Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.85 n.1 Pretoria Jan. 2014
http://dx.doi.org/10.4102/jsava.v85i1.1077
CASE REPORT
Enterococcal-related vertebral osteoarthritis in South African broiler breeders: A case report
Henry Aitchison; Petrus Poolman; Marilette Coetzer; Caron Griffiths; Johan Jacobs; Mignon Meyer; Shahn Bisschop
Avimune (Pty) Ltd, Centurion, South Africa
ABSTRACT
Infections in broilers and broiler breeders by Enterococcus cecorum, causing clinical disease, have increasingly been described in various countries in the Northern Hemisphere over the past decade. This case report describes an outbreak of enterococcal-associated vertebral osteoarthritis (EVOA) in male broiler breeders in several flocks in South Africa. Male birds aged 4 and 9 weeks displayed the common presentation of lameness, paresis or complete paralysis. Autopsies of culled birds revealed masses on caudal thoracic vertebrae T5-T7, with vertebral osteomyelitis and spondylitis. Microbiological assays identified E. cecorum cultured from spondylitic lesions. Affected flocks were treated with amoxycillin at 25 mg/kg in the drinking water for 5 days, resulting in decreased numbers of lame birds and culls. The origin and pathogenesis of EVOA are poorly understood, which limits prevention to environmental factors that may inhibit systemic access by the enteric bacteria. Skeletal growth trends of male birds are thought to increase their susceptibility to bacterial colonisation at sites of skeletal strain, resulting in abscesses and lesions. Evidence points to the emergence of E. cecorum strains with increased pathogenicity; this highlights the need for greater understanding of the origins, treatment and prevention of EVOA to minimise its economic impact on poultry operations.
Introduction
Enterococcus species are gram-positive, catalase-negative facultative anaerobes. Whilst a number of species from this group, including Enterococcus cecorum, are known to be part of the normal gastrointestinal flora of mammals and birds (Devriese et al. 1991; Gong et al. 2002), several clinical conditions, including septicaemia, mastitis, enteritis, respiratory disease and urinary tract infections, have been reported to be associated with Enterococcus spp. (Quinn et al. 1994; Songer & Post 2005). Enterococcus cecorum has recently been isolated from osteomyelitic, spondylitic, arthritic and femoral-head lesions in broilers and broiler breeders in Scotland, the Netherlands, Belgium, Canada and the United States (Armour, Collet & Williams 2011; De Herdt et al. 2008; Devriese et al. 2002; Stalker et al. 2010; Wood et al. 2002). They showed clinical signs of lameness and died as a result of starvation and dehydration. Enterococcus cecorum is recognised as an emerging avian pathogen (Aziz & Barnes 2009; Robbins et al. 2012) with significant economic consequences for the poultry industry.
Case presentation
Sudden onset of lameness (mostly hock sitting) to complete paralysis (Figure 1a and 1b) was reported in 9-week-old male birds only, at three different sites in the Bloemfontein area. Birds placed at another site at a later date also developed similar but milder clinical signs at the age of 4 weeks. Signs at the second site disappeared and reappeared at 9 weeks of age. Floor feeding was practiced on all of the affected sites. The birds did not receive any medication prior to onset of disease, although one site did suffer from a mild coccidiosis outbreak. Losses of males of up to 4% were mainly due to culling of affected birds.

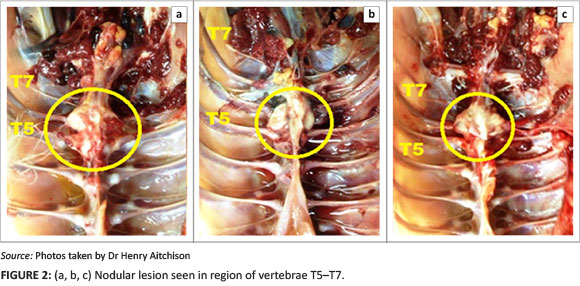
Diagnosis
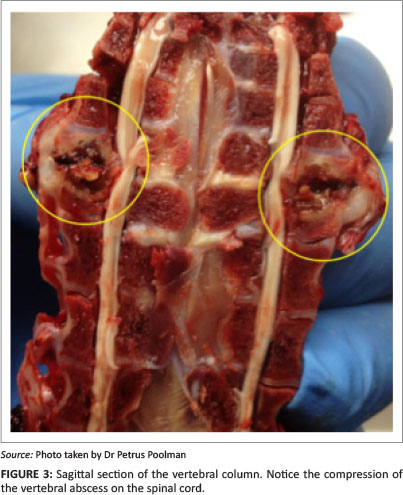
Autopsies were performed on birds showing clinical signs of hock sitting, lameness or paralysis. Nodular masses were observed on the caudal thoracic vertebral column (T5-T7) immediately anterior to the kidneys in all birds (Figure 2a, 2b and 2c), with lung tissue adherent to these vertebral enlargements. Severely affected cases showed a reduction of the space between the last two ribs, as a result of vertebral collapse. Sagittal sections of the vertebral columns revealed vertebral osteomyelitis with necrosis and abscessation, leading to compression of the overlying spinal cord (Figure 3).
