Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.85 n.1 Pretoria Jan. 2014
ORIGINAL RESEARCH
Changes in motility, morphology, plasma membrane and acrosome integrity during stages of cryopreservation of buck sperm
Mushtaq Ahmad; Rashad Nasrullah; Hasan Riaz; Abdul Sattar; Nasim Ahmad
Department of Theriogenology, University of Veterinary and Animal Sciences, Pakistan
ABSTRACT
Changes in sperm structure and function occur during the processing of semen. The present study was designed to investigate the effect on buck sperm during different stages of semen preparation including dilution, cooling, equilibration and freeze-thawing. Semen ejaculates from three mature bucks (replicates = 5) were diluted with tris-citric acid egg yolk glycerol extender at 37 °C, cooled to 4 °C over 90 min, equilibrated at 4 °C for 2 h, transferred to 0.5 mL straws, placed in nitrogen vapour, frozen and thawed and then analysed. Sperm samples were assessed for percentage motility, acrosomal and plasma membrane integrity, live sperm, and morphology after dilution, cooling, equilibration and thawing. Mean percentage motility after dilution (86.0 ± 1.4%) was reduced significantly (p < 0.05) due to cooling and equilibration (77.6 ± 1.3% and 74.6 ± 1.4% respectively); furthermore, it decreased significantly (p < 0.05) after freezing and thawing (42.3 ± 2.5%). Mean percentage of live sperm was higher (p < 0.05) after dilution (89.3 ± 1.4%) compared with cooling (84.8 ± 1.8%) and equilibration (80.2 ± 2.5%) and further reduced (p < 0.05) after freezing and thawing (56.0 ± 3.4%). Sperm morphology dropped significantly (p < 0.05) from 96.4 ± 0.3% after dilution to 88.8 ± 1.3% at cooling and further decreased (p < 0.05) after freezing and thawing (81 ± 1.9%). Mean percentage of sperm with normal plasma membrane after dilution (82.2 ± 1.1%) was significantly reduced (p < 0.05) at cooling or equilibration (73.8 ± 1.8) and further decreased (p < 0.05) after freezing and thawing (50.1 ± 2.9%). The percentage of sperm with normal acrosomes did not differ significantly due to dilution, cooling or equilibration (85.8 ± 1.7%, 83.2 ± 1.6%, 81.7 ± 1.8%) but was significantly reduced after freezing and thawing (45.2 ± 2.8%). In conclusion, frozen thawed sperm showed maximum damage to motility, morphology, plasma membrane and acrosome integrity following cooling.
Introduction
Globally, about 90% of goats are found in Asian countries, including China, India, Pakistan and Bangladesh (Iqbal et al. 2008). In tropical regions, goats are kept to provide milk and meat. Goats are embedded in the culture and are socially accepted to alleviate poverty, particularly in developing countries. Against this background, research has been undertaken on reproductive biotechnology including artificial insemination using fresh or frozen semen.
Cryopreservation facilitates the supply of genetic material for artificial insemination (AI) in goats (Leboeuf, Restall & Salamon 2000). The greatest obstacle to the exploitation of frozen semen is that the freeze-thawing process of mammalian sperm generally leads to a decrease in motility and viability of sperm cells as a result of damage to membrane integrity and ultrastructure (Watson 2000). Frozen thawed sperm are subjected to chemical, osmotic, thermal, and mechanical trauma that occurs during dilution, cooling, equilibration and thawing. It has been demonstrated that cryopreservation leads to a decrease in sperm motility in the goat (Dorado et al. 2009). However, the extent of damage at consecutive stages of cryopreservation of buck sperm has not been determined. Some of the key semen assays related to functional significance and fertility include hypo-osmotic swelling as a measure of plasma membrane integrity (Jeyendran et al. 1984), the presence of a normal acrosomal ridge (Gillan, Evans & Maxwell 2005), and sperm morphology, which has been considered as a robust clinical test (Blom 1973).
