Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.84 n.1 Pretoria Jan. 2013
REVIEW ARTICLE
Laryngeal paralysis in dogs: An update on recent knowledge
Adriaan M. KitshoffI; Bart Van GoethemI; Ludo StegenI; Peter VandekerckhoveII; Hilde de RoosterI
IDepartment of Small Animal Medicine and Clinical Biology, University of Ghent, Belgium
IIVeterinary Centre Malpertuus, Heusden, Ghent, Belgium
ABSTRACT
Laryngeal paralysis is the effect of an inability to abduct the arytenoid cartilages during inspiration, resulting in respiratory signs consistent with partial airway obstruction. The aetiology of the disease can be congenital (hereditary laryngeal paralysis or congenital polyneuropathy), or acquired (trauma, neoplasia, polyneuropathy, endocrinopathy). The most common form of acquired laryngeal paralysis (LP) is typically seen in old, large breed dogs and is a clinical manifestation of a generalised peripheral polyneuropathy recently referred to as geriatric onset laryngeal paralysis polyneuropathy. Diagnosing LP based on clinical signs, breed and history has a very high sensitivity (90%) and can be confirmed by laryngeal inspection. Prognosis after surgical correction depends on the aetiology: traumatic cases have a good prognosis, whereas tumour-induced or polyneuropathy-induced LP has a guarded prognosis. Acquired idiopathic LP is a slow progressive disease, with dogs reaching median survival times of 3-5 years after surgical correction.
Introduction
It is the authors' opinion that the incidence of laryngeal paralysis (LP) is higher than commonly perceived. This is mainly a result of incorrect diagnosis because of a failure to recognise the typical clinical signs. The authors' experience has shown that many cases that are correctly diagnosed are given an improper grave prognosis. New findings regarding idiopathic LP make the disease progression and response to therapy easier to comprehend (Stanley et al. 2010). Adaptations of the surgical techniques and the use of the unilateral arytenoid lateralisation drastically decreased the associated complications (MacPhail & Monnet 2001; White 1989).
The aim of this article is to sensitise the reader to the clinical signs and treatment options for LP. An update will also be given on the laryngeal anatomy, aetiology and the diagnosis of LP in dogs. The most commonly encountered complications are also discussed.
Anatomy
The larynx is a semi-rigid organ composed mainly of hyaline cartilage and muscles (Evans 1993). During inspiration, contraction of the cricoarytenoideus dorsalis (CAD) muscle results in abduction of the arytenoid cartilages and vocal cords, opening up the glottic lumen and allowing air to pass freely (Evans 1993). Failure of the CAD muscle to contract will result in narrowing of the glottic lumen and respiratory stridor (Monnet & Tobias 2012).
The cartilages of the larynx include the epiglottic, arytenoid (paired), sesamoid, inter-arytenoid, thyroid and cricoid cartilages (Figure 1). The arytenoid cartilages have the most complex structure. Their irregular shape is the result of the corniculate, cuneiform, muscular and vocal processes (Evans 1993). The muscular process is situated just lateral to the cricoarytenoid articulation and acts as an insertion site for the CAD muscle (Evans 1993). The corniculate process is the longer of the two dorsal processes and forms the dorsal margin of the laryngeal inlet. The other dorsal process, the cuneiform process, is situated more rostroventrally than the corniculate process (Evans 1993). The ventral part of this process lies in the aryepiglotic fold forming most of the lateral boundary of the laryngeal inlet (Evans 1993). The ring shape of the cricoid cartilage creates a rigid structure that supports the more elastic thyroid and arytenoid cartilages (Monnet & Tobias 2012).
The thyropharyngeus (TP) muscle is situated on the dorsal and lateral aspect of the larynx (Hermanson & Evans 1993). This muscle originates on the lateral aspect of the thyroid cartilage and it extends dorsally to the pharynx to insert on the median plane (Hermanson & Evans 1993). Contraction of this muscle, together with the cricothyroideus muscle, results in constriction of the middle pharyngeal area that assists in swallowing and prevents air from entering the oesophagus (Hermanson & Evans 1993). Opening of the glottis is caused by contraction of the CAD muscle (Hermanson & Evans 1993). This muscle originates from the dorsolateral surface of the cricoid cartilage and inserts on the muscular process of the arytenoid cartilage (Hermanson & Evans 1993). Contraction of the muscle results results in caudodorsal displacement of the arytenoid cartilage (abduction).
All the intrinsic muscles of the larynx, except the cricothyroideus muscle, are innervated by the caudal laryngeal nerve (terminal portion of the recurrent laryngeal nerve) (Hermanson & Evans 1993). The left recurrent laryngeal nerve (RLN) arches around the aorta and ascends on the left side of the trachea, whereas the right RLN arches around the right subclavian artery and ascends on the right side of the trachea (Evans & Kitchell 1993). As the recurrent laryngeal nerves ascend, they give rise to the paralaryngeal recurrent nerves that run parallel to the RLN (Evans & Kitchell 1993). The paralaryngeal recurrent nerves supply sensory innervation to the oesophagus and the trachea (Evans & Kitchell 1993).
Aetiologies and classification
Laryngeal paralysis can be congenital or acquired and, depending on the aetiology, it occurs unilaterally or bilaterally (Monnet & Tobias 2012; Stanley et al. 2010). A hereditary form of LP has been described in Siberian huskies and bouviers des Flandres (O'Brien & Hendriks 1986; Venker-van Haagen 1982). A loss of motor neurons in the nucleus ambiguus as a result of an autosomal dominant trait, with secondary Wallerian degeneration of the recurrent laryngeal nerves, has been identified in the bouvier des Flandres (Parnell 2010). This disease results in either unilateral or bilateral paralysis and, generally, presents in dogs less than 12 months of age (Burbidge 1995; O'Brien & Hendriks 1986; Ridyard et al. 2000; Venker-van Haagen 1982).
