Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.85 n.1 Pretoria Jan. 2014
http://dx.doi.org/10.4102/jsava.v85i1.996
CLINICAL COMMUNICATIONS
Prevalence of select vector-borne disease agents in owned dogs of Ghana
Lorelei L. ClarkeI; Lora R. BallweberII; Kelly AllenIII; Susan E. LittleIII; Michael R. LappinIV
IDepartment of Pathology, University of Georgia, United States
IIVeterinary Diagnostic Laboratory, Colorado State University, United States
IIICenter for Veterinary Health Sciences, Veterinary Pathobiology, Oklahoma State University, United States
IVDepartment of Clinical Sciences, Colorado State University, United States
ABSTRACT
Ticks, sera and ethylenediaminetetraacetic acid (EDTA) blood were collected from dogs evaluated at the Amakom Veterinary Clinic in Kumasi, Ghana. Sera were evaluated for Dirofilaria immitis antigen and antibodies against Borrelia burgdorferi, Anaplasma phagocytophilum and Ehrlichia canis. Conventional polymerase chain reaction assays designed to amplify the deoxyribonucleic acid (DNA) of Ehrlichia spp. or Anaplasma spp. or Neorickettsia spp. or Wolbachia spp., Babesia spp., Rickettsiaspp., Hepatozoon spp., Bartonella spp. and the haemoplasmas were performed on DNA extracted from EDTA blood and all positive amplicons were sequenced. This small survey shows that the following vector-borne pathogens are present in urban Ghanian dogs: Ehrlichia canis, Hepatozoon canis, Dirofilaria immitis and Anaplasma platys. Bartonella henselae was isolated from ticks but not from the dogs.
Introduction
Previous studies in southern and eastern Africa show relatively high rates of canine vector-borne infections (10% - 49%) (Matjila et al. 2008; Sasaki et al. 2008). In addition, domestic dogs can be used as sentinels for human diseases caused by potentially zoonotic agents. The objective of this study was to screen for the presence of selected vector-borne agents in Kumasi, Ghana by analysing a limited sample set from domestic dogs.
One of the investigators collected blood samples and ticks from dogs presented to the Amakom Veterinary Clinic in Kumasi, Ghana, during a 28 day period between December 2010 and January 2011. Blood was collected from those dogs whose owners gave consent for venopuncture. This was the only selection criterion. No travel history was documented. Sampled dogs were presented for a variety of complaints, including lethargy, inappetance and vomiting, or for vaccination. Blood was obtained and 1 mL was placed into a 1.5 mL ethylenediaminetetraacetic acid (EDTA) tube and allowed to clot for serum separation. Ticks, if present, were removed from the dogs that were sampled and placed in isopropanol. Ticks, sera and blood were refrigerated until transport to the United States of America and then stored at -80 °C until assayed.
Sera were evaluated for Dirofilaria immitis antigen and antibodies against Borrelia burgdorferi,Anaplasma phagocytophilum, and Ehrlichia canis (SNAP® 4DX, IDEXX Laboratories, Portland ME). Ticks were identified by microscopy and total deoxyribonucleic acid (DNA) was extracted from the ticks and blood using QIAGEN® DNeasy Blood and Tissue Kits. Polymerase chain reaction assays designed to amplify the DNA of Ehrlichia spp. or Anaplasma spp. or Neorickettsia spp. or Wolbachia(multi-species primers), Babesia spp., Bartonella spp., Rickettsia spp., Hepatozoon spp. and the haemoplasmas were performed on total DNA extracted from EDTA blood and the ticks as previously described (Allen et al. 2008; Birkenheuer, Levy & Breitschwerdt 2003; Jensen et al. 2000, 2001; Lappin et al. 2004). Positive amplicons were sequenced (Macromolecular Resources, Colorado State University, Fort Collins, Colorado) and compared with sequences reported in the National Center for Biotechnology Information database (GenBank).
Ticks from individual dogs (n = 8; all Rhipicephalus spp.) as well as sera and blood (n = 17) were collected. Ten dogs (58.8%) and three (38.0%) ticks showed evidence of carrying blood-borne organisms. Positive Polymerase chain reaction (PCR) and 4DX results are presented in Table 1.
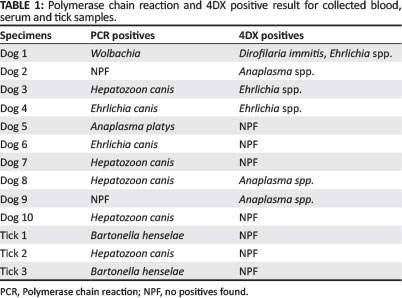
The commercial laboratory kit used to detect A. phagocytophilum antibodies is known to cross-react with Anaplasma platys (IDEXX Laboratories 2008). Anaplasma phagocytophilum is commonly transmitted by Ixodes spp. ticks, which were not detected in this study, whilst A. platys is suspected to most likely be carried by Rhipicephalus sanguineus. The presence of E. canis, also vectored by R. sanguineus, was documented by PCR and serology. Thus, the two dogs that were seropositive for A. phagocytophilum antibodies but PCR negative may have been infected with alternative Anaplasmaspecies such as A. platys, which was isolated by PCR and confirmed by sequencing in a different animal. Discrepancies between serology and PCR results could potentially be attributed to an acute infection or effects of storage.
