Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the South African Veterinary Association
versión On-line ISSN 2224-9435
versión impresa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.93 no.2 Pretoria 2022
http://dx.doi.org/10.36303/JSAVA.001
ORIGINAL RESEARCH
Temporally specific adrenocorticotropic hormone reference intervals for horses in South Africa
D Fisher; E-C Schliewert; EH Hooijberg
Department of Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, South Africa
ABSTRACT
An endogenous adrenocorticotropic hormone (ACTH) concentration above the reference interval (RI) is commonly used as means for diagnosing equine pituitary pars intermedia dysfunction (PPID). Basal ACTH concentrations are highly dependent on photoperiod and RIs should be month- and location-specific. To date, no ACTH RIs have been specifically established for South Africa.
This study aimed to determine geographically and seasonally relevant RIs for equine ACTH in the Gauteng province of South Africa.
A longitudinal prospective study was conducted over twelve months to determine ACTH RIs for a representative population of healthy South African horses in the Gauteng province. Eighty clinically healthy horses under 12 years of age were recruited for monthly venous blood sample collection, from July 2019 to June 2020. ACTH was measured using a chemiluminescent assay. RIs were constructed for each month of the year.
This South African population showed similar temporal changes in ACTH concentrations to those previously observed in other locations. Upper reference limits were at their lowest in early summer (21.4 pg/ml, 90% CI 20.8-21.7) with a pronounced increase in autumn (60.6 pg/ml, 90% CI 53.1-62.7), and tapered off in winter (22.3 pg/ml, 90% CI 19.9-23.2).
The month-specific ACTH RIs generated in this study will improve the accuracy of diagnosis and monitoring of PPID in the local equine population. These results highlighted the previously recommended need for seasonal and location-specific RIs.
Keywords: ACTH, pars pituitary intermedia dysfunction, endocrine, veterinary
Introduction
Pituitary pars intermedia dysfunction (PPID) is a serious endocrine condition in equines affecting older horses and ponies in particular (Ireland & McGowan 2018; McFarlane 2019; McGowan et al. 2013). The measurement of basal plasma adrenocorticotropic hormone (ACTH) concentrations is commonly used to confirm a diagnosis of PPID (Durham et al. 2021; Horn et al. 2021; McFarlane 2019). The ACTH concentration is interpreted in light of clinical signs and reference interval (RI) or diagnostic cut-off for the particular season or time of year, enabling practitioners to confirm or rule out a diagnosis of PPID (Durham et al. 2021; Horn et al. 2021; McFarlane 2019). Where ACTH concentrations are borderline, and clinical signs are indicative of potential PPID status, additional testing, by means of thyrotropin-releasing hormone (TRH) stimulation testing is advocated (Byrne et al. 2018; Durham et al. 2021; McFarlane 2019).
Equine ACTH RIs have been established for several locations in the Northern Hemisphere (Copas & Durham 2012; Durham et al. 2021; Frank et al. 2010); however, seasonal changes in photoperiod at these locations are opposite to those in Southern Hemisphere countries like South Africa. Recent studies from Australia have confirmed the presence of circannual rhythms in ACTH concentrations in horses in that country, and furthermore reported differences in ACTH concentrations from horses sampled in two geographic locations within Australia (Horn et al. 2021; Secombe et al. 2017). These findings emphasise the importance of using location- and season-specific RIs for ACTH.
There are no published data concerning expected seasonal ACTH concentrations in healthy horses in South Africa, and RIs currently in use in the authors' laboratory have been adapted from seasonally-equivalent data derived from Northern Hemisphere studies.
The objective of this study was to generate geographically and seasonally relevant RIs for equine ACTH in the Gauteng province of South Africa. Location-specific ACTH RIs would provide a superior diagnostic tool for practitioners in this area, leading to improved diagnosis and management of PPID. Information generated here will also add to the body of evidence concerning physiological seasonal secretion of ACTH in diverse horse populations. The hypotheses were that plasma ACTH concentrations would show seasonal fluctuations and that RIs would differ from month to month.