Congenital LP polyneuropathy has been reported in Rottweilers, bouviers des Flandres, bull terriers, Dalmatians, German shepherd dogs, Afghan hounds, cocker spaniels, dachshunds, miniature pinchers and Siberian huskies (Bennnett & Clarke 1997; Braund 1994; Braund et al. 1994; Braund et al. 1989; Eger et al. 1998; Harvey & O'Brien 1982; Mahony et al. 1998; O'Brien & Hendriks 1986; Ridyard et al. 2000; Venker-van Haagen 1982). Clinical signs indicating the presence of a polyneuropathy can also be present. These clinical signs include hyporeflexia (all four limbs), decreased postural reactions, hypotonia and appendicular muscle atrophy (Braund 1994; Braund et al. 1994; Davies & Irwin 2003; Gabriel et al. 2006; Mahony et al. 1998; Ridyard et al. 2000).
In young Dalmatians and Rottweilers, axonal degeneration together with loss of myelinated nerve fibres of the RLN and paralaryngeal recurrent nerves are observed (Braund et al. 1994; Braund et al. 1989; Mahony et al. 1998). Phenotypic characteristics, such as white coat, freckles and blue eyes, have been linked to LP in Siberian huskies and German shepherd dogs (O'Brien & Hendriks 1986; Polizopoulou et al. 2003; Ridyard et al. 2000).
Acquired LP can be caused by trauma to the RLN or vagus nerves in the cervical or thoracic region (e.g. bite wounds, surgical trauma, mediastinal tumour) (Monnet & Tobias 2012). Diseases such as neuropathies, caudal brainstem disease, endocrine diseases (hypothyroidism and hypoadrenocorticism), myasthenia gravis, paraneoplastoc syndromes, idiopathic myositis, systemic lupus erythematosus and organophosphate toxicity can also result in LP (Burbidge 1995; Dewey et al. 1997; Kvitko-White et al. 2012; MacPhail & Monnet 2001; Michael 2002; Monnet & Tobias 2012; White 1989). The term geriatric onset laryngeal paralysis polyneuropathy (GOLPP) has recently been used to described the commonly encountered syndrome of acquired idiopathic laryngeal paralysis (AILP) (Monnet & Tobias 2012; Parnell 2010; Stanley et al. 2010). Strong evidence exists that this form is a prominent clinical sign of a generalised peripheral polyneuropathy (Jeffery et al. 2006; Stanley et al. 2010). It commonly occurs in breeds such as Labrador retrievers, Rottweilers, Afghan hounds, Irish setters, golden retrievers, Saint Bernards, Irish setters and standard poodles (Gaber, Amis & Le Couteur 1985; Monnet & Tobias 2012).
In contrast to the congenital form, AILP is typically seen in middle-aged to older large breed dogs (Burbidge 1995; Parnell 2010). Male dogs are presented about twice as often as females (Burbidge, Goulden & Jones 1991; MacPhail & Monnet 2001; White 1989).
Clinical signs
Dogs with unilateral LP (mostly left-sided) will only display clinical signs during strenuous activities (i.e. working dogs) (Monnet & Tobias 2012). Failure to abduct the arytenoid cartilages during inspiration results in increased resistance to airflow and turbulence through the rima glottidis leads to the typical inspiratory stridor (Stanley et al. 2010; Venker-van Haagen 1982). Dysphonia is caused by the inability to tense the vocal cords, which results in the dog's voice changing to a weak, hoarse bark (Parnell 2010). Partial obstruction of the upper airways by the paralysed arytenoids leads to exercise intolerance (Burbidge 1995; Parnell 2010).
Respiratory distress (which can lead to cyanosis) can easily be exacerbated by excitement, exercise, elevated environmental temperatures, pulmonary oedema or the presence of bronchopneumonia (Millard & Tobias 2009; Monnet & Tobias 2012; Parnell 2010). The functional airway obstruction can also be worsened by secondary laryngeal oedema and inflammation (Harvey & O'Brien 1982; Millard & Tobias 2009). Overweight dogs with LP present with more severe clinical signs than normally conditioned animals (Broome, Burbidge & Pfeiffer 2000).
Advanced diagnostics can reveal the presence of concurrent bronchopneumonia, megaoesophagus, hiatal hernia or gastro-oesophageal reflux (Burnie, Simpson & Corcoran 1989; Stanley et al. 2010). These can lead to excessive coughing, gagging and regurgitation in affected patients. In one study, oesophageal motility was decreased in all 32 dogs with AILP (Stanley et al. 2010). This was a result of a peripheral neuropathy and was more pronounced if a liquid diet was fed (Stanley et al. 2010). A decrease in oesophageal motility can be clinically silent (Stanley et al. 2010). Dysphagia can be a symptom of peripheral polyneuropathy and can sometimes be seen in patients with LP (Monnet & Tobias 2012). Congenital LP in dogs is usually the result of a polyneuropathy complex and presents in dogs less than 12 months of age (Monnet & Tobias 2012). This form of the disease is characterised by signs of LP together with lenticular cataracts and neurological signs such as tetraparesis (worse in the pelvic limbs), hyporeflexia in all four limbs, decreased postural reactions, hypotonia and appendicular muscle atrophy (Braund 1994; Braund et al. 1994; Davies & Irwin 2003; Gabriel et al. 2006; Mahony et al. 1998; Ridyard et al. 2000). Concurrent diseases, such as megaoesophagus and aspiration pneumonia, can also be present or can develop during the course of the disease (Braund 1994; Braund et al. 1994; Mahony et al. 1998; Ridyard et al. 2000).
