Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.85 n.1 Pretoria Jan. 2014
ORIGINAL RESEARCH
The creation of a measurable contusion injury in skeletal muscle
Margaret N. DeaneI; Michael GregoryII; Maurice MarsIII
IDepartment of Physiotherapy, University of KwaZulu-Natal, South Africa
IISchool of Life Sciences, University of KwaZulu-Natal, South Africa
IIIDepartment of Telehealth, University of KwaZulu-Natal, South Africa
ABSTRACT
The effect that compressed air massage (CAM) has on skeletal muscle has been ascertained by the morphological and morphometric evaluation of healthy vervet monkey and rabbit skeletal muscle. How CAM may influence the process of healing following a contusion injury is not known. To determine how CAM or other physiotherapeutic modalities may influence healing, it is necessary to create a minor injury that is both reproducible and quantifiable at the termination of a pre-determined healing period. An earlier study described changes in the morphology of skeletal muscle following a reproducible contusion injury. This study extended that work in that it attempted to quantify the 'severity' of such an injury. A 201 g, elongated oval-shaped weight was dropped seven times through a 1 m tube onto the left vastus lateralis muscle of four New Zealand white rabbits. Biopsies were obtained 6 days after injury from the left healing juxta-bone and sub-dermal muscle and uninjured (control) right vastus lateralis of each animal. The tissue was fixed in formal saline, embedded in wax, cut and stained with haematoxylin and phosphotungstic haematoxylin. The muscle was examined by light microscopy and quantification of the severity of injury made using a modified, 'in-house' morphological index and by the comparative morphometric measurement of the cross-sectioned epimysium and myofibres in injured and control muscle. The results showed that a single contusion causes multiple, quantifiable degrees of injury from skin to bone - observations of particular importance to others wishing to investigate contusion injury in human or animal models.
Introduction
Ethical considerations and volunteer apathy are the primary difficulties in creating muscle injury models in humans (James, Scott & Allen 1993; Potera 1995; Staples 1996). It is rare that volunteers are prepared to be experimentally injured and subsequently biopsied. Furthermore, athletes who are injured in the course of training or participating in sporting events are unlikely to subject themselves to invasive biopsy during treatment and healing. Most data regarding muscle injuries, therefore, have been obtained from studies in animals, which allows control over the degree of injury and enables mechanisms of recovery, with and without treatment, to be studied (Fisher, Hiller & Rennie 2003). The effect that compressed air massage (CAM) has on rabbit vastus lateralis and healthy vervet monkey tibialis anterior muscle has been reported by the team undertaking the current study (Gregory & Mars 2003, 2005). The reports described and compared the cross-sectional fibre diameters (FD) and capillary diameters of untreated and CAM-treated muscle sequentially over a period of 6 days after massage. Based on these results, the mechanism of action of CAM was postulated (Gregory & Mars 2005). To date, it has not been possible to determine how or whether CAM may influence the physiological mechanisms of healing following an injury.
In order to determine this and perhaps quantify the effect that CAM or any other physiotherapeutic modality has on an injured muscle, the comparative study would have to meet three important criteria:
• provide a reproducible injury
• establish the changes in the morphology associated with the injury at a specified time during healing
• quantify such changes in such a manner as to enable comparison without or between different treatments.
Deane, Gregory, Mars and Bester (2013) created a reproducible contusion injury in rabbit vastus lateralis muscle. Later, whilst describing the morphological appearance of the muscle 6 days after injury, it appeared that depending on position within the muscle, sub-dermal (SD) or juxta-bone (JB), a single contusion caused injuries of at least two degrees of magnitude (Deane, Gregory & Mars 2013). This study complements the earlier work by attempting to quantify the positional degree of healing and muscle regeneration at this time. The authors have endeavoured to do this by developing a morphological index that numerically describes the residual degree of injury or stage of healing in SD and JB rabbit vastus lateralis muscle 6 days post trauma. It is postulated that the numeric data from such a study could be used as a baseline against which the severity of a contusion and/or the efficacy of CAM or other physiotherapeutic modalities can be compared and perhaps quantified.
