antimicrobial agents. Paris: OIE. [Online] Available from: https://web.oie.int/delegateweb/eng/ebook/AF-book-AMR-ANG_FULL.pdf?WAHISPHPSESSID=03152ead00d06990fa9066b7b71fcabc. Accessed 10 Jul 2020. [ Links ]
World Organization for Animal Health (OIE), 2016a, The OIE Strategy on antimicrobial resistance and the prudent use of antimicrobials. [Online] Available from: https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf. Accessed 6 Apr 2020. [ Links ]
World Organization for Animal Health (OIE), 2016b, OIE Annual report on antimicrobials agents intended for use in animals: Better understanding of the global situation. [Online] Available from: https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/Survey_on_monitoring_antimicrobial_agents_Dec2016.pdf. Accessed 25 Jun 2020. [ Links ]
World Organization for Animal Health (OIE), 2017, OIE Annual report on antimicrobials agents intended for use in animals: Better understanding of the global situation. [Online] Available from: https://www.oie.int/fileadmin/home/eng/our_scientific_expertise/docs/pdf/amr/annual_report_amr_2.pdf. Accessed 25 Jun 2020. [ Links ]
World Organization for Animal Health (OIE),2018, OIE Annual report on antimicrobials agents intended for use in animals: Better understanding of the global situation. [Online] Available from: https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/A_Third_Annual_Report_AMR.pdf. Accessed 25 Jun 2020. [ Links ]
World Organization for Animal Health (OIE), 2019, OIE List of Antimicrobial Agents of Veterinary Importance. [Online] Available from: https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/A_OIE_List_antimicrobials_July2019.pdf. Accessed 2 May 2020. [ Links ]
 Correspondence:
Correspondence:
SA Maruve
Email: simbaiallenmaruve@gmail.com