In 15 dogs with AILP that underwent a full physical neurological examination in one study, all showed neurological abnormalities in addition to respiratory-related problems (Jeffery et al. 2006). These abnormalities included decreased postural reactions, deficits in spinal reflexes and deficits in cranial nerve function (Jeffery et al. 2006). Clinical signs related to the generalised polyneuropathy can be subtle and care should be taken as they can be overlooked when dealing with a dyspnoeic dog (Jeffery et al. 2006). Neurological dysfunction (ataxia) of the hindlimbs in these older dogs is often misinterpreted as weakness or as an orthopaedic condition (Jeffery et al. 2006). This generalised polyneuropathy is a slowly progressive degenerative condition that affects peripheral nerves (Stanley et al. 2010). Obvious clinical signs of general polyneuropathy and dysphagia can take months to years to develop (Jeffery et al. 2006; Stanley et al. 2010).
Diagnosis
Laryngeal paralysis should be suspected in every patient displaying inspiratory stridor, hoarse voice changes and exercise intolerance. The inspiratory dyspnoea does not resolve with open mouth breathing and will worsen with mild lateral compression over the larynx (Monnet & Tobias 2012).
Clinical signs and signalment are integral parts when diagnosing LP. Bouviers des Flandres and Siberian huskies less than 12 months of age with only respiratory problems are suspected to suffer from hereditary LP (O'Brien & Hendriks 1986; Venker-van Haagen 1982). Middle-aged dogs with respiratory problems consistent with LP combined with neurological dysfunction are suspected of having congenital LP, which is mostly the result of a peripheral polyneuropathy (Monnet & Tobias 2012). Older dogs with exercise intolerance, inspiratory stridor and dysphonia are suspected of AILP. The signalment, together with the history, has a specificity of 91.6% and a sensitivity of 98.5% in all dogs with grade 3 and 4 laryngeal paralysis (Broome et al. 2000).
Laryngeal inspection is essential in order to rule out other causes of laryngeal stridor (e.g. laryngeal tumour) and confirm the suspected diagnosis of LP (Broome et al. 2000). Direct visualisation of the larynx can be achieved via transnasal or peroral laryngoscopy. As the latter has a 95% interobserver agreement, it is considered the gold standard of diagnosis (Broome et al. 2000; Radlinsky et al. 2009; Smith 2000). Transnasal laryngoscopy has the advantage that it can be performed in large breed dogs using only sedation and local anaesthesia (Radlinsky, Mason & Hodgson 2004).
Prior to laryngeal examination, an intravenous catheter is placed and the dog is preoxygenated for at least 3-5 min (Millard & Tobias 2009; Smith 2000). The dog is placed in sternal recumbency and the head is held in a normal anatomic position (Gross et al. 2002; Jackson et al. 2004; Smith 2000). To prevent a false positive diagnosis, only a light plane of anaesthesia is maintained (Gross et al. 2002; Jackson et al. 2004; Monnet & Tobias 2012; Smith 2000). The aim is to achieve relaxation of the jaw muscles without affecting the laryngeal reflexes or depressing respiratory movements (Burbidge 1995). Anaesthetic protocols such as diazepam-ketamine combination are avoided because they result in suboptimal laryngeal exposure during laryngoscopy as a result of poor muscle relaxation (Gross et al. 2002). When drug combinations of acepromazine-propofol, acepromazine-thiopental or diazepam-ketamine were used, half of the normal dogs in one study failed to show arytenoid abduction during inspiration (false positive diagnosis) (Jackson et al. 2004). The same study concluded that intravenous thiopental as a sole drug was best for maintaining laryngeal function (Jackson et al. 2004). Although respiratory depression results when using thiopental as induction agent, a very light plane of anaesthesia results in tachypnea, which is ideal to evaluate the larynx (Turner & Ilkiw 1990). Patients suspected of LP should be examined until they almost reach a plane of consciousness (Burbidge 1995). When laryngeal inspection is not conclusive, doxapram HCl (1.1 mg/kg), which induces deep inspiratory movements, can be useful to differentiate normal dogs from dogs with LP (Tobias, Jackson & Harvey 2004). The increased velocity of airflow, however, will result in an increase in the negative airway pressure, which results in paradoxical arytenoid movement that can lead to complete laryngeal obstruction (Tobias et al. 2004).
Laryngeal inspection involves the evaluation of the arytenoid cartilages for active abduction during inspiration and passive adduction during expiration (Monnet & Tobias 2012). Immobile arytenoids and vocal cords in an appropriately anesthetised dog indicate bilateral LP, whereas asymmetrical motion of the arytenoids is indicative of unilateral disease (Monnet & Tobias 2012). To avoid false negative diagnoses of LP in patients with paradoxical movement of the arytenoids, it is helpful if an assistant indicates the inspiration phase to the clinician who is performing the laryngeal inspection. Paradoxical movement in LP patients occurs when the increased negative airway pressure during inspiration results in adduction of the arytenoids and, subsequently, the positive pressure during expiration results in passive return of the arytenoids to their resting position (Burbidge 1995). This is encountered in up to 45% of dogs with LP (Olivieri, Voghera & Fossum 2009). Excessive negative pressure can lead to secondary elongation of the soft palate and eversion of the laryngeal saccules (Millard & Tobias 2009). The constant rubbing of the arytenoid cartilages against each other can result in mucosal ulcerations and oedema at the level of the corniculate processes (Monnet & Tobias 2012).
Other diagnostic methods, such as sound signature identification, tidal breathing flow-volume loops, electromyography, blood gas analysis and plethysomography, can assist in confirming the diagnosis of LP (Amis & Kurpershoek 1986; Bedenice et al. 2006; Burbidge 1995; Yeon et al. 2005). Echolaryngography has been studied but proved less sensitive for diagnosing LP than direct visualisation (Radlinsky et al. 2009; Rudorf, Barr & Lane 2001).