Ethical approval
The experiments were approved by the Ethics and Research Committees of the University of KwaZulu-Natal (ethical clearance number 018/10 / Animal).
Materials and methods
Procedure
The details of the manner in which the rabbits were prepared for injury and biopsy have been described in detail (Deane, Gregory, Mars & Bester 2013). In brief, following approval, four New Zealand white rabbits (R1-R4) were anaesthetised with an intramuscular injection of the combination of 30 mg/kg body weight (bw) ketamine and 4 mg/kg bw xylazine followed by a subcutaneous injection of 5 mg/kg bw morphine sulphate. The thighs of each animal's left and right hind limbs were shaved and the mid-belly of the vastus lateralis muscle identified by palpations and the skin marked with indelible ink. A guide tube 100 cm in length was placed directly over the mark on the left limb and an elongated oval weight of 201 g was dropped seven times onto the muscle.
Six days after injury the animals were anaesthetised, the skin over the lateral mid-thigh was incised longitudinally and biopsies approximately 8 mm in length and 5 mm in diameter were taken from JB and SD positions within the vastus lateralis muscle from each injured left limb of the animals (R1L-R4L) (Deane, Gregory, Mars & Bester 2013). To serve as morphological and morphometric controls, similar biopsies were taken from the uninjured right limbs of the same animals (R1R-R4R). Following biopsy, the skin was sutured and the rabbits were monitored for any post-operative discomfort during and after recovery from the anaesthetic.
Each biopsy was fixed in 10% formal saline for 24 h, dehydrated through increasing concentrations of ethanol, cleared in xylene and embedded in paraffin wax (Deane, Gregory, Mars & Bester 2013). Sections 4 µm in thickness were cut and stained with haematoxylin (H&E) and phosphotungstic haematoxylin (PTAH). The sections were examined using a Nikon Compound Microscope (Nikon Eclipse 8i) and images of the tissue were captured at W10, W20 and W40 magnification and stored in jpeg format.
Quantitative analyses
The vastus lateralis muscle in adult rabbits is approximately 13 mm in thickness. The biopsies were approximately 5 mm in thickness. Deane, Gregory and Mars (2013) showed that the most severe damage to muscle was invariably within 3 mm of the epimysium in JB biopsies and 1 mm - 2 mm in SD specimens. The area from both JB and SD samples was often circumscribed by various numbers of swollen and/or vacuolated myofibres. The vast majority of myofibres beyond these obviously 'pathological' regions of regeneration or repair invariably appeared morphologically normal by light microscopy. In order to determine the presence and/or extent of injury in the deeper regions of the muscle, myofibres distant from obvious pathology were measured for any possible changes in myofibre size. The morphometric method employed was the 'minimum diameter method' described by Dubowitz (1985). In brief, suitable areas containing transverse or obliquely sectioned myofibres were selected for morphometric evaluation (Figure 1). The myofibres were considered to be nearly cylindrical and the diameter of fibres taken to be the least distance across each cell. A minimum of 100 myofibres were measured from apparently normal areas (assessed by light microscopy) beyond the regions of obvious trauma (usually > 2 mm - 3 mm from the epimysium) of JB and SD biopsies from each injured animal. Measurements were made from stored jpeg images using image analytical software (Image-Pro Plus version 6.2). The number of myofibres measured and means and standard deviation were recorded and compared with data from myofibres in similar positions from SD and JB biopsies in the control limbs.
