Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the South African Veterinary Association
versão On-line ISSN 2224-9435
versão impressa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.93 no.2 Pretoria 2022
http://dx.doi.org/10.36303/JSAVA.128
ORIGINAL RESEARCH
The effect of different dietary flavourants and salt levels on feed intake of juvenile ostriches
TS BrandI, II; A KrugerI, III; PG TheronII
IDirectorate: Animal Sciences, Department of Agriculture, Western Cape Government, South Africa
IIDepartment of Animal Sciences, Stellenbosch University, South Africa
IIIAgricultural Management Unit, School for Natural Resource Management, Nelson Mandela University, South Africa
ABSTRACT
Yearly the ostrich industry loses up to 40% of newly-hatched chicks, partly due to insufficient feed intake. This study was conducted to determine whether the inclusion of various feed flavourants would improve feed intake in ostrich chicks (Trial 1). Ninety-six day-old ostrich chicks were raised in groups of 12 at a Western Cape research farm until 28 days of age. These chicks were provided with free-choice access to a variety of flavoured diets, namely sweet, sour, bitter, salt or an unflavoured control diet. Chicks were found to prefer salty feed, as the salt-flavoured diet had the highest daily feed intake (34% of total) throughout the trial. Subsequently Trial 2 was conducted to determine the preferred level of dietary salt (Experiment 1) as well as the influence dietary salt had on various production parameters (Experiment 2). In Experiment 1, three groups of seven chicks each were offered ad libitum access to diets containing 4 g/kg, 14 g/kg, 24 g/kg, and 34 g/kg of salt respectively. Experiment 1 found that chicks preferred the diet containing 14 g salt/kg (36.4% of total daily feed consumed). For Experiment 2, 56 birds were divided into eight groups of seven. Conversely to the current conventional inclusion of 4 g salt/kg, Experiment 2 showed that chicks reared on a diet containing 14 g salt/kg had a 41.7% higher average daily gain than the group consuming 4 g salt/kg. It can therefore be concluded that ostriches prefer diets with a higher dietary salt level than current conventional diets provide (14 g/kg vs 4 g/kg).
Keywords: dietary salt level, feed flavourants, feed preference, ostrich rearing, palatability, ratites
Introduction
The ostrich (Struthio camelus) is a large, flightless ratite, originating from Africa and the Arabian Desert, and is well adapted to hot, arid environments (Skadhauge et al. 1984). As a domesticated animal, it is farmed commercially around the world for feathers, meat and skins. Currently, the total investment in the South African ostrich industry exceeds R2.1 billion, with export income amounting to R1.2 billion annually (South African Ostrich Business Chamber 2022). These figures show that the ostrich industry plays an important role in commercial South African agriculture. This is further supported by recent research in the field (Barends-Jones & Pienaar 2020; Snyders 2020). Globally the ostrich industry also continues to generate interest, particularly in Asia (Ghaffari Moghadam 2016; Miah et al. 2020; Nikravesh-Masouleh et al. 2018; Okasha et al. 2019; Ramedani 2019).
However, the ostrich industry loses up to 40% of chicks hatched within the first three-month production cycle annually (Deeming 1999). Fericean et al. (2021) reported mortality rates of 50% in ostriches up to four months old, while in the wild, this can be as high as 90%, although that is at least partially due to predation.
This high incidence of mortality has been ascribed to low feed intake, retarded growth and low immunity (Deeming 1999). The ostrich does not eat dry feedstuffs in its natural habitat (Milton et al. 1994), but in a commercial production system, ostrich chicks need to learn to consume dry feed. As this is not a natural behaviour for the chick, it can take time to learn, which leads to reduced feed intake early in its production cycle. The early growth and survival of chicks are of fundamental importance to the ostrich industry (Deeming 1997), as ostriches have efficient feed conversion ratios during the first 210 days of life. Improving the palatability of feed can help increase feed intake and, therefore, production.
Palatability is crucial to stimulate feed ingestion in ostriches (Aganga et al. 2003) and can be improved through the use of feed flavourants. According to Kare and Pick (1960), popular interest in flavouring feeds is based largely on increasing feed intake as feed intake is one of the most limiting factors in animal production (Roura 2003). Forbes and Kyriazakis (1995) stated that animals may select feed based on a pleasurable item such as flavour, while Deyoe et al. (1962) stated that it appears that well-accepted flavours might be used in feeds to increase feed consumption by fowl. Engelmann (1934) observed that fowl can discriminate in choices between acid, salt, sweet and bitter, while a more recent study (Yoshida et al. 2021) indicated that chickens can respond to bitter, umami, sour, salty and sweet taste stimuli. According to Heuser (1952), salt influences the palatability of feed while Kleyn (1994) stated that salt is very palatable to animals in moderate quantities.