The objective of the present study was to assess the damage to sperm motility, plasma membrane integrity (PMI), and the normal apical ridge (NAR) of the acrosome, live-dead and morphology of buck sperm after dilution, cooling to 4 °C, equilibration at 4 °C and thawing after freezing.
Materials and methods
Semen collection
Semen was collected from three mature Beetal bucks maintained under optimal conditions of feeding and management in the animal shed of the Department of Theriogenology, University of Veterinary and Animal Sciences, UVAS, Pakistan during the months of October to December, 2011. Semen collection (replicates = 5) was done twice a week using an artificial vagina maintained at 42 °C. Each ejaculate was transferred to the evaluation room within 5 minutes, kept in a water bath at 37 °C and evaluated for sperm motility and concentration. The ejaculates possessing > 65% percentage motility and > 1.5 billion sperm/mL were used for further processing.
Semen processing and evaluation
Semen was diluted with tris-citric acid egg yolk glycerol extender (TCEYG, pH 6.8) comprised of tris(hydroxymethyl) aminomethane (3.93% w/v, Fluka, Buchs, Switzerland), citric acid (1.70% w/v, Fluka), egg yolk (20.00% v/v), fructose (0.20% w/v, Merck, Darmstadt, Germany), glycerol (8.00% v/v, Merck), and antibiotics (benzyl penicillin 1000 i.u./mL and streptomycin sulphate (100 µg/mL, Sigma) (Rasul et al. 2000) at 37 °C to a final concentration of 100 million sperm/ mL. Diluted semen was cooled to 4 °C in 90 minutes (cabinet, Mini tub, France), equilibrated for 2 h and packaged into 0.5 mL French straws. Semen straws were placed in nitrogen vapour for 7 min (4 cm above the liquid level in a closed container), then plunged into and stored in liquid nitrogen until analysed after thawing at 37 °C for 30 s.
Each individual semen sample (3 bucks x 5 replicates = 15 samples) was evaluated for percentage motility, plasma membrane integrity (PMI), normal apical ridge (NAR), live or dead, and morphology at each stage of cryopreservation, that is, after dilution (AD), after cooling (AC), after equilibration (AE) and after freezing and thawing (AT).
Semen assays
Percentage motility
Semen (10 µl) was placed on a pre-warmed (37 °C) glass slide, covered with a cover slip (22 mm x 22 mm) and placed on the pre-warmed stage of a phase contract microscope (Olympus BX51). The percentage of motile sperm was assessed subjectively by viewing 5-6 fields per slide with the aid of closed-circuit television attached to the microscope (200 x). Sperm possessing linear progressive movement were considered to be motile.
Plasma membrane integrity
Plasma membrane integrity (PMI) of sperm was assessed using the hypo-osmotic swelling (HOS) test (Jeyendran et al. 1984). Fifty µL of each semen sample was mixed with 500 µL of HOS solution (190 mL Osmol/kg) and incubated at 37 °C for 30 min. After incubation, a 5 µL semen sample drop was examined under a phase-contrast microscope (400 x). A minimum of 100 sperm were counted for their swelling ability in HOS solution. The sperm characterised by coiling or swelling of the tail of varying degrees were considered to have an intact plasma membrane.
Normal apical ridge (NAR)
A 500 µL aliquot of each semen sample was fixed in 50 µL of a 1 % formal citrate solution. One hundred sperm were counted with a phase contrast microscope (1000 x) for normal apical ridge (NAR). Presence of crescent shaped acrosomes was considered normal.
Morphology and live-dead
A 10 µL aliquot of each semen sample was mixed with eosin-nigrosin stain (Ahmad et al. 2011). A smear was made and dried and 100 sperm were counted and examined for live-dead and morphology under a phase contrast microscope (400 x). Sperm without penetration of stain were considered as live.