Thoracic radiographs should be taken in all dogs suspected of LP in order to assist in the diagnosis of underlying diseases, such as cervical and cranial mediastinal masses, and to identify other pathologies such as megaoesophagus, aspiration pneumonia and noncardiogenic lung oedema (Monnet & Tobias 2012). In dogs suspected of a megaoesophagus, positive contrast oesophograms could confirm the diagnosis; although, this is not performed routinely because of the increased risk of aspiration (Millard & Tobias 2009). In patients with confirmed laryngeal paralysis, 7% - 14% are subsequently diagnosed with hypothyroidism (Asulp et al. 1997; Dixon, Reid & Mooney 1999; Jaggy et al. 1994; White 1989; Zikes & McCarthy 2012). In dogs showing clinical signs of weakness, megaoesophagus, other peripheral or central neurological signs, exercise intolerance, dermatological abnormalities (hyperpigmentation, alopecia, poor coat quality and pyoderma), lethargy or obesity, free thyroxine and thyroid-stimulating hormone should be tested (Jaggy et al. 1994; Jeffery et al. 2006).
Myasthenia gravis is infrequently associated with LP (Jeffery et al. 2006). In dogs with LP presenting with clinical signs of regurgitation (megaoesophagus), dysphagia, multiple cranial nerve abnormalities, generalised or focal neuromuscular weakness or exercise intolerances, acetylcholine receptor antibody titres need to be measured to rule in or out myasthenia gravis (Shelton 2002). Acquired myasthenia gravis can be associated with hypothyroidism or hypoadrenocorticism, or present as paraneoplastic syndrome associated with thymomas, osteogenic sarcoma, cholangiocellular carcinoma and cutaneous lymphoma (Shelton 2002). An attempt should be made to rule out these primary conditions when a diagnosis of myasthenia gravis has been made.
Medical treatment of respiratory distress
Patients with LP can present in acute respiratory distress, resulting in cyanosis and hyperthermia (Burbidge 1995). Emergency treatment is essential and consists of oxygen supplementation, administration of a sedative and cooling of the patient (Burbidge 1995; Millard & Tobias 2009). The route of oxygen supplementation depends on what is tolerated by the patient and can include an oxygen cage, flow-by oxygen, an oxygen hood, a facemask or a nasal cannula (Mazzaferro 2009). If cyanosis, dyspnoea and hypoxia (SPO2 < 95%) persist despite oxygen supplementation, a temporary tracheostomy or temporary intubation under light anaesthesia should be considered until laryngeal swelling decreases or surgical correction can be performed (Millard & Tobias 2009). Temporary intubation is selected if the time of intubation is expected to be just a couple of hours, whereas tracheostomy tubes are used for longer-term management (Millard & Tobias 2009). Fluids are administered with caution as pulmonary oedema can develop in animals with severe upper respiratory tract obstruction (Monnet & Tobias 2012). Sedation using acepromazine (0.005 mg/kg - 0.020 mg/kg) and butorphanol (0.200 mg/kg - 0.400 mg/kg) has been recommended (Millard & Tobias 2009). Additionally, short-acting corticosteroids, such as dexamethasone (0.100 mg/kg -0.500mg/kg) or prednisolone sodium succinate (0.200 mg/kg - 0.400 mg/kg), can be administered in the case of laryngeal oedema (Millard & Tobias 2009). Hyperthermia should be differentiated from true pyrexia that can occur as a result of aspiration pneumonia. Temperatures lower than 41.0 °C are not life threatening unless prolonged and therapy to cool patients should only be instituted if temperatures are elevated above this level (Mazzaferro 2009; Millard & Tobias 2009). Cooling can be achieved by clipping the fur, by wetting the animal, by applying ice packs over well-vascularised regions (neck, axilla and inguinal region), by fanning the wetted patient or by the rectal administration of cool isotonic fluids (Mazzaferro 2009). Continuous monitoring of the temperature is important and cooling procedures should be discontinued as soon as the body temperature reaches 39.4 °C to prevent iatrogenic hypothermia (Mazzaferro 2009).
Conservative management of LP can be considered in older patients with minimal to moderate clinical signs. This involves anti-inflammatory drugs to decrease laryngeal swelling and a weight loss programme for overweight patients (MacPhail & Monnet 2008). The owners should also be educated on the changes in the patient's routine and environment. A cool area should be prepared for the patient, especially in the warmer months of the year. Patients should not be allowed to perform strenuous exercise. Short walks using a harness can be permitted during the cooler periods of the day.
Surgical treatment by cricoarytenoid cartilage lateralisation
Surgical management is advised in all LP patients with severe clinical signs (MacPhail & Monnet 2008; Monnet & Tobias 2012). The aim of surgery is to increase the size of the rima glottidis (LaHue 1989; Millard & Tobias 2009; Monnet & Tobias 2012). As resistance of airflow is inversely proportional to the radius to the power of four, according to Poiseuille's law, even a small increase in size will make a substantial difference (Monnet & Tobias 2012).