There is a long-held belief among poultry keepers that excessive amounts of salt are poisonous to chickens (Hallpin et al. 1936), with chicks being particularly susceptible (Howell & Gumbrell, 1992). Mason and Clark (2000) stated that solutions with a high salt content are toxic to birds without salt glands. Ostriches, however, possess large, functional nasal salt glands, which may be important for extra-renal salt elimination during dehydration (Schmidt-Nielsen et al. 1963). These salt glands also enable ostriches to utilise saline water (Cloudsley-Thompson & Mohamed 1967). Experiments by Britton (1992) showed that chickens require a dietary salt level of approximately 8 g/kg for maximum body weight. Chicks fed less salt had poorer feed conversions and in general, increasing salt in the diet improved feed conversion. The taste of salt (NaCl) may trigger divergent responses in birds, with high concentrations eliciting aversion and lower concentrations being attractive, particularly in cases where the birds are sodium depleted (Roura et al. 2013). However, since no literature documenting the taste preference of ostriches could be found, it was unclear what the response of ostriches to various flavours might be.
This study was therefore carried out in an attempt to find a possible solution for the challenges facing the ostrich-rearing industry with regards to feed intake in ostrich chicks. To this end, different flavourants were added to ostrich chick feed to determine whether this would influence feed intake. Subsequently, different salt inclusion levels in a basal diet were compared to determine which level of salt is preferred by ostrich chicks, and to determine the effect of dietary salt levels on feed intake, growth rate and feed conversion ratio (FCR).
Materials and methods
This study was carried out in two parts. Trial 1 investigated the effect different feed flavourants had on the feed intake of ostrich chicks, while Trial 2 determined the preferred level of dietary salt for ostrich chicks (Experiment 1), as well as the effect of different dietary salt levels on feed intake, growth rate, and FCR in ostrich chicks (Experiment 2).
Both trials were conducted at a research farm in the Western Cape. Animals in both trials were divided into groups and housed in pens in an environmentally controlled ostrich chick house. Groups of chicks were used in this study due to the observation that ostrich chicks tend to stress when kept single, and this would interfere with feeding habits. The pens were approximately 2.5 m2 with concrete walls and floors. On the floors, raised welded metal mesh platforms were used to prevent excessive ingestion of litter and to isolate birds from urine. These platforms were covered by a thick rubberised mesh, conforming to the shape of the floor, which prevented the chicks from injuring themselves. The temperature of the whole room was controlled between 25-30 °C, either through heating with gas heaters or cooling down by using a wet wall. Ventilation was provided by an extracting fan in the wall near the roof.
For Trial 1, 96 day-old ostrich chicks were used. The chicks were of mixed sex and hatched over two days. Chicks of comparable weight were randomly divided into eight groups of twelve and each group housed in a pen as described above. At the start of the experiment the chicks were restricted to only a small area of the pen.
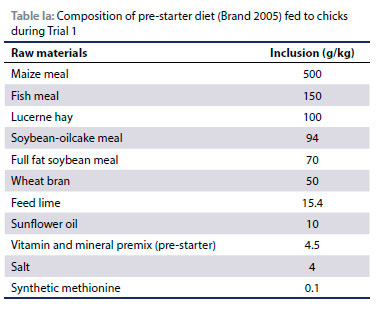
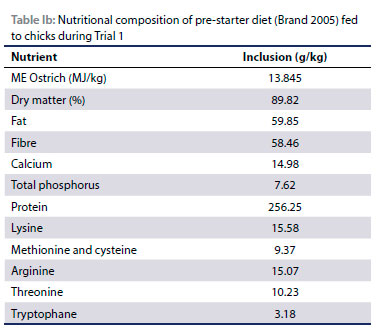
A standard pre-starter diet as per Brand (2005) (Table la and lb) was provided ad libitum from the start of the trial in flat-bottomed, shallow plastic feeders which were placed on the floor. The feeders were spread out to prevent overcrowding and to ensure that each chick had adequate access to feed. Water was provided ad libitum throughout by means of poultry-type plastic drinkers.