Research methods and design
Study design
A longitudinal prospective study was conducted over twelve months to determine ACTH RIs for a representative population of healthy South African horses in the Gauteng province, in accordance with the American Society for Veterinary Clinical Pathology (ASVCP) guidelines for RI studies in veterinary medicine (Friedrichs et al. 2012). Ethical approval was obtained from the University of Pretoria Animal Ethics and Research Ethics Committees (Certificate number REC042-19) prior to candidate recruitment and initiation of data collection, and owner or agent consent was given in writing for all horses included in the study. All procedures followed all international, national, and/or institutional guidelines for the care and use of animals.
Study subjects
Eighty clinically healthy horses under 12 years of age, in work, were recruited in the Gauteng province of South Africa for monthly venous blood sample collection, from July 2019 to June 2020.
As per ASVCP guidelines, the minimum sample size required for RI determination is 40 subjects and the ideal sample size should be > 120 (Friedrichs et al. 2012). Taking both this, and the animal welfare principle of reduction into account, the study aimed to ensure that the minimum number (40) of subjects was still present at the end of the one year sample collection period. Eighty horses were enlisted at the beginning of the study to ensure that even with subject drop-out there would still be at least 40 subjects in the final sample collection.
The properties where subjects were housed and samples were collected, were situated between the latitudes of -25.5782° and -26.2403°, and longitudes of 27.8786° and 28.3134° (within a 90 km catchment area). Horses were sampled at seven properties in total; two of the properties were private properties, four were livery yards, and one a racing yard at a racecourse. The management at each of the properties was similar to industry standards for each type of yard. During the course of the sampling no disease outbreaks or general health problems were noted at any of the yards. All of the horses in the study were in work (hacking, dressage, show jumping, and racing) at various levels for their particular disciplines.
Owner or yard manager history for each horse, a full clinical examination and complete blood count (CBC) were performed on each of the horses prior to the first sample collection. History and clinical examination were performed at each subsequent monthly sampling. Additional assessments were performed prior to the first and last sample collection, and included body condition scoring, palpation of the forefeet and digital pulses, turning in tight circles, and exclusion of other clinical signs of PPID. To be included for the first sampling, subjects had to be older than two and less than 12 years of age, easy to handle, clinically healthy, with no history of chronic disease, recurrent laminitis or chronic lameness. To be included for subsequent samplings, these same horses had to be clinically healthy, and present on the properties. History, clinical examination findings and CBC results (first sampling) were carefully reviewed after each sampling, and subjects were excluded from that data set if there were any abnormal findings detected. Horses that permanently left the properties or that became too difficult to handle were excluded from the time that these events occurred. Horses that left their properties temporarily, or that had minor clinical abnormalities that resolved, were excluded only for those months.
Handling and sampling procedures
Sampling occurred in the first week of each month for a one year period. Previously reported guidelines for blood collection were employed (Couetil et al. 1996). Each horse was restrained with a head halter and lead rein, no chemical restraint was used. Blood was drawn into an EDTA collection tube (BD Vacutainer® K2E [EDTA 7.2 mg], BD-Plymouth, United Kingdom) from the jugular vein via a21G vacutainer needle (BD Vacutainer® Precision Glide™ Multiple Sample Needle) and shoulder and filled via vacuum pressure. The tubes were gently inverted several times and chilled immediately in a cooler box with ice packs and ice blocks. Analyses were performed at the Clinical Pathology Laboratory, Faculty of Veterinary Science, University of Pretoria. All blood samples reached the laboratory for further processing within four hours of collection. Blood samples were centrifuged as soon as possible at 5 000 g for five minutes. The EDTA plasma was aliquoted into cryotubes to be stored at -80 °C until analysis, which took place monthly within 24-72 hrs of each collection (Prutton et al. 2015).
At the first sample collection, a second EDTA sample was obtained in order to perform a CBC as part of the initial health check of each individual. The CBC was run on an automated haematology analyser (Advia 2120i, Siemens Healthcare, Erlangen, Germany) within four hours of collection, with manual blood smear microscopic review.