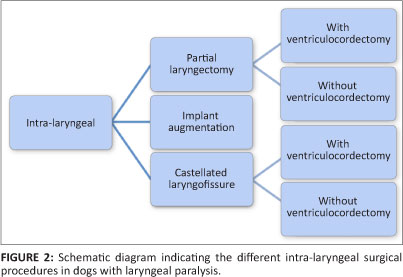
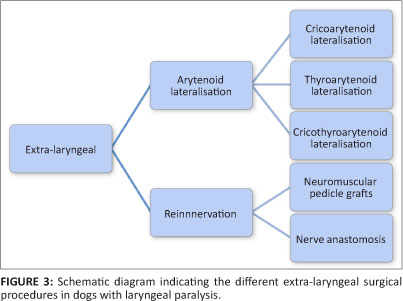
Many surgical techniques have been developed and successfully applied. They can be classified as intra-laryngeal or extra-laryngeal procedures (Figures 2 and 3). Cricoarytenoid cartilage lateralisation is currently considered the procedure of choice (Monnet & Tobias 2012). The objective of this procedure is to prevent passive adduction of the arytenoid cartilage during inspiration by fixing it to a neutral to slightly lateralised position (low tension technique) (Bureau & Monnet 2002). This modification still allows adequate epiglottic coverage of the rima glottidis during swallowing and is believed to reduce aspiration-related complications (Bureau & Monnet 2002).
Unilateral cricoarytenoid lateralisation (UCAL) is performed via a lateral approach (LaHue 1989; Monnet & Tobias 2012). Dogs with unilateral LP are corrected depending on the affected side, whilst dogs with bilateral LP have the lateralisation procedure on the left side if the surgeon is right- handed (MacPhail & Monnet 2001; Monnet & Tobias 2012).
Unilateral correction is sufficient to relieve clinical signs in most bilaterally affected dogs (Monnet & Tobias 2012).
Placing a sandbag under the neck elevates the laryngeal region and the skin incision is made over the larynx, just ventral to the jugular vein (Monnet & Tobias 2012). A combination of blunt and sharp dissection through the subcutaneous muscles (platysma and superficial sphincter colli muscles) and subcutaneous tissue exposes the TP muscle. This is then incised at the dorsocaudal rim of the lamina of the thyroid cartilage, avoiding penetration of the laryngeal mucosa. Alternatively, the TP muscle can be split along the direction of its muscle fibres (Nelissen & White 2011). Cricothyroid disarticulation may be performed in the adult dog when additional exposure is required. As an alternative, a stay suture can be placed through the lamina of the thyroid cartilage to achieve atraumatic lateral retraction. The muscular process of the arytenoid cartilage is usually prominent and easily palpable because of the neurogenic atrophy of the CAD muscle (Griffin & Krahwinkel 2005). A transverse incision is made through the CAD muscle and dissection is continued carefully until the cricoarytenoid articulation is visible (Monnet & Tobias 2012). The cranial part of the joint capsule is left intact during dissection of the cricoarytenoid joint (Bureau & Monnet 2002). A nonabsorbable monofilament suture (e.g. polypropylene) on a tapercut needle is recommended for fixing the arytenoid. Depending on the size of the dog, USP 2/0 (< 40 kg) or USP 0 (> 40 kg) is used (Demetriou & Kirby 2003). The suture is anchored dorsally on the caudal border of the cricoid cartilage, taking care not to penetrate the laryngeal lumen. It is recommended that extubation be attempted after performing this step as inadvertent suturing of the endotracheal tube can occur (Weinstein & Weisman 2010). The needle is then passed through the muscular process of the arytenoid in a medial-to-lateral direction (Monnet & Tobias 2012). Older dogs can have brittle laryngeal cartilages that can tear during suture placement (Monnet & Tobias 2012). For this reason, needle selection is very important to decrease the risk of tearing or even fracturing of the cartilage once the suture is tightened. Some authors advise pre-drilling a small hole in the arytenoid cartilage using an 18-gauge hypodermic needle before needle placement (Monnet & Tobias 2012).
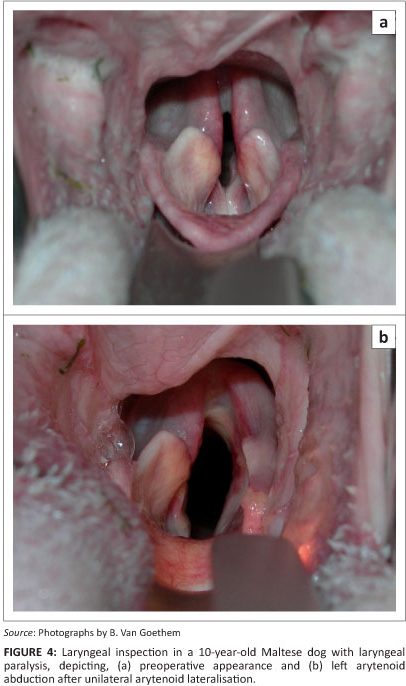
The suture is carefully tied until resistance from the tensed remaining part of the joint capsule is felt (Bureau & Monnet 2002). Alternatively, the suture can be tied under direct visual endoscopic control after temporary extubation (Weinstein & Weisman 2010). Adequate abduction is defined as any degree of abduction resulting in an increase in the glottic diameter without axial displacement of the dependant (non-surgically treated) side (Weinstein & Weisman 2010) (Figure 4). Meticulous apposition of the TP muscle, using a continuous suture pattern with monofilament absorbable suture material is essential to decrease the chance for postoperative dysphagia (Nelissen & White 2011). The subcutaneous tissues are closed in two layers and the skin is closed routinely.
Postoperative complications occur in 10% - 58% of dogs (Gaber et al. 1985; Hammel, Hottinger & Novo 2006; MacPhail & Monnet 2001; Snelling & Edwards 2003). These include gagging or coughing, aspiration pneumonia, recurrence of clinical signs (caused by implant failure or cartilage tearing), residual stridor, respiratory distress, gastric dilatation volvulus, seroma or haematoma formation, and death (Millard & Tobias 2009; Monnet & Tobias 2012). It should be kept in mind that dogs carry a lifelong risk for the development of respiratory tract complications postoperatively (MacPhail & Monnet 2001). Aspiration pneumonia is the most frequently noted complication, occurring in about 8% - 24% of dogs postoperatively (Demetriou & Kirby 2003; Hammel et al. 2006; MacPhail & Monnet 2001; Snelling & Edwards 2003; White 1989). Low-tension techniques are believed to decrease the incidence of postoperative aspiration pneumonia (Bureau & Monnet 2002).