The standard diet was mixed with commercially produced nontoxic food flavourants developed for human consumption, to create four treatment diets (sweet, sour, bitter and salt). Untreated mash was used as a control diet throughout this study. The flavourants were thoroughly mixed into the mash by hand to ensure uniform distribution at 1 kg per 100 kg feed. Due to the fact that the flavourants had different colours, 5 g of chromium dioxide per kg feed was added to all the rations. This ensured that all the rations had the same green colour to eliminate the influence of colour. Each feeder was assigned one of the diets and all the diets were presented to the birds in a free choice situation.
The order in which the feeders were presented was changed every four hours during the day to ensure an even distribution and to overcome the effect of positioning. According to Pick and Kare (1962), frequent switching of the positions of the alternatives is essential if a measure of degree of preference, uncontaminated by the possibility of learning, is desired. The chicks were not deprived of food prior to the testing period and at the time of this study the birds had no taste experience. Factors that could influence preference (light, position, freshness of feed, cleanliness of drinkers and feeding containers) were equalised as far as possible.
Live weight and dry matter intake of birds were recorded every second day over the four-week experimental period. Food preference of the chicks was determined by weighing the feed consumed per feeder on a weekly basis.
For histological purposes, one ostrich chick (two months of age) and one adult ostrich (12 months of age) were slaughtered in order to collect tissue from the mouth to ascertain whether or not taste buds were present. Samples were taken from the tip and the lateral part of the tongue, the mouth cavity underneath the tongue, the palate of the mouth, as well as the first part of the oesophagus. These samples were subsequently mounted on slides and placed under an electron microscope for visual examination to determine whether taste buds were present or not.
A total of 77 chicks were used in Trial 2, respectively 21 chicks in Experiment 1 and 56 chicks in Experiment 2. The chicks were 52 days of age and of mixed sex. Chicks of comparable weight were randomly divided into 11 groups of seven each and housed as described above.
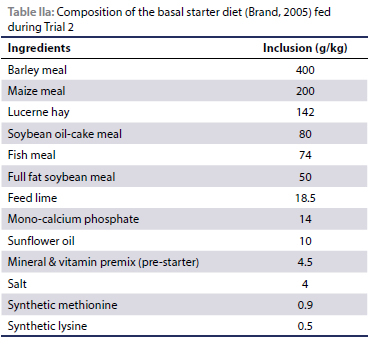
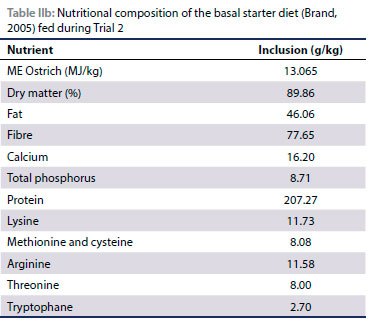
An ostrich starter ration (Table IIa and IIb) as per Brand (2005) was provided, with varying amounts of salt mixed into the ration to provide four different salt levels (4 g/kg, 14 g/kg, 24 g/kg and 34 g/kg respectively). In Experiment 1, three pens with seven chicks per pen were provided with all four diets in a free choice situation to determine the feed preference for diets with varying salt levels. In Experiment 2, each of the four diets was provided ad libitum to two pens of seven chicks each. Water was provided ad libitum by means of metal drinkers, positioned against the wall of the pen.
Dry matter intake and live weight of the chicks were determined weekly for a five-week period during Experiment 2, while average daily gain (ADG) and FCR were subsequently calculated for the chicks.
Results from both preference trials were analysed statistically by one-way analysis of variance, while results from Experiment 2 in Trial 2 were analysed statistically by a repeated measures analysis of variance test where treatment and week were set as main factors and pen as a random effect. Week was modelled as the within-subject effect. The Statgraphics Centurion XV program was used to perform these analyses. Means were considered to be statistically different at a level of p < 0.05. All statistical procedures are described in detail by Snedecor and Cochran (1980).
The study was carried out under the supervision and with the ethical approval of the Western Cape Department of Agriculture.
Results
During Trial 1, it was found that the average daily intake for ostrich chicks up to 28 days of age was 160 ± 17.079 g/chick/day over the entire trial period. Data from the free choice preference test are presented in Table III.
Apart from the salt flavourant, it is clear that the use of flavourants did not influence the feed preference in ostrich chicks. For each subsequent week, the intake for salty feed was higher (p < 0.05) compared to the other treatments. Over the entire trial period, the intake of the salty feed was more than twice as high as any other flavour feed. For the cumulative four-week period, the salty feed was chosen most frequently (34.0%), followed by sweet (17.9%), control (17.1%), bitter (15.7%) and sour (15.4%) feeds, respectively (p < 0.01).