Microbiology
Swab samples were collected aseptically from spondylitic lesions, as well as femoral-head lesions, and submitted for culture. Inoculation was performed using tryptone soy agar with 5% blood, and incubated at 37 °C under micro-aerophilic conditions (candle jar).
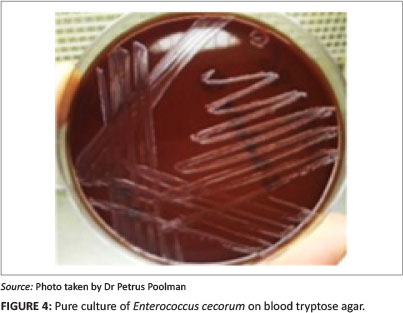
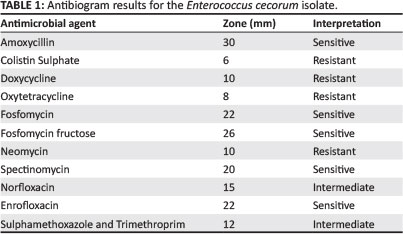
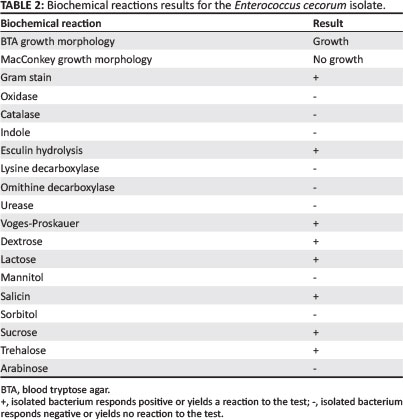
Negative cultures were obtained from femoral-head lesion samples. At 24 h post incubation, cultures from the spondylitic lesion samples were visible as very small colonies (Figure 4) and at 48 h post incubation, convex, non-haemolytic colonies of up to 1.5 mm had grown. The cultures obtained were not visibly mixed. To establish which species of Enterococcus was present, a number of tests were carried out in a South African National Accreditation System (SANAS)-accredited veterinary laboratory (Table 1). The results obtained were indicative of E. cecorum (Holt 1993).
Histopathology
Sections of the affected spinal columns were also submitted for histopathological examination. Severe osteomyelitis was seen, affecting the marrow cavity of the vertebrae. It was characterised by large numbers of heterophils associated with some necrotic bone, as well as haemorrhage. There was extension of the inflammation into the surrounding vertebral osseus tissues. One section showed the presence of eosinophilic fibrinous exudate within a vertebral centre, accompanied by numerous bacterial organisms and surrounded by more chronic fibrosis and infiltration of moderate numbers of heterophils as well as mononuclear leukocytes. Reactive osteoid formation and cartilage metaplasia were evident in these areas that had led to severe thickening of the vertebral body and resultant pressure on the spinal cord with thinning of the spinal canal. In these areas, the neuropil of the spinal cord appeared disorganised with some vacuolisation, most probably indicating pressure necrosis. A final morphological diagnosis of fibrinopurulent to pyogranulomatous vertebral osteomyelitis and spondylitis was made.
Treatment
An antibiogram (Kirby-Bauer testing) was carried out for the cultured E. cecorum. An important modification to the test was that it was carried out on blood tryptose agar (BTA), with very low bacterial growth (Table 2). Similar antibiotic sensitivity profiles have previously been reported for E. cecorum isolates from outbreaks in other countries (De Herdt et al. 2008). It was decided to treat with amoxycillin at 25 mg/kg in the drinking water for 5 days. The number of culls and lame birds decreased after treatment.
Discussion
The exact origin, predisposing factors, and pathogenesis of enterococcal-associated vertebral osteoarthritis (EVOA) are still largely unknown (Kense & Landman 2011; Robbins et al. 2012). Currently, however, the most widely accepted theory is that E. cecorum, which is normally present in the gut, enters the blood stream via a compromised intestinal barrier (Martin, Martin & Barnes 2011; Stalker et al. 2010). Thus, any factor affecting gut health in a negative way or any disturbance in the balance of the gut flora can predispose to E. cecorum entering systemically (Armour et al. 2011; Martin et al. 2011).