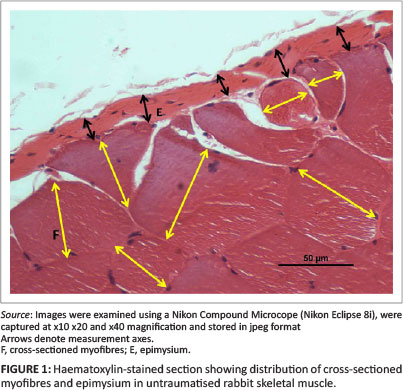
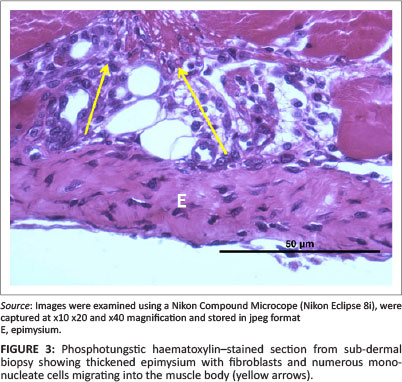
Deane, Gregory and Mars (2013) as well as Deane, Gregory, Mars and Bester (2013) reported that 6 days after injury the cellular structure and thickness of the epimysium appeared to change with the apparent severity of the initial injury. To quantify this subjective observation, the thickness of the epimysium was measured in all control and injured JB and SD specimens. As above, measurements were made from digital images using image analytical software. A minimum of 100 and maximum of 229 measurements were made along the cross-sectioned epimysium from each JB and SD control specimen and from 105 to 123 measurements of JB and SD biopsies from injured animals (Figures 1 and 3).
The morphological index was developed using macroscopic and microscopic features of the injured muscle (see below). The two tailed, unpaired /-tests, assuming unequal variance, were applied to the myofibre and epimysial data between all control and injured samples. Alpha was set at 5% and confidence intervals (CI) were calculated.
Results
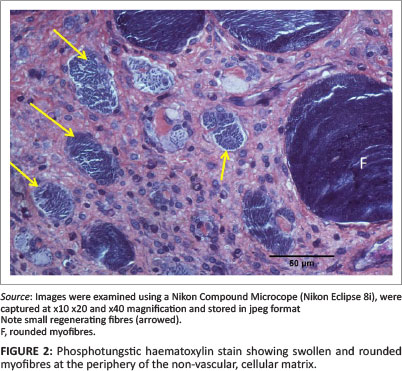
The morphological appearance of the muscle in the JB and SD positions 6 days after injury was detailed by Deane, Gregory and Mars (2013). In brief, in JB muscle, the injury stretched from the epimysium for up to approximately 3 mm into the muscle body. In this region, most myofibres had necrosed and had been replaced by a non-vascular matrix within which were small mononuclear cells, myotubes and small, new, regenerating fibres. In some samples, within and on the periphery of the cellular matrix there were occasional swollen, sometimes vacuolated myofibres that may have been undergoing processes of repair (Figure 2).
Sub-dermal specimens, whilst showing less evidence of severe injury close to the epimysium, also contained occasional swollen, vacuolated myofibres, both within and on the periphery of the necrotic region similar to those described above in Figure 2. In Rabbit 3, however, the injury was more severe. This SD biopsy exhibited a non-vascular, hyperplastic, myofibre-free area up to approximately 2 mm from the epimysium similar to that described above in JB biopsies. In all specimens from JB and SD positions the epimysium was obviously thickened. The morphology of myofibres in all control samples was normal in every respect.
Epimysium thickness
The cross-sectioned epimysium in control specimens appeared as a membrane of relatively uniform thickness at the periphery of bundles of normal myofibres (Figure 1). There were no obvious morphometric differences between the thickness of the epimysium encapsulating the muscle in JB (9.2 µm) or SD (9.1 µm) specimens (Table 1).
In all cases and each position, the thickness of the control epimysium ranged from 4 µm to 18 µm with an overall mean of 9.2 µm ± 3 µm (95% CI: 8.9 µm - 11.6 µm). In injured SD samples, the epimysium averaged 40.6 µm ± 10.01 µm (95% CI: 39.6 µm - 41.5 µm) in thickness with a range from 14.1 µm to 64.0 µm. The epimysium in injured JB samples was greatly thickened (Figure 3), having an average thickness of 59.8 µm ± 22.0 µm with an extended range from 21.5 µm to 100.7 µm.
The overall thickness of the epimysium encapsulating SD samples was compared with that of JB muscle. Following injury, the epimysium of all JB biopsies was significantly thicker than the epimysium of SD biopsies from the same animal, R1 (p < 0.01) and R2-4 (p < 0.001).