No taste buds could be found in any of the samples taken from either the juvenile or the adult ostrich. However, in all the samples taken, mucus-secreting cells were very prominent.
In Trial 2, Experiment 1, it was found that the average dry matter intake of chicks in the free choice experiment was 425 ± 51.282 g/chick/day. Overall growth rate was 265 ± 36.449 g/chick/day with a feed conversion ratio of 1.6 ± 0.113 kg feed/kg live weight gain. Results with regard to the free choice experiment are presented in Table IV.

From this experiment, it is clear that ostrich chicks between seven and 12 weeks of age preferred a level of 14 g salt/kg feed in their diet. Intake of feed was the highest for the diet containing 14 g salt/kg feed (34.62%), followed by the diets containing 34 g salt/kg feed (24.43%), 24 g salt/kg feed (23.99%) and 4 g salt/kg feed (16.95%) (p <0.01).
No significant interaction between dietary salt inclusion level and week were found in Trial 2, Experiment 2. Results for treatment and week are presented separately in Table V and VI. Production data regarding the different dietary salt inclusion levels revealed a significant tendency (p = 0.09) for chicks consuming the diet with 14 g salt/kg feed to have higher end weights than chicks that consumed the diets with 4 g salt/kg feed. Chicks consuming diets with 14 g salt/kg feed grew 41.7% faster than chicks that consumed the diet with 4 g salt/kg feed (312.38 ± 38.698 g/ chick/day vs 220.42 ± 42.297 g/chick/day. This difference was not statistically significant. No differences occurred in the feed intake (average value of 441.2 ± 23.916 g/chicks/day) or FCR (1.9 ± 0.157 kg feed/ kg live weight gain) of chicks that consumed the different diets (p = 0.21).
The feed intake as well as daily gain of ostrich chicks used in the study gradually increased over the experimental period and differed significantly between weeks in most instances.
Discussion
The results from Trial 1 indicated that ostrich chicks with no previous exposure to flavourants have a strong preference for salty feed. Similar results were obtained by El-Boushy and Kennedy (1987), who indicated that chickens have the ability to differentiate between sweet, salt, sour and bitter additives and have an acute sense of taste. This is further supported by the work of Niknafs and Roura (2018) and Yoshida et al. (2021), with the latter indicating that chickens respond to bitter, umami, sour, salty and sweet taste stimuli. The results obtained here also support previous results from Brand et al. (2018), indicating that ostriches can discriminate against feed based on taste.
However, while it has been indicated that chickens clearly preferred solutions of true sugar (sucrose, fructose and maltose) over water (Jacobs & Scott 1957), in this study, it was found that ostrich chicks preferred salty feed to other flavours. Another study (Gentle 1972) indicated that a preference for 5% sucrose solution existed in chickens. Studies on bobwhite quails (Hamrum 1953) and Japanese quails (Brindley 1965; Harriman & Milner 1969) indicated that these species also preferred sweet solutions (sucrose) and rejected salty solutions. Feral pigeons were found to be responsive to taste stimuli derived from sucrose but were indifferent to glucose (Duncan 1960), while jungle fowl preferred sucrose solution to water (Kare & Maller 1967). An overview of previous studies in taste in avian species by Niknafs and Roura (2018), while not including ostriches, indicated that no species previously studied preferred salty solutions to water or sweet solutions. While these studies were conducted using solutions and not solid feeds, it seems reasonable to suppose that ostriches are an anomaly among most domesticated and even some wild bird species in their preference for salty feed.
The exact reason for this preference for salty feed remains unclear, but it could be speculated that it relates to the adaptation of the bird to arid environments where water is limited. In order to excrete nitrogenous waste from the body, urination must take place. However, this is inevitably accompanied by the loss of water, which is undesirable in an arid environment. In birds such as the emu, this problem is overcome by post-renal absorption of water during cloacal mixing of urine and faeces (Skadhauge & Maloney 1991). During this process, salt is also reabsorbed, thus simultaneously conserving water and maintaining salt levels in the body. The ostrich differs from the emu in that urine and faeces are stored separately, with urine being held in the coprodeum (Skadhauge et al. 2003). The epithelium in the coprodeum of the ostrich contains an abundance of goblet cells, which secrete a mucosal layer that largely prevents osmotic exchange between the urine and blood plasma (Laverty & Skadhauge 2008; Skadhauge et al. 2003). This allows the ostrich to produce highly concentrated urine (Laverty & Skadhauge 2008) as an osmotic gradient can be maintained between plasma and urine. Since only a limited exchange of water soluble-ions can take place across this barrier, it also limits the ability of the ostrich to reabsorb salt, thus increasing its dietary salt requirement.