From the observations made in this case it is evident that E. cecorum has a predilection for cartilage and bone, especially the caudal thoracic vertebrae (T5-T7). A possible explanation for this hinges on the fact that the T2-T5 vertebrae are fused, whilst the T7 vertebra is fused to the lumbosacral vertebra. The T6 vertebra is, thus, the only freely moving articulation in this area and subject to more stress. Micro-trauma to this area could lead to altered vascular flow, the formation of micro-thrombi and subsequent sequestration and multiplication of bacteria, if systemically present (Aziz & Barnes 2007; Stalker et al. 2010).
The ages of broiler breeders in recorded outbreaks range from 3-18 weeks (Armour et al. 2011; De Herdt et al. 2008; Robbins et al. 2012), an age span that correlates with a period of rapid skeletal development in the birds. The highly vascularised nature of growth plates in rapidly growing bones, in particular, has been reported to leave such articulating bones susceptible to vascular trauma and bacterial infection during the resultant capillary bed expansion (Collett 2013; Wiseman & Prisby 2013). Furthermore, the higher body weights in male birds and, thus, greater weight borne on joints and increased chance of trauma may also explain the prevalence of the condition in this group (Armour et al. 2011; De Herdt et al. 2008). Nutritional restriction of broilers during the growth period in which outbreaks have been recorded may also contribute to irritation of the gut, allowing the entry of the enteric bacteria to systemic circulation.
Immunosuppression and the environment have also been indicated as contributory factors (Armouret al. 2011; Stalker et al. 2010). Any immunosuppressive condition would naturally impair the ability to combat an opportunistic infection with E. cecorum, which is a normal intestinal commensal. Differences in pathogenicity between clinical and commensal intestinal isolates have also been proposed (Martin et al. 2011; Robbins et al. 2012; Stalker et al. 2010), with evidence reported by Boerlin et al. (2012) suggesting that the emergence of clones is more likely to cause opportunistic infections. It has been observed that the disease can persist in a site from one cycle to the next, implicating the environment as a source for such E. cecorum strains of varying virulence (De Herdtet al. 2008; Kense & Landman 2011).
Treatment and prevention protocols are limited, as the origin and pathogenesis of the disease is still unclear. Although various antibiotics have been shown to be effective against E. cecorum, obtaining adequate concentrations of these in the spine is difficult (Kense & Landman 2011). With regard to management, the following may reduce the risk of the disease developing: avoiding excessive feed restriction; following the suggested weight profiles and nutritional guidelines (Martin et al. 2011); ensuring adequate coccidiosis control (Stalker et al. 2010); avoiding overstocking; providing adequate feeder space; and preventing respiratory disease. Subsequent to the initial outbreak, several interventions can reduce the risk of disease in flocks on affected sites. These include: complete clean-out and disinfection with fumigation of the house; litter replacement; proper cleaning of water lines; and continuous water sanitation (Armour et al. 2011).
Enterococcal-associated vertebral osteoarthritis can be confused with other causes of spinal compression and should be differentiated from spondylolisthesis ('kinky back') caused by vertebral subluxation, and scoliosis, the lateral deviation of the spinal vertebrae (Armour et al. 2011; Robbinset al. 2012). Proper flock monitoring can assist in early detection of any of these conditions, allowing their diagnosis and management.
Conclusion
Disease caused by E. cecorum can have a significant economic impact on any poultry operation, resulting in: increased culling and overall mortality in breeders; poor feed conversion ratios; and increased condemnations in broilers (Armour et al. 2011; De Herdt et al. 2008).
More research is required to determine the origin and pathogenesis of EVOA and until more clarity on the disease is gained, gastro-intestinal health protection and minimising the potential for back injuries is essential. Early detection, differentiation from conditions with similar clinical signs and treatment are important to limit the losses incurred as a result of the disease.
Acknowledgements
Competing interests
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Authors' contributions
H.A. (Avimune) and P.P. (Avimune) first identified the clinical presentation of the condition in birds on the farms. They both worked to diagnose and manage the infections and gave significant intellectual contributions to the preparation of the manuscript. M.C. (Avimune) and C.G. (Avimune) co-wrote the bulk of the manuscript. J.J. (Avimune) and M.M. (Avimune) carried out all microbiological tests to identify the infective organism and contributed microbioligcal reports used in the manuscript. S.B. (Avimune) co-ordinated preparation of the manuscript. He contributed significant intellectual input to its preparation, including editing the work.