Analytical procedures and quality assurance
ACTH analysis was performed by an automated chemiluminescent assay using the Immulite 2000 analyser (Siemens Healthcare, Erlangen, Germany) validated for ACTH in horses (Couetil et al. 1996; Irvine et al. 2016). The manufacturer's determined limit of detection for this assay, as determined from a blank sample, is 5.0 pg/ml and results lower than 5 pg/ml are read out by the analyser as < 5 pg/ml. Adjustment (calibration) was performed as per manufacturer's recommendations, measurement of internal quality control material was conducted on each day that the assay was run, and results were within manufacturer's target limits.
Statistical analysis
The following statistical procedures are those recommended by the ASVCP as well as recent modelling studies for RI generation, and were performed for each monthly data set (Friedrichs et al. 2012; Le Boedec 2016). Descriptive statistics and visual examination of data were performed. Data distribution was assessed with the Shapiro-Wilk test, and was considered Gaussian when p > 0.2. Outlier analysis was performed using the Tukey and Dixon-Reed methods, and identified outliers were removed after review of the analytical and clinical data. Detected outlier results were excluded in the month that they occurred, but those horses were not subsequently excluded from the study if they still met the inclusion and exclusion criteria. The 95% lower and upper reference limits (LRL, URL) for the population were determined as follows: if the p-value of the Shapiro-Wilk test was > 0.2, then the parametric method was used; otherwise the non-parametric method was used (Le Boedec 2016). The 90% confidence intervals (CI) for the RLs were calculated using a bootstrap method. Reference Value Advisor for Excel was utilised for the RI calculations. All results under the detection limit were converted to 5.0 pg/ml for the purposes of the statistical analyses.
A Kruskal-Wallis test with a post-hoc Conover analysis was used to compare ACTH concentrations between the 12 months. This analysis was performed using MedCalc (MedCalc Software version 19.1.7, Ostend, Belgium). The p-value was set at p < 0.05.
Results
Reference population
In total, 80 horses were enrolled for the study. The original study population was comprised of 10 fillies, 28 mares, 33 geldings, 8 colts, and one stallion between the ages of two and 12 years (mean 5.7 years; median 5.0 years). A range of breeds was included: 42 Thoroughbred, 23 Warmblood, 10 cross and mixed breeds (Arab cross, Boerperd cross, Lipizzaner cross, mixed breed pony, Irish Sport Horse cross), one Arabian, two Friesians, one Lusitano, and one Percheron. The living conditions, healthcare routine, and management of the horses recruited in the study were not altered for the purposes of the study, and each remained in their owners' and veterinarians' care during the course of the study.
All horses included in the initial sample group had CBC results within laboratory RIs. Although initially included for sampling, data from three horses were subsequently excluded from the July group as one horse had an enlarged submandibular lymph node, one had a superficial laceration on the right carpus and one had superficial laceration on the left hock. These abnormalities had resolved by the August sampling and these horses were again included. One mare, initially included in the August group, was excluded due to a history of abortion four days prior to blood collection. This horse was clinically normal and reincluded in the September group. One mare foaled during the 12 months of the study, and was not excluded.
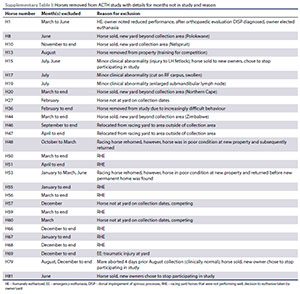
Some horses were removed from their properties during (or for part of) the study to areas outside of the collection zone, either for training, competition or sale, and so the sample group size varied from month to month. Ten horses were euthanised. Of these ten, nine were euthanised due to debilitating musculoskeletal conditions preventing them from competing at the same level and one horse was euthanised following a traumatic incident at the yard. Further details concerning the number of individuals included and excluded each month are shown in Table I. Detailed information concerning the reasons for exclusion of individual horses has been included in Supplementary Table I.
Reference intervals
RI results are presented in Table II. Between 59 and 77 reference individuals were included monthly, with subject numbers decreasing over the study period as outlined in Table I. Data had a non-Gaussian distribution and the non-parametric method was used to determine the RIs, for all months. The number of outliers removed ranged from zero to eight in each month. A total of 19 outliers were detected; 15 horses had only one outlier and two horses had two outliers each. In all cases, these horses had unremarkable clinical examinations, and no recent history of illness.