About 5% of patients require a contralateral procedure because of arytenoid fragmentation, avulsion of the lateralisation suture or inadequate lateralisation (White 1989). Recurrence of clinical signs postoperatively is seen more commonly in small breed dogs (Snelling & Edwards 2003). Complications during the postoperative period can be minimised by sound knowledge of the anatomy, meticulous tissue handling and avoidance of laryngeal lumen penetration (Monnet & Tobias 2012). Factors that negatively influence the surgical outcome include age, concurrent respiratory tract abnormalities, oesophageal disease, neurological disease or neoplastic disease and the placement of a temporary tracheostomy tube (MacPhail & Monnet 2001). Unilateral cricoarytenoid lateralisation has a good clinical outcome, with 88% - 90% of dogs showing an improved quality of life in the postoperative period (Hammel et al. 2006; Snelling & Edwards 2003). Variations of this technique exist in which the arytenoid is also fixed to the thyroid (cricothyroarytenoid lateralisation) or solely to the thyroid (thyroarytenoid lateralisation) (Monnet & Tobias 2012). The latter technique results in a less extensive (but satisfactory) opening of the rima glottidis when compared to cricoarytenoid lateralisation and takes less time to perform (Griffiths, Sullivan & Reid 2001). The clinical outcomes of UCAL and thyroarytenoid lateralisation compare well (Griffiths et al. 2001).
Other surgical techniques
Permanent tracheostomy creates a bypass of the larynx (Monnet & Tobias 2012). It is considered in patients that are at risk for postoperative aspiration pneumonia. This includes patients with generalised myopathy, megaoesophagus, hiatal hernia and gastrointestinal disorders (Monnet & Tobias 2012).
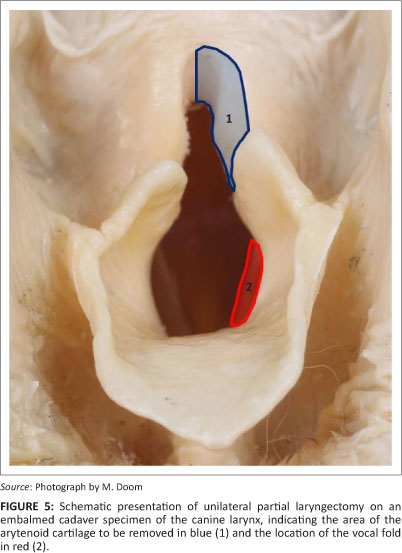
Partial laryngectomy (Figure 5) is an older technique involving removal of the vocal cords and a substantial part of the corniculate and vocal processes (unilateral or bilateral) in order to ensure unobstructed airflow without influencing the protective effect on the airway (Harvey 1983a, 1983b). This procedure can result in significant postoperative swelling that might necessitate placement of a temporary tracheostomy tube. Complications are seen in approximately 50% of the dogs and include persistent upper respiratory stridor, coughing, vomiting, aspiration pneumonia, laryngeal webbing and exercise intolerance (Harvey 1983a; Harvey & O'Brien 1982; MacPhail & Monnet 2001; Ross et al. 1991) (Table 1). This abandoned technique has recently regained popularity since the introduction of diode laser arytenoidectomy via transoral approach. No direct postoperative complications were reported in 20 dogs and only 10% developed aspiration pneumonia in the long term (Olivieri et al. 2009).
A recent retrospective study on ventriculocordectomy via ventral laryngotomy has shown some promising results with limited short-term and long-term complications. The authors of this article concluded that because of the ease of the procedure, the limited complications and minimal surgical trauma, this technique should be considered for routine use (Zikes & McCarthy 2012).
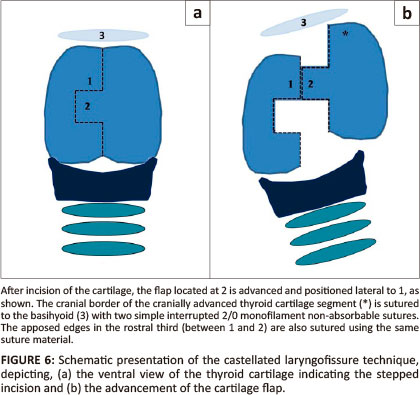
Castellated laryngofissure is another historical procedure that creates an enlargement of the ventral laryngeal ostium by offset closure of a castellated incision on the ventral aspect of the thyroid cartilage (Figure 6). This procedure is technically difficult, results in severe postoperative laryngeal oedema and requires the placement of a temporary tracheostomy tube for 2-3 days postoperatively (Monnet & Tobias 2012). Variable results have been obtained and the procedure was abandoned (Burbidge et al. 1991).
Reinnervation techniques and neuromuscular pedicle grafts have been used in dogs to restore the abductor function in experimentally denervated patients (Greenfield et al. 1988; Paniello, West & Lee 2001; Rice 1982). These techniques might be of use in patients with acquired LP of traumatic origin. It is likely to be ineffective in patients with polyneuropathy or polymyopathy as a primary cause (Monnet & Tobias 2012). Its routine use is also questioned as it takes a minimum of 5 months for restoration of laryngeal function (Greenfield et al. 1988).
Laryngeal augmentation with implantable devices has been reported ex vivo (Cabano et al. 2011) and in vivo (Kwon et al. 2007) in canine patients. No extensive clinical data exist for the current devices and hence their use can currently not be recommended.