Hence it seems reasonable to propose that ostriches may have evolved a preference for salty feed as the taste could serve as an indication of higher dietary salt levels.
No taste buds could be found in either the juvenile or adult ostriches. This is in contrast to other bird species such as the pigeon (37 buds), the chicken (24 buds), the duck (200 buds) and the parrot (350 buds) (Kare & Ficken 1963). More recent studies concluded that previous estimates on the number of taste buds in chickens were far too low and indicated that as many as 767 taste buds may be present in a chicken's oral cavity (Niknafs & Roura 2018). Since no taste buds could be found in the tip and the lateral part of the tongue, the mouth cavity underneath the tongue, the palate of the mouth as well as the first part of the oesophagus in either chicks or adult ostriches, it may be argued that they do not have a strong ability to taste, and probably rely on olfactory sensors to select food. Previous work done on the subject indicated that a combination of visual cues, taste and smell influenced feed preference in ostriches (Brand et al. 2018). However, taste buds may be present in the throat area as histological studies by Lindenmaier and Kare (1959) showed. It is also possible that ostriches possess a taste system that does not rely on visible macrostructures. It has been indicated that the avian nutrient chemosensory system may be partially present in the gastrointestinal tract (GIT) or hypothalamus (Byerly et al. 2010; Niknafs & Roura 2018). These extra-oral taste receptors have been found to play a key role in feed intake and appetite control (Niknafs & Roura 2018). If ostriches, therefore, possess taste and nutrient receptors in the GIT or hypothalamus, it would obviate the need for oral taste receptors (taste buds). However, until further work confirming or disproving the presence of such receptors in the ostrich is done, it is impossible to state with certainty whether ostriches rely primarily on olfactory or gustatory factors to determine feed preference.
As mentioned, mucus-secreting cells were found prominently in the histological samples. These cells may have a connection with thermoregulation in the ostrich, but are unlikely to play a role in taste perception. Skadhauge et al. (1995) noted that excited and heat-stressed ostriches panted, and this panting was accompanied by large amounts of mucus secretion from the mouth and the throat area.
In Experiment 1 of Trial 2, allowing the chicks access to free choice diets revealed that ostrich chicks prefer a diet containing 14 g salt/kg feed, proving that intake may be stimulated by higher dietary salt levels than in conventional diets and therefore it might be necessary to raise dietary salt levels in ostrich feeds (current recommended salt levels are 4-5 g/kg, as adopted from commercial guidelines for poultry). The fact that ostriches can tolerate, and even prefer higher levels of dietary salt, is probably partially due to the epithelial structure of the coprodeum as previously explained. Since very little osmotic exchange takes place across the mucosal barrier (Skadhauge et al. 2003), ostriches cannot recirculate NaCl throughout the body as effectively as other bird species and must therefore rely on dietary salt to maintain salt levels in the body, resulting in a preference for diets with a higher salt content than other poultry species.
The presence of salt glands in the ostrich would also allow for the consumption of diets that are high in salt. Salt glands play a role in extra-renal salt excretion (Schmidt-Nielsen et al. 1963; Laverty & Skadhauge 2008) and allow the birds to utilise saline water (Cloudsley-Thompson & Mohamed 1967). According to Laverty and Skadhauge (2008), salt glands excrete a solution that can have up to twice the osmolality of urine. This allows the birds to excrete excess salt very effectively, and likely prevents salt from reaching toxic levels in the body as may occur in chickens or other birds without salt glands (Hallpin et al. 1936; Howell & Gumbrell 1992; Mason & Clark 2000).
In Experiment 2 of this trial, it was found that feed intake and FCR did not differ significantly, but birds receiving 14 g salt/kg feed grew faster than birds on the standard diet, albeit not significantly so. This seems to imply that the improved growth is not caused by a nutrient dilution effect in the diet, which would lead to increased feed intake. It should be noted that growth rate and feed intake of birds used in the study exceeded values proposed by Brand and Olivier (2011) for birds of the same age. This may be due to the higher salt levels in the experimental diets in this study stimulating feed intake and thereby improving growth. On the other hand, the live weight, ADG, feed consumption and FCR values were all lower than that observed by Miah et al. (2020). These differences could likely be ascribed to dietary and environmental differences between the studies.