Six of the eight horses with outliers in January were racehorses, and sample collection occurred sooner after training (within one hour of returning from track) compared with previous collections (two to three hours after return from track). Three of the four most extreme outliers (ACTH of 102.0, 102.0, 102.0, 123.0 pg/ml) occurred in April; each of the three horses resided at different yards.
Monthly variation in ACTH concentrations
Based on the results of the Kruskal-Wallis test, there were significant changes in monthly ACTH concentrations, as shown in Table III. Figure 1 illustrates the variation in ACTH concentrations with changing daylight lengths. ACTH concentrations are lowest in winter and early summer, as daylight increases, and show a pronounced increase as daylight length decreases in late summer and autumn.
Discussion
To the authors' knowledge, this is the first report establishing reference intervals for ACTH in a healthy South African equine population. The significant increase in ACTH concentrations from late summer to autumn (February to May) is consistent with seasonal variations previously reported in Australia and the Northern Hemisphere autumn (Byrne et al. 2018; Copas & Durham 2012; Durham et al. 2021; Horn et al. 2021 Secombe et al. 2017.). The findings reiterate the need for temporally and geographically established RIs for local horse populations and laboratories.
The pars distalis of the pituitary gland produces the majority of the ACTH in healthy horses apart from in autumn, when the pars intermedia produces more ACTH, increasing the overall ACTH concentrations at this time (McFarlane 2019). These seasonal regulations are thought to be an evolutionary adaptation in the horse in which the animal's body is preparing for more challenging conditions posed by winter. Changes in photoperiod, mediated by circulating melatonin produced during non-daylight hours in the pineal gland, are responsible for these seasonal hormone rhythms (McFarlane 2019). This is why geographically and temporally specific ACTH RIs are necessary, as the location and season will determine the number of (non)-daylight hours experienced by the horse, which will in turn determine the variation and magnitude of the increased ACTH concentrations observed during autumn (McFarlane et al. 2011).
Measurement of basal endogenous ACTH concentration is commonly used as a means for diagnosing PPID. The diagnostic accuracy of basal ACTH for PPID is dependent on the values used to define the absence or presence of disease. A recent study has shown that comparing basal ACTH to a URL has a high specificity (98-100%) but low sensitivity (20-89%) for diagnosing PPID (Horn et al. 2021). Diagnostic cut-offs, generated using results from both healthy and PPID-affected horses, have a higher sensitivity, but lower specificity, in comparison (Durham et al. 2021; Horn et al. 2021). The URLs generated here will therefore be useful to confirm disease, but may not be appropriate for screening older horses with mild or no clinical signs, as a high proportion of false-negative results are possible. It was also found in the Australian study that, in contrast to Northern Hemisphere studies, accuracy of both URLs and diagnostic cut-offs was decreased in March (autumn) (Horn et al. 2021). The authors warn that these findings may be relevant to other sub-tropical locations in the Southern Hemisphere. The aim of our study was solely to generate RIs, and ACTH results from a PPID-affected population were not included, so the diagnostic accuracy of these new South African RIs is as yet unknown.
The number of horses included in this study, between 59 and 77 at each sampling point, is sufficient for generation of RI and similar to the Secombe et al. (2017) ACTH RI determination study in which two populations of 40 and 41 horses were sampled at different locations (Friedrichs et al. 2012; Secombe et al. 2017). A limitation of the reference population is that the horses were younger than the population of horses affected by PPID, which are usually older than 15 years of age (Durham et al. 2021; McFarlane 2019; Mc Gowan et al. 2013). Older horses have higher plasma ACTH concentrations (Horn et al. 2021). In our study, a younger population was selected specifically to reduce the risk of including early or subclinical PPID cases, as the presence of this disease in younger horses is very low and the aim was to determine RIs for a non-diseased population (Mc Gowan et al. 2013; Secombe et al. 2017). Selecting older horses may lead to the inclusion of results from subclinical cases of PPID into the dataset (Ireland & McGowan 2018). As there is no ante-mortem gold standard test for the exclusion or diagnosis of PPID, selection of a truly healthy, age-appropriate reference population for this disease is challenging (Durham et al. 2021; Ireland & McGowan 2018; McFarlane 2019.).