Postoperative care
After surgical treatment, partial obstruction of the larynx will be relieved and the respiratory dyspnoea will resolve immediately. Oxygen therapy should be administered as necessary and perioperative dexamethasone sodium phosphate (0.1 mg/kg - 1.0 mg/kg) can be helpful to decrease laryngeal swelling and oedema (Monnet & Tobias 2012). Food and water is withheld until 12 h after the operation. Heavy sedation in the postoperative period is avoided to preserve the swallowing reflexes (Monnet & Tobias 2012). The patient is first offered canned food rolled into balls (Monnet & Tobias 2012). If no coughing or gagging is observed, small amounts of water can be offered (Monnet & Tobias 2012). The decision to administer postoperative antibiotic is usually case based.
Prognosis and conclusion
A clear distinction needs to be made between the different forms of the disease. Prognosis for hereditary LP is excellent as dogs are cured by surgery. Congenital LP neuropathy has a poor prognosis and most dogs tend to be euthanased within 10 weeks as a result of worsening clinical signs (Davies & Irwin 2003). The prognosis for acquired LP will vary depending on the cause: trauma cases can be cured; neoplasia-induced LP will depend on the tumour type.
Evidence strongly suggests that the most common form of LP in dogs is, in fact, an early stage of GOLPP (Stanley et al. 2010). Even though all complications should be considered when making a prognosis in any dog developing LP as a component of polyneuropathy, this condition progresses slowly, making short-term prognosis more favourable.
Acknowledgements
The authors would like to the Department of Morphology at the Faculty of Veterinary Medicine, Ghent University for supplying the embalmed canine larynxes for the photographs shown in Figures 1 and 5.
Competing interests
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this article.
Authors' contributions
A.M.K. (University of Ghent) wrote the manuscript. H.d.R. (University of Ghent), B.v.G. (University of Ghent), L.S. (University of Ghent) and P.V. (Veterinary Centre Malpertuus) made conceptual contributions.
References
Amis, T.C. & Kurpershoek, C., 1986, 'Tidal breathing flow-volume loop analysis for clinical assessment of airway obstruction in conscious dogs', American Journal of Veterinary Research 47, 1002-1006. PMid:3717718 [ Links ]
Alsup, J.C., Greenfeld, C.L., Hungerford, McKiernan, B.C. & Whiteley, H.E., 1997, 'Comparison of unilateral arytenoid lateralization and ventral ventriculocordectomy for the treatment of experimentally induced laryngeal paralysis in dogs', Canadian Veterinary Journal 38(5), 287-293. [ Links ]
Bedenice, D., Rozanski, E., Bach, J., Lofgren, J. & Hoffman, A.M., 2006, 'Canine awake head-out plethysmography (HOP): Characterization of external resistive loading and spontaneous laryngeal paralysis', Respiratory Physiology and Neurobiology 151, 61-73. http://dx.doi.org/10.1016/j.resp.2005.05.030, PMid:16055393 [ Links ]
Bennnett, P.F. & Clarke, R.E., 1997, 'Laryngeal paralysis in a Rottweiler with neuroaxonal dystrophy', Australian Veterinary Journal 75, 784-786. http://dx.doi.org/10.1111/j.1751-0813.1997.tb15650.x [ Links ]
Braund, K.G., 1994, 'Pediatric neuropathies', Seminars in Veterinary Medicine and Surgery (Small Animal) 9, 86-98. [ Links ]
Braund, K.G., Shores, A., Cochrane, S., Forrester, D., Kwiecien, J.M. & Steiss, J.E., 1994, 'Laryngeal paralysis-polyneuropathy complex in young Dalmations', American Journal of Veterinary Research 55, 534-542. PMid:8017700 [ Links ]
Braund, K.G., Steinberg, H.S., Shores, A., Steiss, J.E., Mehta, J.R., Toiviokinnucan, M. et al., 1989, 'Laryngeal paralysis in immature and mature dogs as one sign of a more diffuse polyneuropathy', Journal of the American Veterinary Medical Association 194, 1735-1740. PMid:2546908 [ Links ]
Broome, C., Burbidge, H.M. & Pfeiffer, D.U., 2000, 'Prevalence of laryngeal paresis in dogs undergoing general anaesthesia', Australian Veterinary Journal 78, 769-772. http://dx.doi.org/10.1111/j.1751-0813.2000.tb10449.x, PMid:11194723 [ Links ]
Burbidge, H., 1995, 'A review of laryngeal paralysis in dogs', British Veterinary Journal 151, 71-82. http://dx.doi.org/10.1016/S0007-1935(05)80066-1 [ Links ]
Burbidge, H.M., Goulden, E. & Jones, B.R., 1991, 'An experimental evaluation of castellated laryngofissure and bilateral arytenoid lateralisation for the relief of laryngeal paralysis in dogs', Australian Veterinary Journal 68, 268-272. http:// dx.doi.org/10.1111/j.1751-0813.1991.tb03239.x, PMid:1953550 [ Links ]
Bureau, S. & Monnet, E., 2002, 'Effects of suture tension and surgical approach during unilateral arytenoid lateralization on the rima glottidis in the canine larynx', Veterinary Surgery 31, 589-595. http://dx.doi.org/10.1053/jvet.2002.34671, PMid:12415529 [ Links ]