It may therefore be concluded that ostrich chicks prefer salt flavoured diets and that providing birds with a diet containing 14 g/salt kg feed could potentially improve growth rate. This would imply that current standard industry diets, containing only 4 g salt/kg feed, are insufficient to meet the needs of growing ostrich chicks.
Conclusion
Ostrich chicks preferred a salt-flavoured diet in a free choice situation. Flavouring commercial diets with salt may therefore help to alleviate problems with feed intake in ostrich chicks which could potentially help to decrease production losses suffered as a result of early mortalities in the production cycle.
Building on this finding, it was then found that the optimal dietary salt level for ostrich chicks was 14 g salt/kg feed. According to preference studies, the current standard inclusion of 4 g salt/kg feed in ostrich feed may therefore be below the preferred threshold of the birds. Future studies with more birds at different ages are necessary to verify the optimum amount of dietary salt inclusion between 4-14 g salt/kg feed in the diet that is needed to optimise production for ostriches. It can, however, be stated with reasonable certainty that, until further studies are completed, an inclusion level of 14 g salt/kg feed is advisable for use in the industry.
Acknowledgements
Labourers at the Kromme Rhee Experimental Farm for feeding and care of the experimental animals.
Conflict of interest
The authors declare that they have no financial or personal relationship(S) that may have inappropriately influenced them in writing this article and that no competing interests exist.
Funding source
The project was partly funded by the Western Cape Agricultural Research Trust.
ORCID
TS Brand: https://orcid.org/0000-0003-0160-6605
A Kruqer: https://orcid.org/0000-0001-8328-4560
PG Theron: https://orcid.org/0000-0002-8999-8772
References
Aganga, A.A., Aganga, A.O., Omphile, U.J., 2003, Ostrich feeding and nutrition, Pakistan Journal of Nutrition 2(2), 60-67. https://doi.org/10.3923/pjn.2003.60.67. [ Links ]
Barends-Jones, V. & Pienaar, L., 2020, The South African ostrich industry footprint, Western Cape Department of Agriculture (WCDoA): Elsenburg, South Africa. [ Links ]
Brand, T.S., 2005, Ostrich Feeding, Technical Brochure 1, Elsenburg Research Centre, Private bag X1, Elsenburg, 7607. [ Links ]
Brand, T.S., Engelbrecht, J.A., Van der Merwe, J., Hoffman, L.C., 2018, Feed preference of grower ostriches consuming diets differing in Lupinus angustifolius inclusion levels, S Afr J Anim Sci 48(1), 170-185. https://doi.org/10.4314/sajas.v48i1.19. [ Links ]
Brand, T.S. & Olivier, A., 2011, Ostrich nutrition and welfare, in P. Glatz, C. Lunan & I. Maleck (eds), The Welfare of Farmed Ratites, Springer, London. https://doi.org/10.1007/978-3-642-19297-5_5. [ Links ]
Brindley, L.D., 1965, Taste discrimination in bobwhite and Japanese quail, Anim Behav 13(4), 507-512. https://doi.org/10.1016/0003-3472(65)90114-4. [ Links ]
Britton, W.M., 1992, Effect of dietary salt intake on water and feed consumption of chicks. Proceedings of the Georgia Nutrition Conference for the Feed Industry, pp. 48-53. [ Links ]
Byerly, M.S., Simon, J., Cogburn, L.A., et al., 2010, Transcriptional profiling of hypothalamus during development of adiposity in genetically selected fat and lean chickens, Physiol Genomics 42(2), 157-167. https://doi.org/10.1152/physiolgenomics.00029.2010. [ Links ]
Cloudsley-Thompson, J.L. & Mohamed, E.R.M., 1967, Water economy of the ostrich, Nature 216, 1040. https://doi.org/10.1038/2161040a0. [ Links ]
Deeming, D.C., 1997, Ratite egg incubation: a practical guide, Ratite Conference. [ Links ]