Myofibre morphometry
Analyses showed that mean FD approximately 2 mm or more from the epimysium in both SD and JB biopsies were generally reduced in diameter 6 days after injury (Table 2).
With the exception of animal R4 (-1.2%), whose FD appeared statistically normal (p > 0.01), all FD in injured SD specimens were significantly reduced (p < 0.01). In the case of JB biopsies, there was little difference in mean FD between control and injured samples in animals R1 (2.8%) and R2 (5.9%), whilst the mean FD in animals R3 (26.1%) and R4 (28.1%) was significantly reduced (p < 0.001). Taken as groups, the average reduction of FD in SD specimens was -14.2% (p < 0.01) and in JB specimens -11.4% (p < 0.01).
Morphological index
The relative severity of features noted in SD and JB biopsies is quantified numerically by means of the morphological index (MI). Variations in the morphological appearance of the muscle obtained from the superficial and deep regions of the contused vastus lateralis suggested a pattern of damage and healing, various factors of which could be used to numerically describe the severity of the initial injury and phase of healing. These observations were incorporated into the MI, which is comprised of five features that included the macroscopic appearance of the tissue at the time of biopsy and appearance of the tissue when observed with the light microscope (Table 3). Included in features 2 and 5 were the thickness of the epimysium and the percentage change in FD. A score of 0 was considered normal, and the maximum abnormality (degree of injury or phase of healing) for each feature was scored 3, giving a maximum 'degree of injury' total of 15 (Table 3). The MI for the SD and JB biopsies of control and injured muscle is shown in Table 4. The MI was expressed as a percentage of maximum possible score, with 0% representing normality and 100% representing severe pathology or abnormality. It should be noted that the severity of the overall injury to each animal may depend on a number of factors. Whilst every precaution was taken to ensure the area of impact was the same in each animal, the position of the vastus lateralis in relation to point of impact and bone could result in minor differences in the 'crushing' effect of the blow. This could explain the variation in apparent severity of injury between individual JB and SD specimens within their particular groups.
The numeric scores based on MI for the 'severity' of pathology in samples from the SD and JB regions of the injured muscle are shown in Table 4. The mean scores for the SD biopsies of 6.9 ± 1.2 (95% CI: 4.9-8.7) and for the JB biopsies of 12.5 ± 2.6 (95% CI: 8.3-16.7) were significantly different (p < 0.05). Note that the mean relative 'severity' of pathology (expressed as a percentage of the maximum score of 15) was 83.3% in JB and 46% in SD specimens.
Discussion
The drop-mass technique has been used by others to create a controlled contusion injury. Fisher et al. (2003) dropped a solid aluminium bar weighing 700 g through a tube, from a height of 125 mm, onto the medial gastrocnemius muscle of rats. Markert et al. (2005) and McBrier et al. (2007) used the drop-mass technique on rats to determine the effects of therapeutic ultrasound. Deane, Gregory, Mars and Bester (2013) created a reproducible contusion injury in rabbit vastus lateralis by modifying the drop-mass method employed by Bunn et al. (2004). The final objective of the study by Deane, Gregory, Mars and Bester (2013) was to determine whether a treatment modality such as compressed air massage (CAM) influences muscle healing after a contusion injury. This further required the development of means of assessing muscle injury and repair. Biochemical analysis of blood for muscle injury markers has been used to indicate the state of healing following an injury (Armstrong, Ogilvie & Schwane 1983). In the current study, the light microscopic appearance of the injured tissue was used to both assess and quantify the degree of healing and/or muscle regeneration following a regulated contusion injury.
This modus operandi has been successfully employed in the past. Tovey, Husband and Yiu (1989) and Gregory et al. (1991) compared the effect of two drugs on the ulcerated duodenal mucosa by comparing mucosal abnormalities. Gregory (1994) extended this work by evaluating the morphological appearance of the mucosa surrounding healing and healed duodenal ulcers by creating a morphological index (MI) to quantify the 'quality' of mucosal healing after various types of pharmaceutical interventions. Mars and Gregory (1991) and Gregory and Mars (1992), in their studies to determine the effect of tourniquet-mediated ischaemic reperfusion injury on skeletal muscle, used morphometry to measure the FD of control and injured myofibres to determine a quantifiable, morphometric 'baseline' against which the effect of a particular pharmaceutical preparation could be compared (Gregory & Mars 1991).