Preparation of chemicals
A hypo-osmotic solution of 190 mL Osmol/kg was prepared by dissolving Tri-sodium citrate dehydrate (Merck, Germany) and D (-) fructose (Sigma, USA) in 100 mL of de-ionised distilled water (Rasul et al. 2000). Formal citrate solution was prepared by dissolving 2.9% (w/v) tri-sodium citrate dehydrate (Merck, Germany) with 1.0% v/v commercial formaldehyde (37.0%, Merck, Germany) (Cabrera et al. 2005).
Statistical analysis
Data were presented as mean ± SEM and analysed using one-way ANOVA to assess differences amongst stages of cryopreservation, that is, AD, AC, AE and AT for motility, PMI, NAR, live sperm and morphology. Normality of data was determined through the Shapiro-Wilk test. A probability level of < 0.05 was considered to be significant. The least significant difference (LSD) test was used for multiple comparisons. All analysis was performed using statistical software SPSS (Version 13).
Results
Changes in motility, plasma membrane integrity, normal acrosome, live sperm and morphology at consecutive stages of cryopreservation of buck semen are presented below (Figure 1).
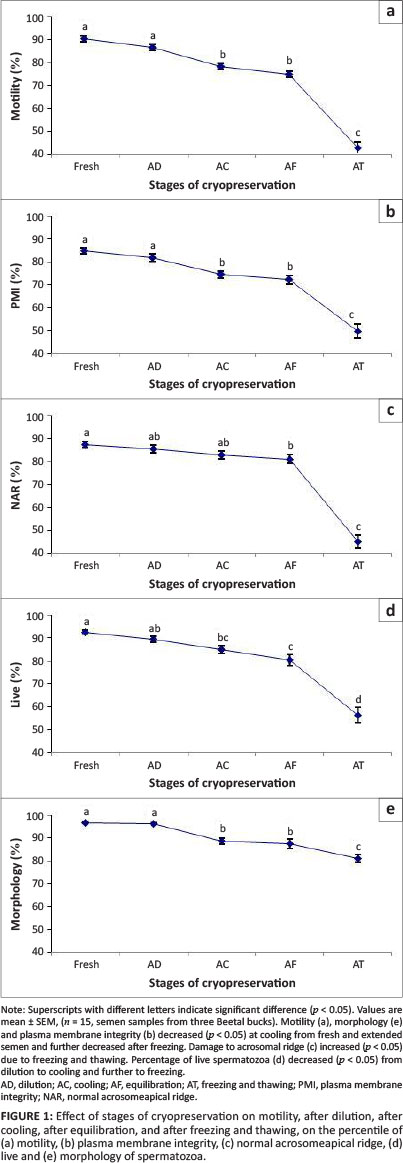
Percentage motility
Fresh semen possessed 89.8 ± 1.26% progressive motility. This declined to 86 ± 4.2% after dilution, which was higher (p < 0.05) than at the stages of cooling (77.6 ± 3.9) and equilibration (74.6 ± 4.2). However, mean motility of sperm after freezing and thawing (42.3 ± 7.5) significantly declined (p < 0.05) compared with all other stages of cryopreservation.
Plasma membrane integrity
Fresh semen contained 85.3 ± 0.92% sperm with an intact plasma membrane. The mean percentage of swollen sperm after dilution (82.2 ± 3.3) differed significantly at cooling (75.0 ± 5.1) and after equilibration (72.6 ± 5.7). However, the mean percentage of swollen sperm after freezing and thawing (50.1 ± 8.7) declined (p < 0.05) significantly compared with other stages of cryopreservation.
Normal acrosome
Sperm with normal acrosomes were 87.7 ± 1.3% in fresh semen. The percentage of sperm with normal acrosomes did not differ significantly as a result of dilution, cooling or equilibration (85.8 ± 5.4%, 83.2 ± 4.8%, 81.7 ± 5.4 respectively) but decreased significantly (p < 0.05) after freezing and thawing (45.2 ± 8.4%).
