Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.85 n.1 Pretoria Jan. 2014
ORIGINAL RESEARCH
Internal parasites and health management of pigs in Burayu District, Oromia Regional State, Ethiopia
Bersissa KumsaI, II; Elias KifleI
IDepartment of Parasitology, Addis Ababa University, Ethiopia
IIFaculty of Medicine, Aix Marseille Universite, France
ABSTRACT
The study determined the prevalence and major types of gastrointestinal parasites in pigs and assessed the health management practices on farms in Burayu District in West Shoa Zone of Oromia Regional State, Ethiopia. The study was performed from November 2007 to April 2008 using standard coprological examination and a well-organised questionnaire survey. Of the 272 pigs examined for the presence of gastrointestinal parasites, 36 (13.2%) were infected with one or more types of parasite. Neither age nor management system proved to be a statistically significant factor in the prevalence of parasites. The highest prevalence of parasites was recorded in December, January and April, whereas the lowest was observed in February. Significant variation in the prevalence of parasites was noticed amongst study months. The majority of farmers did not use acaricides to treat and control external parasites. Anthelmintics were not used by any of the farmers. Some 76.1% of the farmers never used any type of treatment for sick pigs; 21.7% of the farmers used modern treatment and 2.2% of the farmers used traditional medicines. More than 95.0% of pigs were kept on soil floors and only 10.9% of the housing systems had good ventilation. Dung was removed at least every three days, with the majority of farmers (91.2%) removing it every morning. This study provided evidence for the occurrence of internal parasites in pigs kept in Burayu District in Oromia. Further epidemiological studies are needed to determine the zoonotic and economic importance of pig parasites in other parts of Ethiopia.
Introduction
In domestic pigs, internal parasites are particularly important in tropical and subtropical countries, where nutrition and sanitation are generally poor (Eijck & Borgsteede 2005). Infection by parasites negatively affects productivity of pigs and leads to poor growth rates, decreased litter size, reduced weight gain, poor feed utilisation and conversion, reduced fertility, condemnation of affected organs, high treatment costs and mortality (Kagira et al. 2012). In domestic animals, chronic subclinical parasitic diseases are much more common and are responsible for greater economic losses than acute diseases and many other lethal infectious diseases (Kumsa, Tolera & Nurfeta 2010).
The prevalence, worm burden and species composition of helminths largely depend on the agroecology and type of swine production systems. For instance, in highly intensive production systems, the infection levels are usually low and involve only a few species. In contrast, in most traditional systems, the poor hygienic and nutritional conditions favour a higher prevalence, burden and rate of helminth transmission, similar to the situation in extensive outdoor management systems without anthelmintic interventions (Ng'ang'a, Karanja & Mutune 2008; Tamboura et al. 2006). The roaming of pigs favours the uptake of infective stages of parasites, making the pigs particularly susceptible to infection with internal parasites. Moreover, the warm and humid conditions of the tropics and infrequent treatment against parasitic diseases are conducive to a high worm burden (Marufu et al. 2008).
Efficient and profitable pig production depends on an understanding of the concepts of genetics, environment, herd health, management and nutrition. These factors interact with one another and the net output determines the level of production and profitability (Nansen & Roepstorff 1999). Information on prevalence, types of parasite and management practices helps to formulate pig development and extension programmes. In addition, knowledge about parasite species can be used as baseline data to design effective parasite control measures. In Ethiopia, no previous study has been carried out to determine the prevalence and species composition of parasites in pigs kept under different management systems. Therefore, the objectives of the current study were to identify the major types of internal parasite of pigs in the study area and assess the health management practices on pig farms in Burayu District in Oromia Regional State.
Materials and methods
Study area assessed
The study was undertaken in Burayu District, which is located in West Shoa Zone of Oromia Regional State, from November 2007 to April 2008. The area is geographically situated at 9°3'N and 38°30'E, at an altitude of 2400 m a.s.l. The annual average rainfall is 1060 mm, of which 70% falls during the long rainy season (June to September), whilst about 30% falls during the short rainy season (February to April). The mean minimum and maximum temperatures are 4.0 °C and 23.3 °C, respectively. The mean relative humidity and rainfall are 50.4% and 43.4 mm, respectively (Ministry of Agriculture 2004).
Study animals
Pigs owned by smallholder farmers in Burayu District were examined for intestinal parasites. The health management practices of pigs in the area. The study area had an estimated pig population of 7000 (Ministry of Agriculture 2004).
Study design
Sampling procedure
A simple random sampling procedure was used to select animals for participation. A total of 46 smallholder pig farms, with a total of 5000 pigs, were randomly selected from the list of all pig farms in the area to assess health management practices. The sample size for the study was determined as described by Thrusfield (2005). Accordingly, a 92.7% expected prevalence of nematode infection was used from findings in neighbouring Kenya (Kagira et al. 2012) and an acceptable error rate and confidence level were set at 5.0% and 95.0%, respectively, to determine the sample size. Based on this and the pig population of the district, a total of 272 pigs of different ages and both sexes were examined for internal parasites on smallholder pig farms in the study area.