Morphological indices are formulated about positive and negative criteria of the phenomenon to be studied (Gregory 1994). In formulating the MI for this study, five criteria were identified and scores given for the degree of injury or phase of healing. Negative phenomena were given high scores, maximum 3, and normal or near normal muscle were given low scores, minimum 0. In addition, where possible, cross-myofibre and epimysial measurements have been included to supplement subjective observations. The subsequent MI has been designed to provide a numerical description of the severity or phase of healing through the full thickness of rabbit vastus lateralis muscle 6 days after a contusion injury.
At biopsy, it was evident from simple macroscopic observation that the contusion had not caused an injury of uniform 'severity' through the full thickness of the muscle. Subjective examination of muscle morphology by Deane, Gregory and Mars (2013) showed that 6 days after injury, the muscle remained more severely traumatised in regions close to the bone than those just beneath the skin. The central portions of the muscle appeared essentially normal. In this study, the MI confirmed numerically that JB tissue 6 days after injury remained significantly more damaged or abnormal than SD muscle. The morphometric data, however, showed that the intermediate region was also abnormal, suggesting at least three levels of injury from a single contusion.
The vastus lateralis muscle in adult healthy rabbits is approximately 13 mm in thickness. The biopsies from both the SD and JB positions were approximately 5 mm in diameter. Each biopsy was delimited on its outer extremity by a portion of epimysium. This meant that the myofibres distant from the epimysium in both SD and JB biopsies were largely located near the centre of the muscle. Myofibres in this region appeared morphologically normal. However, on average the fibres ranged from marginally larger than those in control muscle to those that were up to 28% reduced in diameter. On average, mean FD from SD and JB biopsies were significantly smaller than their control counterparts, whilst those in JB biopsies were significantly smaller than those in SD samples. It is possible that reduction in FD is a consequence of an injury-mediated process of metabolic dysfunction resulting in myofibre atrophy. A simpler explanation is that such reduction is primarily a consequence of simple disuse atrophy.
Each rabbit had been traumatised as a consequence of the biopsy, and movement was probably impaired. The animal would, therefore, favour the uninjured limb for any movement, causing myofibres in that limb to be marginally enlarged as a consequence of exercise-mediated hypertrophy and myofibres in the injured limb to be reduced as a consequence of disuse atrophy. This could explain the disparity in FD between control and injured muscle. It is interesting to note, however, that the myofibres in the bundles closest to the more severely injured JB muscle, on average, are significantly smaller than those in SD muscle. As any movement of the limb would most probably require the use of the whole muscle, disuse atrophy on its own could not explain this discrepancy. It does suggest, therefore, that at least some reduction of fibre size was a consequence of an injury-mediated impairment of myofibre metabolism.
Based on the MI data, there appears to be a continuum of injury through the muscle, from bone to skin, which could be graded from 5 to 1. It is postulated that on impact, in a small muscle the size of the rabbit vastus lateralis, the tissue is essentially crushed against the bone. Here, capillaries are ruptured and myofibres are damaged. The loss of microcirculatory perfusion to the area causes the damaged myofibres in that region to die; the necrotic regions are invaded by neutrophils and the remnants of myofibres and disrupted connective tissue removed by scavenging phagocytes (level 5). Myofibres, connective tissue and capillaries are replaced from the pool of satellite cells, myoblasts and fibroblasts, some of which may emanate from the hyperplastic epimysium. On the periphery of this severely damaged region (level 4) are myofibres that, whilst damaged, are still supplied with a limited blood supply. These myofibres may be repaired and remodelled by satellite cell or sarcolemmal fusion (Gregory & Mars 2004). The central region of the muscle is essentially cushioned from the crush component of the contusion and the vascular bed remains largely intact. Some degree of fibre atrophy occurs as a consequence of the reasons described above with a more and less extreme response being exhibited in JB and SD biopsies respectively (levels 2 and 1). Near the surface, the immediate impact causes damage to the sub-dermal tissue, but far less than that near the bone. Some vascular damage may cause small areas of fibre necrosis, which in turn necessitates fibre replacement, but most injured myofibres are able to be repaired through normal processes (levels 4 and 3).