Deeming, D.C., 1999, Introduction, in D.C. Deeming (ed.), The Ostrich: Biology Production and Health, CABI, London. [ Links ]
Deyoe, C.W., Davies, R.E., Krishnan, R., et al., 1962, Studies on the taste preference of the chick, Poult Sci 41, 781-784. https://doi.org/10.3382/ps.0410781. [ Links ]
Duncan, C.J., 1960, Preference tests and the sense of taste in the feral pigeon (Columba livia var gmelin), Anim Behav 8(1-2), 54-60. https://doi.org/10.1016/0003-3472(60)90009-9. [ Links ]
El-Boushy, A.R. & Kennedy D.A., 1987, Flavouring agents improve feed acceptability (I), Poultry, June/July, 32-33. [ Links ]
Engelmann, C., 1934, Versuche uber den Geschmackssinn von Taube, Ente und Huhn[Experiments on the taste of pigeon, duck and chicken], Z Vgl Physiol 20(5), 626-645. https://doi.org/10.1007/BF00339157. [ Links ]
Fericean, L.M., Ostan, M., Rada, O., et al., 2021, Behaviour of captive ostrich chicks from 6 weeks to 1 year, Research Journal of Agricultural Science 53(2), 126-132. [ Links ]
Forbes, J.M. & Kyriazakis, I., 1995, Food preferences in farm animals: why don't they always choose wisely?, Proc Nutr Soc 54(2), 429-440. https://doi.org/10.1079/PNS19950012. [ Links ]
Gentle, M.J., 1972, Taste preference in the chicken (Gallus domesticus l.), Br Poult Sci 13(2), 141-155. https://doi.org/10.1080/00071667208415928. [ Links ]
Ghaffari Moghadam, Z., 2016, Economic evaluation of ostrich production using fuzzy approach in Sistan, Iranian Journal of Applied Animal Science 6(3), 685-690. [ Links ]
Hallpin, J.G., Holmes, C.E., Hart, E.B., 1936, Salt requirements of poultry, Poult Sci 15, 99-103. https://doi.org/10.3382/ps.0150099. [ Links ]
Hamrum, C.L., 1953, Experiments on the senses of taste and smell in the bob-white quail (Colinus virginianus virginianus), Am Midl Nat 49(3), 872. https://doi.org/10.2307/2485214. [ Links ]
Harriman, A.E. & Milner, J.S., 1969, Preference for sucrose solutions by Japanese quail (Coturnix coturnix japonica) in Two-Bottle Drinking Tests, Am Midl Nat 81(2), 575. https://doi.org/10.2307/2423991. [ Links ]
Heuser, G.F., 1952, Salt additions to chick rations, Poult Sci 31, 85-88. https://doi.org/10.3382/ps.0310085. [ Links ]
Howell, J. & Gumbrell, R.C., 1992, Salt poisoning in broiler chickens, N Z Vet J 40(2), 85. https://doi.org/10.1080/00480169.1992.35706. [ Links ]
Jacobs, H.L. & Scott, M.L., 1957, Factors mediating food and liquid intake in chickens, Poult Sci 36(1), 8-15. https://doi.org/10.3382/ps.0360008. [ Links ]
Kare, M.R. & Pick, H.L., 1960, The influence of the sense of taste on feed and fluid consumption, Poult Sci 39, 697-706. https://doi.org/10.3382/ps.0390697. [ Links ]
Kare, M.R. & Ficken, M.S., 1963, Comparative studies on the sense of taste, in Y. Zotterman (ed), Olfaction and taste, Pergamon Press Oxford. https://doi.org/10.1016/B978-1-4831-9834-7.50026-3. [ Links ]
Kare, M.R. & Maller, O., 1967, Taste and food intake in domesticated and jungle fowl, J Nutr 92(2), 191-196. https://doi.org/10.1093/jn/92.2.191. [ Links ]
Kleyn, R., 1994, Minerals, in Commercial Poultry Nutrition in Southern Africa. Spesfeed, Rivonia. [ Links ]
Laverty, G. & Skadhauge, E., 2008, Adaptive strategies for post-renal handling of urine in birds, Comp Biochem Physiol A Mol Integr Physiol 149(3), 246-254. https://doi.org/10.1016/j.cbpa.2008.01.014. [ Links ]
Lindenmaier, P. & Kare, M.R., 1959, 'The taste end-organs of the chicken', Poult Sci 38, 545-550. https://doi.org/10.3382/ps.0380545. [ Links ]
Mason, R. & Clark, J., 2000, The chemical senses in birds, in G. Causey Whittow (ed), Sturkie's Avian Physiology, pp. 39-56, Academic Press, San Diego. https://doi.org/10.1016/B978-012747605-6/50004-3. [ Links ]
Miah, A.G., Abdulle, K.M., Rahman, M. et al., 2020, Growth performance and survivability of ostrich chicks in Bangladesh, J Vet Res Adv 2(2), 32-40. [ Links ]