Questionnaire survey
Data on farm size, production system, frequency of dung removal, deworming and acaricide application, feeding and watering, any treatment of sick animals and housing system were obtained by means of a well-organised questionnaire survey. The questionnaires were completed during visits to farms registered by the district and directly interviewing the farmers to avoid any misunderstanding.
Faecal sample collection and examination
All faecal samples were taken directly from the rectum, transferred to pre-labelled containers and transported to the parasitology laboratory of the Faculty of Veterinary Medicine, Addis Ababa University (based at Bishoftu) and, if possible, examined within four hours of collection. Samples not examined within four hours were stored at 4 °C. Age of the animal, farm type and dates were recorded for each animal during sample collection. The faecal samples were processed by direct flotation and sedimentation techniques and examined under a light microscope for the presence of parasite ova. Zinc sulphate (ZnSO4) was used as flotation fluid. Procedures described elsewhere (Taylor, Coop & Wall 2007) were used to prepare the flotation solution. Eggs or parasites were identified using key morphological features as described by Taylor et al. (2007).
Data analysis
Data were coded and stored in Microsoft Excel spreadsheets. Descriptive analyses such as frequency and percentage as well as chi-square tests were computed using a statistical package (SPSS for Windows, 15). Significance was set at p < 0.05.
Results
Prevalence of parasites
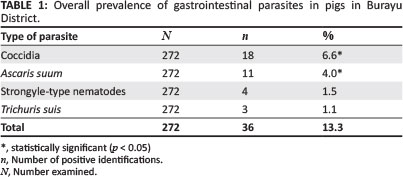
Of the total 272 pigs examined, 36 (13.2%) were positive for one or more species of ova of internal parasites. Four types of parasite were identified, namely oocysts of coccidia, eggs of Ascaris suum, strongyle-type nematodes and Trichuris suis (Table 1). Coccidia oocysts showed the highest overall prevalence, followed by A. suum, and the lowest prevalence was recorded for T. suis (Table 1). The prevalence of coccidia was significantly higher (p < 0.05) than the prevalence of T. suis and strongyle-type nematodes. Statistically significant variation was not observed in the prevalence of parasites amongst pigs of different age groups (p > 0.05) and pigs kept on different farm types. Prevalence of parasites in young and adult pigs, as well as in pigs kept by different farm type is presented in Table 2 and Table 3, respectively. The highest prevalence of parasites in pigs was recorded in December, followed by January and April, whilst the lowest was recorded in February (Table 4). Statistically significant (p < 0.05) variation in the prevalence of parasites was observed amongst study months.
Management practices on pig farms
Farm characteristics
During the study period, 46 farms were visited to collect data on production and parasite control practices. The study indicated that there were 37 rearing farms, two fattening farms and seven rearing and fattening farms in the area. Between 1 and 32 pigs were kept on rearing farms, between 33 and 42 pigs were kept on fattening farms and between 42 and 241 pigs were kept on rearing-and-fattening farms.
Health management on farms
The questionnaire survey revealed that in addition to roaming in the vicinity of their houses during the rainy season, when green pastures are abundant, pigs on all the farms were provided with supplementary feeds. Pigs were provided with feed and water between one and three times a day, as allowed for by the financial status of farmers. Wheat bran was the most commonly provided supplementary feed because it was cheaper than other feed types.
The questionnaire survey also showed that 39.0% of the farmers used acaricides for treatment of ectoparasites; however, 61.0% of the respondents had never used any acaricides. All the farmers interviewed stated that they had never used any anthelmintics against gastrointestinal parasites. A total of 76.1% of the farmers did not use any treatment for sick pigs, but 21.7% responded that sick pigs received modern treatment in the nearby veterinary clinics, with oxytetracycline and ivermectin being the most commonly used treatment. Some 2.2% of the farmers used traditional treatments for sick animals.
Furthermore, the questionnaire survey revealed that the majority of the housing systems (87.0%) were poorly ventilated; only 10.9% of the houses had good ventilation. It was noted that 95.7% of the pigs were kept on soil floors whereas the rest (4.3%) were kept on concrete floors. In the study area, 91.2% of the farmers removed the dung every morning whilst the rest (8.7%) removed the dung every two or three days.
Discussion
Since no previous information on the prevalence and significance of parasites in pigs in Ethiopia is available, this study provided the first evidence for the occurrence of parasites in pigs in Burayu District in Oromia. The overall prevalence recorded (13.2%) is lower than the prevalence of parasites in pigs reported in Tanzania (Esrony et al. 1997), Zimbabwe (Marufu et al. 2008), Burkina Faso (Tamboura et al. 2006) and Kenya (Kagira et al. 2012). This difference can probably be attributed to differences in management systems, age of pigs studied, agroecology and the season when the studies were performed. This study was conducted during the dry season of the year, when parasites are at their lowest level. The low stocking rates, due to pig farming only recently having been introduced in Ethiopia, could be another possible explanation.