In summary, just beneath the impact zone there is a moderate injury (level 3 to 4) that rapidly diminishes to level 1. This in turn progresses to level 2, which then extends to level 4 and 5 in the immediate vicinity of the bone. This study was designed to create a controlled, measurable injury for a particular muscle in rabbits. Our preconception was that beneath the point of impact, the injury would be essentially uniform throughout the muscle. This is patently not the case. Our results show that if a contusion essentially 'crushes' the muscle between skin and bone, multiple levels of injury are extant, depending on the position of myofibre bundles within the muscle.
Conclusion
In order to deduce how physiotherapeutic modalities reduce pain and influence healing after injury it is necessary to create an injury against which a modality can be examined and compared. The drop-mass method for creating an experimental contusion is probably the simplest way to create an experimental injury. In rabbits, a single contusion causes injuries of different magnitudes at different positions within the muscle. This phenomenon was used to create an MI that enabled the relative severity of injury or muscle repair to be quantified. It is hoped that this MI will prove useful in assessing the severity of an injury and/or thereafter comparing the efficacy of different physiotherapeutic modalities when applied to injured muscle.
Acknowledgements
Gratitude is due to the University of KwaZulu-Natal for teaching relief and financial support; Mrs Linda Bester, senior technologist, and the staff of the Biomedical Resource Unit for monitoring the rabbits during and after recovery from anaesthetic; the staff of the Biomedical Resource Unit and Electron Microscope Unit for their technical assistance; and the late Professor R. Mpofu, from the Department of Physiotherapy of the University of Western Cape, for her useful comments on the manuscript.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this article.
Authors' contributions
M.N.D. (University of KwaZulu-Natal) was the project leader, was responsible for the experimental and project design, made conceptual contributions and wrote the manuscript. M.G. (University of KwaZulu-Natal) and M.M. (University of KwaZulu-Natal) made conceptual contributions to experimental processes and to the writing of the manuscript.
References
Armstrong, R.B., Ogilvie, R.W. & Schwane, J.A., 1983, 'Eccentric exercise-induced injury to rat skeletal muscle', Journal of Applied Physiology 54, 80-93. [ Links ]
Bunn, J.R., Canning, J., Burke, G., Mushipe, M., Marsh, D. & Li, G., 2004, 'Production of consistent crush lesions in murine quadriceps muscle - A biomechanical, histolomorphological and immunohistochemical study', Journal of Orthopaedic Research 22, 1336-1344. http://dx.doi.org/10.10167j.orthres.2004.03.013, PMid:15475218. [ Links ]
Deane, M., Gregory, M., Mars, M. & Bester, L., 2013, 'Creation of a contusion injury in rabbit skeletal muscle using a drop mass technique', Journal of the South African Veterinary Association 84(1), 1-6. http://dx.doi.org/10.4102/jsava.v84i1.957 [ Links ]
Deane, M., Gregory, M. & Mars, M., 2013, 'The effect of a contusion injury on rabbit skeletal muscle - A morphological study', Open Access Journal of Science and Technology 1(1), 1-8. http://dx.doi.org/10.11131/2013/100012 [ Links ]
Dubowitz, V., 1985, 'Normal muscle', in V. Dubowitz, C.A. Sewry & R.B. Fitzsimons (eds.), Muscle biopsy: A practical approach, pp. 82-128, Bailliere Tindall, London. [ Links ]