References
Armour, N.K., Collet, S.R. & Williams, S.M., 2011, 'Enterococcus cecorum-related arthritis and osteomyelitis in broilers and broiler breeders', The Poultry Informed Professional 117, 1-7. [ Links ]
Aziz, T. & Barnes, H.J., 2007, 'Is spondylitis an emerging disease of broilers?', World Poultry 23, 44-45. [ Links ]
Aziz, T. & Barnes, H.J., 2009, 'Spondylitis is emerging in broilers', World Poultry 25(9), 19. [ Links ]
Boerlin, P., Nicholson, V., Brash, M., Slavic, D., Boyen, F., Sanei, B. et al., 2012, 'Diversity ofEnterococcus cecorum from chickens', Veterinary Microbiology 157, 405-411. http://dx.doi.org/10.1016/j.vetmic.2012.01.001 [ Links ]
Collett, S., 2013, 'Lameness in broilers - osteomyelitis', The Poultry Informed Professional 128, 1-5. [ Links ]
De Herdt, P., Defoort, P., Van Steelant, J., Swam, H., Tanghe, L., Van Goethem, S. et al., 2008, 'Enterococcus cecorum osteomyelitis and arthritis in broiler chickens', Vlaams Diergeneeskundig Tijdschrift 78, 44-48. [ Links ]
Devriese, L.A., Hommez, J., Wijfels, R. & Haesebrouck, F., 1991, 'Composition of the enterococcal and streptococcal intestinal flora of poultry', Journal of Applied Bacteriology 71, 46-50. http://dx.doi.org/10.1111/j.1365-2672.1991.tb04480.x [ Links ]
Devriese, L.A., Cauwerts, K., Hermans, K. & Wood, A.M., 2002, 'Enterococcus cecorum septicaemia as a cause of bone and joint lesions resulting in lameness in broiler chickens', Vlaams Diergeneeskundig Tijdschrift 71, 219-221. [ Links ]
Gong, J., Forster, R.J., Yu, H., Chambers, J.R., Wheatcroft, R., Sabour, P.M. et al., 2002, 'Molecular analysis of bacterial populations in the ileum of broiler chickens and comparison with bacteria in the cecum', FEMS Microbiological Ecology 41, 171-179. http://dx.doi.org/10.1111/j.1574-6941.2002.tb00978.x [ Links ]
Holt, J.G. (ed.), 1993, Bergey's Manual of Determinative Bacteriology, 9th edn., Williams & Wilkins Co., Baltimore, p. 538. [ Links ]
Kense, M.J. & Landman, W.J.M., 2011, 'Enterococcus cecorum infections in broiler breeders and their offspring: Molecular epidemiology', Avian Pathology 40(6), 603-612. http://dx.doi.org/10.1080/03079457.2011.619165 [ Links ]
Martin, L.T., Martin, M.P. & Barnes, H.J., 2011, 'Experimental reproduction of ente rococcal spondylitis in male broiler breeder chickens', Avian Diseases 55, 273-278. http://dx.doi.org/10.1637/9614-121410-Reg.1 [ Links ]
Quinn, P.J., Carter, M.E., Markey, B. & Carter, G.R., 1994, Clinical veterinary microbiology, Mosby, Philadelphia. [ Links ]
Robbins, K.M., Suyemoto, M.M., Lyman, R.L., Martin, M.P., Barnes, H.J. & Borst, L.B., 2012, 'An outbreak and source investigation of enterococcal spondylitis in broilers caused by Enterococcus cecorum', Avian Diseases 56, 768-773. http://dx.doi.org/10.1637/10253-052412-Case.1 [ Links ]
Songer, J.G. & Post, K.W., 2005, Veterinary microbiology: Bacterial and fungal agents of animal disease, Elsevier Saunders, Philadelphia. [ Links ]
Stalker, M.J., Brash, M.L., Weisz, A., Ouckama, R.M. & Salvic, D., 2010, 'Arthritis and osteomyelitis associated with Enterococcus cecorum infection in broiler and broiler breeder chickens in Ontario, Canada', Journal of Veterinary Diagnostic Investigation 22, 643-645. http://dx.doi.org/10.1177/104063871002200426 [ Links ]
Wiseman, R.F. & Prisby, R.D., 2013, 'Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: A translational model for the pathogenesis of femoral head necrosis', Frontiers in Endocrinology 3, 1-14. [ Links ]
Wood, A.M., MacKenzie, G., McGillveray, N.C., Brown, L., Devrieses, L. & Baele, M., 2002, 'Isolation of Enterococcus cecorum from bone lesions in broiler chickens', Veterinary Record 150(1), 27. [ Links ]
 Correspondence:
Correspondence:
Petrus Poolman
248 Jean Avenue
Lyttelton
Centurion 0148
South Africa
Email: petrus@avimune.co.za
Received: 23 June 2013
Accepted: 07 Jan. 2014
Published: 06 Nov. 2014