Only one (recent) study has reported a possible sex effect on baseline ACTH concentrations (Horn et al. 2021). The numbers of males and females were roughly equal in the present study, and sex was not a consideration in the selection of the reference individuals. Some studies have indicated a potential tendency for breed disposition to PPID, with ponies and Morgan horses reported to be predisposed (Durham et al. 2021; McFarlane 2019; Ireland & McGowan 2018). Only one mixed breed pony was enrolled in the study and no outlying ACTH concentrations were observed in that pony.
Similar to previous studies, this study showed a statistically significant increase in plasma ACTH concentrations from the summer to winter months. The dramatic increase in ACTH levels coincided with the beginning of the decrease in daylight length, as has been previously reported (Copas & Durham 2012; McFarlane et al. 2011; Secombe et al. 2017), with a general increase evident from the months of February to May. As is the case elsewhere, ACTH levels then decreased again in winter, when daylight length is shortest (Durham et al. 2021). In our study, the peak URL in autumn (60.6 pg/ml), differed from peak autumnal reference limits or diagnostic thresholds in other studies using the Immulite 1000 (77.4 - 138.0 pg/ml in Southern Hemisphere studies [Horn et al. 2021; Mc Gowan et al. 2013; Secombe et al. 2017]; 49.7-75.2 pg/ml in Northern Hemisphere studies [Copas & Durham 2012; Donaldson et al. 2005; Durham et al. 2021.]). The Immulite 2000 method has been reported to have a negative bias of approximately 11% compared to the Immulite 1000 (unpublished data; Durham et al. 2021). This method-associated bias may account for some of the difference, but these findings reinforce the point that ACTH reference intervals should be not only season- and method-specific, but also latitude-specific (Secombe et al. 2017).
Due to the labile nature of ACTH, consideration of sample stability is important if these results are to be used for wider equine populations (Prutton et al. 2015). Equine ACTH has been reported to be stable when frozen for up to 30 days, and a single freeze-thaw cycle, as employed in this study, has been reported to have no statistically significant effect on ACTH concentrations in healthy horses without PPID (Hu et al. 2020; Prutton et al. 2015). Based on this, preanalytical effects are unlikely to have caused inaccurate results in this study, and consequently these RIs can be used to evaluate ACTH concentrations in patient samples collected under similar conditions.
There were an unusually high number of outliers (eight) in January (Table I). Six of these eight outliers were race horses in training. At the January sample collection, training was delayed on the morning of collections and samples were taken from all the racing yard horses less than one hour after their return from the track for training, as opposed to two to three hours post-training in other months. Studies have verified that short bursts of intense exercise can result in a transient increase in ACTH secretion which returns to pre-exercise values within thirty minutes (Alexander et al. 1991). This is presumed to be the reason for the outlier values of these six horses in January.
The mare that foaled during the fifth month (November 2019) of the study was not excluded as no outlier values were obtained from this horse during the course of the year and the mare remained clinically healthy. Studies report peak plasma ACTH concentrations one week pre-partum, but that in the weeks following parturition, mares have a lower ACTH concentration when compared with controls (Cudd et al. 1995; Piccione et al. 2017). The mare was sampled less than two weeks after parturition and was retained in the study as all the mare's ACTH values fell well within the RI, including the sampling prior to parturition.
The nine horses that were euthanised during the course of the study due to musculoskeletal conditions were not laminitic at the time of euthanasia or at any collection point prior to the euthanasia, and had no ACTH values above the RI at any single time point prior to being removed from the study.