Fisher, B.D., Hiller, C.M. & Rennie, S.G.A., 2003, 'A comparison of continuous ultrasound and pulsed ultrasound on soft tissue injury markers in the rat', Journal of Physical Therapeutics Science 15, 65-70. http://dx.doi.org/10.1589/jpts.15.65 [ Links ]
Gregory, M., 1994, 'The value of morphological analysis in duodenal ulcer therapy', PhD thesis, Electron Microscope Unit, University of KwaZulu-Natal, South Africa. [ Links ]
Gregory, M. & Mars, M., 1991, 'Pharmacological manipulation of the ischaemic reperfusion injury: A histological, morphometric study', 29th Congress of the South African Microscopy Society, Cape Town, December, Communications of the Electron Microscopy Society of South Africa 21, 217-218. [ Links ]
Gregory, M.A. & Mars, M., 1992, 'Serial morphological changes in primate skeletal myofibres following 3 hours of ischaemia and 24 hours of reperfusion', South African Medical Journal 81, 473-478. [ Links ]
Gregory, M. & Mars, M., 2003, 'The effect of compressed air massage on the morphology of untraumatised rabbit skeletal muscle', Microscope Microanalysis 9(Suppl. 2), 1440-1441. [ Links ]
Gregory, M. & Mars, M., 2004, 'Mobilisation of satellite cells following ischaemia and reperfusion in primate skeletal muscle', South African Journal of Sports Medicine 16(1), 17-24. [ Links ]
Gregory, M. & Mars, M., 2005, 'Compressed air massage causes capillary dilation in untraumatised skeletal muscle: A morphometric and ultrastructural study', Physiotherapy 91(3), 131-137. http://dx.doi.org/10.10167j.physio.2004.11.007 [ Links ]
Gregory, M.A., Pettengell, K.E., Spitaels, J.M. & Simjee, A.E., 1991, 'Alterations in the cytological composition of the juxta-scar villous mucosa following curative therapy with sucralfate or cimetidine', South African Medical Journal 80, 450-453. [ Links ]
James, P.B., Scott, B. & Allen, M.W., 1993, 'Hyperbaric oxygen therapy in sports injuries: A preliminary study', Physiotherapy 79, 571-572. http://dx.doi.org/10.1016/S0031-9406(10)60302-1 [ Links ]
Markert, C.D., Merick, M.A., Kirby, T.E. & Devor, S.T., 2005, 'Nonthermal ultrasound and exercise in skeletal muscle regeneration', Archives of Physical Medicine and Rehabilitation 86, 1304-1310. http://dx.doi.org/10.1016Zj.apmr.2004.12.037, PMid: 16003655. [ Links ]
Mars, M. & Gregory, M.A., 1991, 'A histometric analysis of skeletal myofibres following 90 minutes of ischaemia and 3 hours of reperfusion', Journal of Surgical Research 50, 191-195. http://dx.doi.org/10.1016/0022-4804(91)90246-I [ Links ]
McBrier, N.M., Lekan, J.M., Druhan, L.J., Devor, S.T. & Merrick, M.A., 2007, 'Therapeutic ultrasound decreases mechano-growth factor messenger ribonucleic acid expression after muscle contusion injury', Archives of Physical Medicine and Rehabilitation 88(7), 936-940. http://dx.doi.org/10.1016/j~.apmr.2007.04.005 [ Links ]
Potera, C., 1995, 'Healing under pressure', Physician and Sports Medicine 25(11), 46-47. [ Links ]
Staples, J.R., 1996, 'Effects of intermittent hyperbaric oxygen on pain perception and eccentric strength in a human injury model', PhD thesis, Department of Kinesiology, University of British Columbia, Canada. [ Links ]
Tovey, F.I., Husband, E.M. & Yiu, Y.C., 1989, 'Comparison of relapse rates and mucosal abnormalities after healing of duodenal ulceration and after 1 year's maintenance with cimetidine or sucralfate: A light and electron microscopic study', Gut 30, 586-593. http://dx.doi.org/10.1136/gut.30.5.586 [ Links ]
 Correspondence:
Correspondence:
Margaret Deane
Private Bag X54001
Durban 4000
South Africa
Email: rhodem@ukzn.ac.za
Received: 05 Apr. 2013
Accepted: 29 Mar. 2014
Published: 26 Aug. 2016