In contrast, the types of parasite recorded in the current study are similar to those reported by previous workers (Nansen & Roepstorff 1999). In our study, the prevalence of coccidia was higher in pigs kept on rearing farms and rearing-and-fattening farms than pigs kept on the fattening farms. This could probably be attributed to the relatively higher stocking rates on rearing and rearing-and-fattening farms than on fattening farms. This observation is in line with the earlier work of Lisbeth et al. (2001) and Eijck and Borgsteede (2005), who reported higher prevalence of coccidia in pigs on farms with high stocking rates.
The low overall prevalence recorded for A. suum is in agreement with a previous report by Obonyo et al. (2012) in Kenya, who suggested that the large number of exposed pigs without patent infection may lead to a considerable underestimation of the true prevalence in pigs. Furthermore, studies on the immunological response of pigs to infection with A. suum showed that it stimulates the development of strong protective immunity, depending on the extent and duration of exposure (Nansen & Roepstorff 1999).
The observation of low overall prevalence of strongyle-type nematodes noted in the present study could be explained by the fact that low numbers of eggs are excreted during the dry months (Christensen, Barnes & Nansen 1997; Pattison, Smith & Thomas 1998), owing to reduced parasite load and decline in fecundity of female worms. The use of ivermectin for the treatment of ectoparasites might have reduced the prevalence of helminths in pigs in the study area. The finding of significantly higher overall prevalence of parasites (p < 0.05) in some months of the study period can be attributed to the variations in temperature and humidity between months, some of which may favour the development and survival of different stages of parasites of pigs, as has been suggested previously (Obonyo et al. 2012; Tamboura et al. 2006). The significantly lower overall prevalence of T. suis than the other internal parasites in the current study agrees with the low prevalence of T. suis previously reported in pigs in Kenya (Ng'ang'a, Karanja & Mutune 2008). The observation of higher overall prevalence of A. suum, T. suis and strongyle-type nematodes in young pigs in the present study agrees with the previous reports by Esrony et al. (1997) and Obonyo et al. (2012), which is probably a manifestation of the lower immunity in young pigs (Nansen & Roepstorff 1999). The findings regarding the limited use of acaricides and anthelmintics and the nature of management practices recorded during the questionnaire survey implied that the production management and the overall health care of pigs in the study area are generally poor. This observation is in line with that of a recent study in Kenya (Obonyo et al. 2013). It can probably be attributed to a lack of education, awareness and access to quality extension services amongst farmers in the study area, as has been suggested before (Kagira et al. 2003).
The poor ventilation observed in pig housing in the study area may have exposed the animals to respiratory diseases that might exacerbate the damage caused by the migratory stages of A. suum and pig lungworms (Obonyo et al. 2013). The finding that the majority of pigs (95.7%) were kept on soil floors also suggests that the pigs could have been exposed to a higher risk of infection by parasites than pigs kept on concrete floors (Kagira et al. 2012).
Conclusion
This study has provided evidence of the occurrence of internal parasites in pigs kept in Burayu District in Oromia. The results imply that nematode infections may be one of the contributing factors to low productivity of pigs in the study area. There is, therefore, an urgent need to institute strategic control measures that integrate better nutrition with anthelmintic treatments against these parasites in order to increase pig productivity. Farmers should be educated and encouraged to improve management and husbandry practices and productivity of pigs in Ethiopia. Further epidemiological studies in different animal management systems and agroecological zones are urgently needed in other parts of Ethiopia to determine the potential zoonotic and economic importance of pig parasites.
Acknowledgements
This study was financially supported by the School of Veterinary Medicine, Addis Ababa University. We gratefully acknowledge the willingness and cooperation of pig farmers in Burayu District in Oromia.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
B.K. (Addis Ababa University) was responsible for the concept and design of the study, data analysis and scientific correction of the manuscript. E.K. (Addis Ababa University) was responsible for administration of the questionnaires, data collection and laboratory work. Both authors contributed to the writing and final approval of the manuscript.