Milton, S.J., Dean, W.R.J., Siegfried, W.R., 1994, Food selection by ostrich in Southern Africa, J Wildl Manage 58, 234-248. https://doi.org/10.2307/3809386. [ Links ]
Niknafs, S. & Roura, E., 2018, Nutrient sensing, taste and feed intake in avian species, Nutr Res Rev 31(2), 256-266. https://doi.org/10.1017/S0954422418000100. [ Links ]
Nikravesh-Masouleh, T., Seidavi, A., Kawka, M. et al., 2018, The effect of dietary energy and protein levels on body weight, size, and microflora of ostrich chicks, Trop Anim Health Prod 50(3), 635-641. https://doi.org/10.1007/s11250-017-1480-8. [ Links ]
Okasha, M.A.M., Attia, A.I. & Mahrose, K.M., 2019, Ostrich breeding in China, Zagazig Journal of Agricultural Research 46(5), 1583-1591. https://doi.org/10.21608/zjar.2019.48177. [ Links ]
Pick, H.L.(Jr) & Kare, M.R., 1962, The effect of artificial cues on the measurement of taste preference in chicken, J Comp Physiol Psychol 55, 342-345. https://doi.org/10.1037/h0042924. [ Links ]
Ramedani, Z., Alimohammadian, L., Kheialipour, K., et al., 2019, Comparing energy state and environmental impacts in ostrich and chicken production systems, Environ Sci Pollut Res Int 26(27), 28284-28293. https://doi.org/10.1007/S11356-019-05972-8. [ Links ]
Roura, E., 2003, Recent studies on the biology of taste and olfaction in mammals and aves. New approaches in swine nutrition, Congreso Latinoamericano de avicultura y suinocultura, Florianopolis, pp. 12-16. [ Links ]
Roura, E., Baldwin, M.W., Klasing, K.C., 2013, The avian taste system: Potential implications in poultry nutrition, Anim Feed Sci Technol 180(1-4), 1-9. https://doi.org/10.1016/j.anifeedsci.2012.11.001. [ Links ]
Schmidt-Nielson, K., Borut, A., Lee, P. et al., 1963, Nasal salt excretion and the possible function of the cloaca in water conservation, Science 142, 1300-1301. https://doi.org/10.1126/science.142.3597.1300. [ Links ]
Skadhauge, E. & Maloney, S.K., 1991, Osmotic adaptation of the emu (Dromaius novaehollandiae), J Comp Physiol B 161(2), 173-178. https://doi.org/10.1007/BF00262881. [ Links ]
Skadhauge, E., Erlwanger, K.H., Ruziwa, S.D., et al., 2003, Does the ostrich (Struthio camelus) coprodeum have the electrophysiological properties and microstructure of other birds?, Comp Biochem Physiol A Mol Integr Physiol 134(4), 749-755. https://doi.org/10.1016/S1095-6433(03)00006-0. [ Links ]
Skadhauge, E., Prys-Jones, R.P., Swart, D., et al., 1995, Colonic SCFA and electrolyte absorption and nasal gland development, in relation to NaCl intake in the ostrich (Struthio camelus), European Journal of Physiology 430. [ Links ]
Skadhauge, E., Warüi, J.M., Kamau, J.M.Z. & Maloiy, G.M.O., 1984, Function of the lower intestine and osmoregulation in the ostrich: Preliminary anatomical and physiological observations, Q J Exp Physiol 69, 809-818. https://doi.org/10.1113/expphysiol.1984.sp002870. [ Links ]
Snedecor, G.W. & Cochran, W.G., 1980, Statistical Methods (7th edn), Iowa State University Press, Ames. [ Links ]
Snyders, M., 2020, Perceptions about commercial ostrich farming: views of consumers, farmers and secondary stakeholders, MScAgric thesis, Dept. of Animal Science, University of Stellenbosch. [ Links ]
South African Ostrich Business Chamber, 2022, South African Ostrich Business Chamber, Available from: from https://ostrichsa.co.za/. Accessed 22 May 2022. [ Links ]
Yoshida, Y., Kawabata, F., Tabata, S., et al., 2021, Evolvement of taste sensitivity and taste buds in chickens during selective breeding, Poult Sci 100(6), 101113. https://doi.org/10.1016/j.psj.2021.101113. [ Links ]
 Correspondence:
Correspondence:
TS Brand
Email: tertius.brand@westerncape.gov.za