Live dead
Fresh semen had 92.6 ± 0.68% live sperm that did not take up the stain.The mean percentage of live sperm after dilution (89.3 ± 4.2) differed significantly (p < 0.05) from that after cooling (84.8 ± 5.4) and equilibration (80.2 ± 7.5). However, it declined further (p < 0.05) after freezing and thawing (56.0 ± 10.5).
Morphology
Fresh semen possessed 96.8 ± 0.36% morphologically normal sperm. This was similar at dilution (96.4 ± 5.2), but was significantly (p < 0.05) lower after cooling (88.8 ± 5.7) and equilibration (87.6 ± 7.5). However, morphology declined significantly (p < 0.05) compared with all other stages of cryopreservation after freezing and thawing (81.0 ± 5.7).
Discussion
To the best of the authors' knowledge, this study reports for the first time in detail the changes in the motility of caprine semen at successive stages of cryopreservation that included dilution, cooling, equilibration and freezing or thawing. In this study, the percentage motility was significantly reduced by up to 42% after freezing and thawing. Similar findings for a decline in motility due to freezing and thawing compared with fresh semen have been reported in Boer (Tuli & Holtz 1994) and Florida goats (Dorado, Munoz-Serrano & Hidalgo 2010). There maybe two reasons for the reduction in sperm motility: firstly, biophysical injuries as a result of formation of ice crystals in the extra- and intracellular environment and increasing solute concentration (Mazur 1984), and secondly, biochemical oxidative stress resulting in irreversible damage to sperm structure, changes in membrane fluidity and enzymatic activity (Aitken, Clarkson & Fishel 1989). More than 50% of mammalian sperm are usually injured by the cryopreservation process (Watson 2000). Similarly, the motility pattern decreased significantly after freezing and thawing in bull (Budworth, Amann & Chapman 1988) and buffalo (Bubalis bubalis) sperm (Rasul et al. 2000). In order to minimise the cryodamage in goats, altering the freezing rate or addition of membrane stabilisers might be potential areas for future investigation.
Plasma membrane integrity is of prime importance for the freezing and fertility of the sperm cells. The hypo-osmotic swelling assay has been described as a useful test for assessing functional integrity of the plasma membrane in humans (Jeyendran et al. 1984). In the existing study, the plasma membrane integrity of the sperm was lowered by equilibration and declined further after freezing and thawing, as has previously been reported in goat semen (Azerédo, Esperb & Resendec 2001). A similar decrease in the membrane integrity of sperm after freezing and thawing has been reported in bulls (Correa & Zavos 1994), boars (Vazquez et al. 1997) and stallions (Neild et al. 1999). Biochemically, these finding are supported by the fact that the lipid components of the plasma membrane of buck semen are significantly reduced after freezing (Holt & North 1984).
The significance of an acrosomal cap is known to be a prerequisite for successful fertilisation. In the current study, the percentage of sperm with a normal acrosome did not change due to the other stages of cryopreservation, but it was reduced by up to 50% after freezing and thawing. Earlier researchers suggested that cryopreservation induces reduction in acrosome integrity of frozen semen samples of goats (Aboagla & Terada 2004). Acrosome membrane-intact sperm ranged between 40% and 70% in Canary (Cabrera et al. 2005) and Florida bucks (Dorado, Rodriguez & Hidalgo 2007). It is presumed that loss of the acrosomal cap during freezing and thawing of buck sperm is similar to that demonstrated in bull sperm (Bamba & Cran 1988). Greater release of the acrosomal enzyme hyaluronidase was noticed after freezing and thawing in the sperm of buffalo bulls (Akhtar & Chaudhry 1989). Therefore, it would be meaningful to study the relationship between fertility and acrosome damage due to freezing and thawing in goat semen.