Limitations of the current study include the relatively low number of horses sampled. Moreover, horses were lost to attrition and therefore twelve months of data is not recorded for each horse that was enlisted in the study. No ante-mortem test is able to confirm PPID status and although unlikely based on the inclusion and exclusion criteria, some of the participants included in the study may have had subclinical PPID or other diseases affecting ACTH production. No necropsies were permitted on subjects that were euthanised and no TRH stimulation testing was performed to confirm non-PPID status. As discussed previously, the age group sampled was selected in an attempt to ensure subclinical PPID cases were unlikely to be included, however, this limits the extrapolation of the results in a diseased population (Durham et al. 2021). Additionally, as the breed composition in the sample population was not specifically selected for the overall population the RI will be applied to, under- or overrepresentation of some breeds may be another limiting factor (Horn et al. 2021; McFarlane 2019). As also discussed, there was no attempt in this study to include PPID-infected horses, generate diagnostic cutoffs, or determine the diagnostic accuracy of these RIs.
Conclusion
This study contributes to the body of literature illustrating the need for temporally and seasonally determined RIs for ACTH to aid in the diagnosis of PPID in equines. There is still no "gold standard" test to confirm the diagnosis. However, a locally relevant ACTH RI used in conjunction with prudent decision-making based on horse signalment and clinical signs will aid practitioners in diagnosing PPID, recommending additional testing or testing at another time of year. Further temporal studies in South Africa that examine the diagnostic utility of these RIs in a population that includes healthy and PPID horses are needed.
Acknowledgements
The authors thank Siemens Healthcare, South Africa, for donating two ACTH reagent kits for this study.
Conclift of interest
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Funding source
Funding was provided by the Department of Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, and by a postgraduate bursary from the Health and Welfare Sector Education and Training Authority of South Africa.
ORCID
D Fisher: https://orcid.org/0000-0002-6203-2353
E-C Schliewert: https://orcid.org/0000-0003-3763-8203
EH Hooijberg: https://orcid.org/0000-0002-4367-799X
References
Alexander, S.L., Irvine, C.H.G., Ellis, M.J. & Donald, R.A., 1991, The effect of acute exercise on the secretion of corticotropin-releasing factor, arginine vasopressin, and adrenocorticotropin as measured in pituitary venous-blood from the horse, Endocrinology 128(1), 65-72. https://doi.org/10.1210/endo-128-1-65. [ Links ]
Byrne, D.P., Secombe, C.J., Tan, R.H.H., et al., 2018, Circannual variability in adrenocorticotropic hormone responses to administration of thyrotropin-releasing hormone in clinically normal horses in Australia, Vet J 238, 58-62. https://doi.org/10.10167j.tvjl.2018.07.008. [ Links ]
Copas, V.E.N. & Durham, A.E., 2012, Circannual variation in plasma adrenocorticotropic hormone concentrations in the UK in normal horses and ponies, and those with pituitary pars intermedia dysfunction, Equine Vet J 44(4), 440-443. https://doi.org/10.1111/j.2042-3306.2011.00444.x. [ Links ]
Couetil, L., Paradis, M.R. & Knoll, J., 1996, Plasma adrenocorticotropin concentration in healthy horses and in horses with clinical signs of hyperadrenocorticism, J Vet Intern Med 10(1), 1-6. https://doi.org/10.1111/j.1939-1676.1996.tb02016x [ Links ]
Cudd, T.A., Leblanc, M., Silver, M., et al., 1995, Ontogeny and ultradian rhythms of adrenocorticotropin and cortisol in the late-gestation fetal horse, J Endocrinol 144(2), 271-283. https://doi.org/10.1677/joe.0.1440271. [ Links ]