Hepatozoon canis is also thought to be carried predominantly by Rhipicephalus spp. ticks. In the present study, the identity amongst the H. canis sequences from the dogs and reference sequences from GenBank ranged from 99.2% to 100% related to H. canis sequences. Sequences were 91.7% - 92.5% identical to Hepatozoon americanum sequences, but it is more likely that the Ghanaian isolates are H. canis given that H. americanum has never been detected outside of the USA, has a low level of parasitaemia (making it difficult to amplify by PCR) and is vectored by Amblyomma maculatum, which has not been documented in this region of Africa.
DNA of Babesia spp., Rickettsia spp., the haemoplasmas, Neorickettsia spp. and Bartonella spp. were not amplified from the blood of the dogs tested in this study and no dogs were seropositive for B. burgdorferi. Since Babesia canis vogeli, Mycoplasma haemocanis and possibly Babesia gibsoni are associated with Rhipicephalus spp. ticks and have all been documented in Africa, the negative findings for these organisms probably relates to the sample size. These organisms are not always associated with clinical disease, but could pose a threat in immunocompromised or young animals.
Overall, the present study provides evidence that a diverse array of vector-borne agents is present in client-owned dogs of Kumasi, Ghana. Also, the evidence of Bartonella henselae being carried in ticks indicates a potential zoonotic risk in the region. Further studies are needed to sample larger canine populations and also to compare regions of western Africa. Documentation of clinical disease correlating to detection of infection will also be important to determine the impact these agents have on dog populations in the region.
Acknowledgements
The authors would like to thank the veterinarians, staff, clients and dogs of Amakom Veterinary Clinic, as well as Jennifer Hawley, Melissa Brewer and Arianne Morris for helping with the lab work. This study was funded by the Center for Companion Animal Studies at Colorado State University and the Krull-Ewing Endowment in Veterinary Parasitology at Oklahoma State University.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this article.
Authors' contributions
L.L.C. (University of Georgia) conceived the study, collected the samples, carried out the immunoassays and the majority of the polymerase chain reactions, and drafted the manuscript. L.R.B. (Colorado State University) identified the ticks. K.A. (Oklahoma State University) and S.E.L. (Oklahoma State University) carried out Hepatozoon spp. polymerase chain reactions and sequence alignment. M.R.L. (Colorado State University) helped conceive the study, coordinated laboratory and collaboration efforts and helped draft the manuscript. All authors read and approved the final manuscript.
References
Allen, K.E., Yihang, L., Kaltenboeck, B., Johnson, E.M., Reichard, M.V., Panciera, R.J. et al., 2008, 'Diversity of Hepatozoon species in naturally infected dogs in the southern United States', Veterinary Parasitology 154(3-4), 220-225. http://dx.doi.org/10.1016/j.vetpar.2008.03.027 [ Links ]
Birkenheuer, A.J., Levy, M.G. & Breitschwerdt, E.B., 2003, 'Development and evaluation of seminested PCR for detection and differentiation of Babesia gibsoni (Asian Genotype) and B. canisDNA in canine blood samples', Journal of Clinical Microbiology 41(9), 4172-4177. http://dx.doi.org/10.1128/JCM.41.9.4172-4177.2003 [ Links ]
IDEXX Laboratories, 2008, SNAP® 4DX test kit insert, IDEXX, Portland. [ Links ]
Jensen, W.A., Fall, M.Z., Rooney, J., Kordick, D.L. & Breitschwerdt, E.B., 2000. 'Rapid identification and differentiation of Bartonella species using a single-step PCR assay', Journal of Clinical Microbiology 38(5), 1717-1722. [ Links ]
Jensen, W.A., Lappin, M.R., Kamkar, S. & Reagan, W.J., 2001, 'Use of a polymerase chain reaction assay to detect and differentiate two strains of Haemobartonella felis in naturally infected cats',American Journal of Veterinary Research 62(4), 604-608. http://dx.doi.org/10.2460/ajvr.2001.62.604 [ Links ]
Lappin, M.R., Breitschwerdt, E.B., Jensen, W.A., Dunnigan, B., Rha, J., Williams, C.R. et al., 2004, 'Molecular and serologic evidence of Anaplasma phagocytophilum infection in cats in North America',Journal of the American Veterinary Medical Association 225(6), 893-896. http://dx.doi.org/10.2460/javma.2004.225.893 [ Links ]
Matjila, P.T., Leisewitz, A.L., Jongejan, F. & Penzhorn, B.L., 2008, 'Molecular detection of tick-borne protozoal and ehrlichial infections in domestic dogs in South Africa', Veterinary Parasitology 155, 152-157. http://dx.doi.org/10.1016/j.vetpar.2008.04.012 [ Links ]
Sasaki, M., Omobowale, O., Ohta, K., Tozuka, M., Matsuu, A., Hirata, H. et al., 2008, 'A PCR-based epidemiological survey of Hepatozoon canis in dogs in Nigeria', Journal of Veterinary Medical Science70(7), 743-745. http://dx.doi.org/10.1292/jvms.70.743 [ Links ]
 Correspondence:
Correspondence:
Michael Lappin
300 West Drake Road
Colorado 80523
United States
Email: mlappin@colostate.edu
Received: 26 Jan. 2013
Accepted: 20 Nov. 2013
Published: 11 Sept. 2014