Conclusion
In conclusion, the cooling of buck sperm from 37 °C to 4 °C has a negative effect on motility. However, equilibration of buck sperm at 4 °C for two hours seems to have very little effect on semen characteristics. Freezing and thawing cause considerable damage to motility, plasma membrane integrity, acrosomal cap, live-dead ratio and morphology of the sperm. Further studies should focus on determining the ultra-biochemical changes at different stages of cryopreservation to enhance the understanding of their more detailed effects on buck sperm.
Acknowledgement
The authors are thankful to Mr. Ali Raza for the help in semen collection and processing.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this article.
Authors' contributions
M.A. (University of Veterinary and Animal Sciences) and R.N. (University of Veterinary and Animal Sciences) performed most of the experiments. A.S. (University of Veterinary and Animal Sciences) and N.A. (University of Veterinary and Animal Sciences) provided the conceptual design for the experiments. H.R. (University of Veterinary and Animal Sciences) and M.A. were involved in writing this manuscript.
References
Aboagla, E.M. & Terada, T., 2004, 'Effects of egg yolk during the freezing step of cryopreservation on the viability of goat spermatozoa', Theriogenology 62, 11601172. http://dx.doi.org/10.1016/j.theriogenology.2004.01.013 [ Links ]
Ahmad, E., Ahmad, N., Naseer, Z., Aleem, M., Khan, M.S., Ashiq, M. & Younis, M., 2011, 'Relationship of age to body weight, scrotal circumference, testicular ultrasonograms, and semen quality in Sahiwal bulls', Tropical Animal Health and Production 43, 159-164. http://dx.doi.org/10.1007/s11250-010-9668-1 [ Links ]
Aitken, R.J., Clarkson, J.S. & Fishel, S., 1989, 'Generation of reactive oxygen species, lipid peroxidation, and human sperm function', Biology of Reproduction 41, 183197. http://dx.doi.org/10.1095/biolreprod41.L183 [ Links ]
Azerédo, G., Esperb, C. & Resendec, K., 2001, 'Evaluation of plasma membrane integrity of frozen-thawed goat spermatozoa with or without seminal plasma', Small Ruminant Research 41, 257-263. http://dx.doi.org/10.1016/S0921-4488(01)00189-4 [ Links ]
Bamba, K. & Cran, D.G., 1988, 'Effect of rapid warming of bull and rabbit semen', Journal of Reproduction and Fertility 82, 501-507. http://dx.doi.org/10.1530/jrf.0.0820501 [ Links ]
Blom, E., 1973, 'The ultrastructure of some characteristic sperm defects and a proposal for a new classification of bull spermiogram', Nordisk Veterinaer Medicin 25, 383-391. [ Links ]
Budworth, P., Amann, R. & Chapman, P., 1988, 'Relationships between computerized measurements of motion of frozen-thawed bull spermatozoa and fertility', Journal of Andrology 9, 41-54. [ Links ]
Cabrera, F., Gonzalez, F., Batista, M., Calero, P., Medrano, A. & Gracia, A., 2005, 'The effect of removal of seminal plasma, egg yolk level and season on sperm freezability of canary buck (Capra hircus)', Reproduction in Domestic Animals 40, 191-195. http://dx.doi.org/10.1111/j.1439-0531.2005.00544.x [ Links ]
Correa, J.R. & Zavos, P.M., 1994, 'The hypoosmotic swelling test: its employment as an assay to evaluate the functional integrity of the frozen-thawed bovine sperm membrane', Theriogenology 42, 351-360. http://dx.doi.org/10.1016/0093-691X(94)90280-1 [ Links ]