References
Christensen, C.M., Barnes, E. & Nansen, P., 1997, 'Experimental Oesophagostomum dentatum infection in the pig: worm populations at the dry season of the year', International Journal of Parasitology 25, 399-408. [ Links ]
Eijck, I. & Borgsteede, F., 2005, 'A survey of gastrointestinal pig parasites on free-range, organic and conventional pig farms in the Netherlands', Veterinary Research Communications 29, 407-414. http://dx.doi.org/10.1007/s11259-005-1201-z, PMid:16195935 [ Links ]
Esrony, K., Kambarage, D.M., Mtambo, M.M.A., Muhairrva, A.P. & Kusiluka, L.J.M., 1997, 'Helminthosis in local and cross-bred pigs in Morogoro Region of Tanzania,' Preventive Veterinary Medicine 32, 41-46. http://dx.doi.org/10.1016/S0167-5877(97)00011-1 [ Links ]
Kagira, J.M., Kanyari, P.N., Githigia, S.M., Maingi, N., Ng'ang'a, J.C. & Gachohi, J.M., 2012, 'Risk factors associated with occurrence of nematodes in free range pigs in Busia District, Kenya,' Tropical Animal Health and Production 44, 657-664. http://dx.doi.org/10.1007/s11250-011-9951-9, PMid:21833678 [ Links ]
Kagira, J.M., Kanyari, P.W.N., Munyua, W.K. & Waruiru, R.M., 2003, 'The control of parasitic nematodes in commercial piggeries in Kenya as reflected by a questionnaire survey on management practices', Tropical Animal Health and Production 35, 79-84. http://dx.doi.org/10.1023/A:1022031806486, PMid:12636362 [ Links ]
Kumsa, B, Tolera, A. & Nurfeta, A., 2010, 'Comparative efficacy of seven brands of albendazole against naturally acquired gastrointestinal nematodes in sheep in Hawassa, southern Ethiopia', Turkish Journal of Veterinary and Animal Sciences 34, 417-425. [ Links ]
Lisbeth, E., Thomsen, K., Mejer, H., Wendt, S., Roepstorff, A. & Hindsbo, O., 2001, 'The influence of stocking rate on transmission of helminth parasites in pigs on permanent pasture during two consecutive summers', Veterinary Parasitology 99, 120-146. [ Links ]
Marufu, M.C.M., Chanayiwa, P., Chimonyo, M. & Bhebhe, E., 2008, 'Prevalence of gastrointestinal nematodes in Mukota pigs in a communal area of Zimbabwe', African Journal of Agricultural Research 3, 91-95. [ Links ]
Ministry of Agriculture, 2004, Ethiopian agricultural sample survey report on livestock and livestock characteristics (Volume II), Ministry of Agriculture, Addis Ababa. [ Links ]
Nansen, P. & Roepstorff, A., 1999, 'Parasitic helminths of the pig: factors influencing transmission and infection levels', International Journal of Parasitology 29, 877891. http://dx.doi.org/10.1016/S0020-7519(99)00048-X [ Links ]
Ng'ang'a, C.J, Karanja, D.N. & Mutune, M.N., 2008, 'The prevalence of gastrointestinal helminth infections in pigs in Kenya', Tropical Animal Health and Production 40, 331-334. http://dx.doi.org/10.1007/s11250-007-9112-3 [ Links ]
Obonyo, F.O., Maingi, N., Githigia, S.M. & Ng'ang'a, C.J., 2012, 'Prevalence, intensity and spectrum of helminths of free range pigs in Homabay District, Kenya', Livestock Research for Rural Development 24, Article #48, viewed 12 March 2013, from http://www.lrrd.org/lrrd24/3/obon24048.htm [ Links ]
Obonyo, F.O., Maingi, N., Githigia, S.M. & Ng'ang'a, C.J., 2013, 'Farming practices and risk factors for transmission of helminths of free range pigs in Homabay District, Kenya', Livestock Research for Rural Development 25, Article #36, viewed 13 March 2013, from http://www.lrrd.org/lrrd25/3/obon25036.htm. [ Links ]
Pattison, H., Smith, W. & Thomas, R., 1979, 'The effect of sub-clinical nematode parasitism on the reproductive performance of sows', Animal Production 29, 321-326. http://dx.doi.org/10.1017/S0003356100023588 [ Links ]
Tamboura, I-LH., Banga-Mboko, H., Maes, D., Youssao, L., Traore, A., Bayala B. et al., 2006, 'Prevalence of common gastrointestinal nematode parasites in scavenging pigs of different ages and sexes in eastern centre province, Burkina Faso', Onderstepoort Journal of Veterinary Research 73, 53-60. http://dx.doi.org/10.4102/ojvr.v73i1.169, PMid:16715878 [ Links ]
Taylor, M.A., Coop, R.L. & Wall, R., 2007, Veterinary parasitology, Wiley-Blackwell, Oxford. [ Links ]
Thrusfield, M., 1995, Veterinary epidemiology, 2nd edn, Blackwell Science, Oxford. PMid:7610538 [ Links ]
 Correspondence:
Correspondence:
Bersissa Kumsa
PO Box 34
Bishoftu, Ethiopia
Email: bersissak@yahoo.com
Received: 30 July 2012
Accepted: 25 Mar.
2013 Published: 26 Feb. 2014