Donaldson, M.T., McDonnell, S.M., Schanbacher, B.J., et al., 2005, Variation in plasma adrenocorticotropic hormone concentration and dexamethasone suppression test results with season, age, and sex in healthy ponies and horses, J Vet Intern Med 19(2), 217-222. https://doi.org/10.1111/j.1939-1676.2005.tb02685.x. [ Links ]
Durham, A.E., Clarke, B.R., Potier, J.F.N., et al., 2021, Clinically and temporally specific diagnostic thresholds for plasma ACTH in the horse, Equine Vet J 53(2), 250-260. https://doi.org/10.1111/evj.13292. [ Links ]
Frank, N., Elliott, S.B., Chameroy, K.A., et al., 2010, Association of season and pasture grazing with blood hormone and metabolite concentrations in horses with presumed pituitary pars intermedia dysfunction, J Vet Intern Med 24(5), 1167-1175. https://doi.org/10.1111/j.1939-1676.2010.0547.x. [ Links ]
Friedrichs, K.R., Harr, K.E., Freeman, K.P., et al., 2012, ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics, Vet Clin Path 41(4), 441-453. https://doi.org/10.1111/vcp.12006. [ Links ]
Horn, R., Stewart, A.J., Jackson, K.V., et al., 2021, Clinical implications of using adrenocorticotropic hormone diagnostic cutoffs or reference intervals to diagnose pituitary pars intermedia dysfunction in mature horses, J Vet Intern Med 35(1), 560-570. https://doi.org/10.1111/jvim.16017. [ Links ]
Hu, K., Stewart, A.J., Yuen, K.Y., et al., 2020, The effect of freeze-thaw cycles on determination of immunoreactive plasma adrenocorticotrophic hormone concentrations in horses, J Vet Intern Med 34(3), 1350-1356. https://doi.org/10.1111/jvim.15771. [ Links ]
Ireland, J.L. & McGowan, C.M., 2018, Epidemiology of pituitary pars intermedia dysfunction: A systematic literature review of clinical presentation, disease prevalence and risk factors. Vet J 235, 22-33. https://doi.org/10.1016/j.tvjl.2018.03.002. [ Links ]
Irvine, K.L., Burt, K., Hill, A.J., et al., 2016, Initial analytic quality assessment and method comparison of an immunoassay for adrenocorticotropic hormone measurement in equine samples, Vet Clin Path 45(1), 154-163. https://doi.org/10.1111/vcp.12326. [ Links ]
Le Boedec, K., 2016, Sensitivity and specificity of normality tests and consequences on reference interval accuracy at small sample size: a computer-simulation study, Vet Clin Path 45(4), 648-656. https://doi.org/10.1111/vcp.12390. [ Links ]
Mc Gowan, T.W., Pinchbeck, G.P. & Mc Gowan, C.M., 2013, Evaluation of basal plasma a-melanocyte-stimulating hormone and adrenocorticotrophic hormone concentrations for the diagnosis of pituitary pars intermedia dysfunction from a population of aged horses, Equine Vet J 45(1), 66-73. https://doi.org/10.1111/j.2042-3306.2012.00575.x. [ Links ]
McFarlane, D., 2019, Diagnostic testing for equine endocrine diseases confirmation versus confusion, Vet Clin North Am Equine Pract 35(2), 327-338. https://doi.org/10.1016/j.cveq.2019.03.005. [ Links ]
McFarlane, D., Paradis, M.R., Zimmel, D., et al., 2011, The effect of geographic location, breed, and pituitary dysfunction on seasonal adrenocorticotropin and a-melanocyte-stimulating hormone plasma concentrations in horses, J Vet Intern Med 25(4), 872-881. https://doi.org/10.1111/j.1939-1676.2011.0745x [ Links ]
Piccione, G., Arfuso, F., Abbate, F., et al., 2017, Adrenocorticotrophic hormone and cortisol levels during late pregnancy and post-foaling period in mares, Anim Sci Pap Rep 35, 173-180. [ Links ]
Prutton, J.S.W., Kass, P.H., Watson, J.L. & Pusterla, N., 2015, Pre-analytical stability of adrenocorticotrophic hormone from healthy horses in whole blood, plasma and frozen plasma samples, Vet J 204(1), 123-124. https://doi.org/10.1016/j.tvjl.2015.02.010. [ Links ]
Secombe, C.J., Tan, R.H.H., Perara, D.I., et al., 2017, The effect of geographic location on circannual adrenocorticotropic hormone plasma concentrations in horses in Australia, J Vet Intern Med 31(5), 1533-1540. https://dx.doi.org/10.1111/jvim.14782. [ Links ]
Thorsen S., Time and date AS, Available from: https://www.timeanddate.com/. Accessed 19 Jan 2021. [ Links ]
 Correspondence:
Correspondence:
EH Hooijberg
Email: emma.hooijberg@up.ac.za