Dorado, J., Hidalgo, M., Munoz, A. & Rodriguez, I., 2009, 'Assessment of goat semen freezability according to the spermatozoa characteristics from fresh and frozen samples', Animal Reproduction Science 112, 150-157. http://dx.doi.org/10.1016/j. anireprosci.2008.04.005 [ Links ]
Dorado, J., Munoz-Serrano, A. & Hidalgo, M., 2010, 'The effect of cryopreservation on goat semen characteristics related to sperm freezability', Animal Reproduction Science 121, 115-123. http://dx.doi.org/10.1016/j.anireprosci.2010.04.182 [ Links ]
Dorado, J., Rodriguez, I. & Hidalgo, M., 2007, 'Cryopreservation of goat spermatozoa: comparison of two freezing extenders based on post-thaw sperm quality and fertility rates after artificial insemination', Theriogenology 68, 168-177. http:// dx.doi.org/10.1016/j.theriogenology.2007.04.048 [ Links ]
Gillan, L., Evans, G. & Maxwell, W.M., 2005, 'Flow cytometric evaluation of sperm parameters in relation to fertility potential', Theriogenology 63, 445-457. http:// dx.doi.org/10.1016/j.theriogenology.2004.09.024 [ Links ]
Holt, W.V. & North, R.D., 1984, 'Partially irreversible cold-induced lipid phase transitions in mammalian sperm plasma membrane domains: Freeze-fracture study', Journal of Experimental Zoology 230, 473-483. http://dx.doi.org/10.1002/jez.1402300316 [ Links ]
Iqbal, A., Khan, B.B., Tariq, M. & Mirza, M., 2008, 'Goat - A potential dairy animal: Present and future prospects', Pakistan Journal of Agricultural Sciences 45, 227-230. [ Links ]
Jeyendran, R.S., Van der Ven, H.H., Perez-Pelaez, M., Crabo, B.G. & Zaneveld, L.J., 1984, 'Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics', Journal of Reproduction and Fertility 70, 219-228. http://dx.doi.org/10.1530/jrf.0.0700219 [ Links ]
Leboeuf, B., Restall, B. & Salamon, S., 2000, 'Production and storage of goat semen for artificial insemination', Animal Reproduction Science 62, 113-141. http://dx.doi.org/10.1016/S0378-4320(00)00156-1 [ Links ]
Mazur, P., 1984, 'Freezing of living cells: Mechanisms and implications', American Journal of Physiology 247, 125-142. [ Links ]
Neild, D., Chaves, G., Flores, M., Mora, N., Beconi, M. & Aguero, A., 1999, 'Hypoosmotic test in equine spermatozoa', Theriogenology 51, 721-727. http://dx.doi.org/ 10.1016/S0093-691X(99)00021-7 [ Links ]
Rasul, Z., Anzar, M., Jalali, S. & Ahmad, N., 2000, 'Effect of buffering systems on post-thaw motion characteristics, plasma membrane integrity, and acrosome morphology of buffalo spermatozoa', Animal Reproduction Science 59, 31-41. http://dx.doi.org/10.1016/S0378-4320(00)00070-1 [ Links ]
Tuli, R.K. & Holtz, W., 1994, 'Effect of glycerolization procedure and removal of seminal plasma on post-thaw survival and got-release from Boer goat spermatozoa', Theriogenology 42, 547-555. http://dx.doi.org/10.1016/0093-691X(94)90692-C [ Links ]
Vazquez, J.M., Martinez, E.A., Martinez, P., Garcia-Artiga, C. & Roca, J., 1997, 'Hypoosmotic swelling of boar spermatozoa compared to other methods for analysing the sperm membrane', Theriogenology 47, 913-922. http://dx.doi.org/10.1016/S0093-691X(97)00046-0 [ Links ]
Watson, P.F., 2000, 'The causes of reduced fertility with cryopreserved semen', Animal Reproduction Science 60-61, 481-492. http://dx.doi.org/10.1016/S0378-4320(00)00099-3 [ Links ]
 Correspondence:
Correspondence:
Nasim Ahmad
Department of Theriogenology
Faculty of Veterinary Science
University of Veterinary and Animal Sciences
Outfall Road, Lahore 54000, Pakistan
Email: drnasim@yahoo.com
Received: 20 Dec. 2012
Accepted: 26 July 2013
Published: 28 Feb. 2014