Burnie, A., Simpson, J. & Corcoran, B., 1989, 'Gastrooesophageal reflux and hiatus- hernia associated with laryngeal paralysis in a dog', Journal of Small Animal Practice 30, 414-416. http://dx.doi.org/10.1111/j.1748-5827.1989.tb01595.x [ Links ]
Cabano, N.R., Greenberg, M.J., Bureau, S. & Monnet, E., 2011, 'Effects of bilateral arytenoid cartilage stenting on canine laryngeal resistance ex vivo', Veterinary Surgery 40, 97-101. http://dx.doi.org/10.1111/j.1532-950X.2010.00753.x, PMid:21062323 [ Links ]
Davies, D.R. & Irwin, P.J., 2003, 'Degenerative neurological and neuromuscular disease in young rottweilers', Journal of Small Animal Practice 44, 388-394. http://dx.doi.org/10.1111/j.1748-5827.2003.tb00173.x, PMid:14510327 [ Links ]
Demetriou, J.L. & Kirby, B.M., 2003, 'The effect of two modifications of unilateral arytenoid lateralization on rima glottidis area in dogs', Veterinary Surgery 32, 62-68. http://dx.doi.org/10.1053/jvet.2003.50000, PMid:12520491 [ Links ]
Dewey, C., Bailey, C., Shelton, G., Kass, P. & Cardinet, G., 1997, 'Clinical forms of acquired myasthenia gravis in dogs: 25 cases (1988-1995)', Journal of Veterinary Internal Medicine 11, 50-57. http://dx.doi.org/10.1111/j.1939-1676.1997. tb00073.x, PMid:9127290 [ Links ]
Dixon, R.M., Reid, S.W.J. & Mooney, C.T., 1999, 'Epidemiological, clinical and biochemical characteristics of canine hypothyroidism', Veterinary Record 145, 481-487. [ Links ]
Eger, C.E., Huxtable, C.R.R., Chester, Z.C. & Summers, B.A., 1998, 'Progressive tetraparesis and laryngeal paralysis in a young Rottweiler with neuronal vacuolation and axonal degeneration: An Australian case', Australian Veterinary Journal 76, 733-737. http://dx.doi.org/10.1111/j.1751-0813.1998.tb12301.x, PMid:9862062 [ Links ]
Evans, H.E., 1993, 'The respiratory system', in M.E. Miller & H.E. Evans (eds.), Miller's anatomy of the dog, 3rd edn., pp. 463-493, Saunders, Philadelphia. PMid:8403598 [ Links ]
Evans, H.E. & Kitchell, R.L., 1993, 'Cranial nerves and cutaneous innervation of the head', in M.E. Miller & H.E. Evans (eds.), Miller's anatomy of the dog, 3rd edn., pp. 953-987, Saunders, Philadelphia. [ Links ]
Gaber, C., Amis, T. & Le Couteur, R., 1985, 'Laryngeal paralysis in dogs - A review of 23 cases', Journal of the American Veterinary Medical Association 186, 377-380. PMid:3972696 [ Links ]
Gabriel, A., Poncelet, L., Van Ham, L., Clercx, C., Braund, K.G., Bhatti, S. et al., 2006, 'Laryngeal paralysis-polyneuropathy complex in young related Pyrenean mountain dogs', Journal of Small Animal Practice 47, 144-149. http://dx.doi.org/10.1111/j.1748-5827.2006.00058.x, PMid:16512846 [ Links ]
Greenfield, C.L., Walshaw, R., Kumar, K., Lowrie, C.T. & Derksen, F.J., 1988, 'Neuromuscular pedicle graft for restoration of arytenoid abductor function in dogs with experimentally induced laryngeal hemiplegia', American Journal of Veterinary Research 49, 1360-1366. PMid:3178033 [ Links ]
Griffin, J. & Krahwinkel, D., 2005, 'Laryngeal paralysis: Pathophysiology, diagnosis, and surgical repair', Compendium on Continuing Education for the Practicing Veterinarian 27, 857-869. [ Links ]
Griffiths, L.G., Sullivan, M. & Reid, S.W., 2001, 'A comparison of the effects of unilateral thyroarytenoid lateralization versus cricoarytenoid laryngoplasty on the area of the rima glottidis and clinical outcome in dogs with laryngeal paralysis', Veterinary Surgery 30, 359-365. http://dx.doi.org/10.1111/j.1532-950X.2001.00359.x, PMid:11443597 [ Links ]
Gross, M.E., Dodam, J.R., Pope, E.R. & Jones, B.D., 2002, 'A comparison of thiopental, propofol, and diazepam-ketamine anesthesia for evaluation of laryngeal function in dogs premedicated with butorphanol-glycopyrrolate', Journal of the American Animal Hospital Association 38, 503-506. PMid:12428879 [ Links ]
Hammel, S.P., Hottinger, H.A. & Novo, R.E., 2006, 'Postoperative results of unilateral arytenoid lateralization for treatment of idiopathic laryngeal paralysis in dogs: 39 cases (1996-2002)', Journal of the American Veterinary Medical Association 228, 1215-1220. http://dx.doi.org/10.2460/javma.228.8.1215, PMid:16618225 [ Links ]
Harvey, C.E., 1983a, 'Partial laryngectomy in the dog I. Healing and swallowing function in normal dogs', Veterinary Surgery 12, 192-196. http://dx.doi.org/10.1111/j.1532-950X.1983.tb00741.x [ Links ]
Harvey, C.E., 1983b, 'Partial laryngectomy in the dog II. Immediate increase in glottic area obtained compared with other laryngeal procedures', Veterinary Surgery 12, 197-201. http://dx.doi.org/10.1111/j.1532-950X.1983.tb00742.x [ Links ]
Harvey, C.E. & O'Brien, J.A., 1982, 'Treatment of laryngeal paralysis in dogs by partial laryngectomy', Journal of the American Animal Hospital Association 18, 551-556. [ Links ]
Hermanson, J.W. & Evans, H.E., 1993, 'The muscular system', in M.E. Miller & H.E. Evans (eds.), Miller's anatomy of the dog, 3rd edn., pp. 258-384, Saunders, Philadelphia. [ Links ]
Holt, D. & Harvey, C., 1994, 'Idiopathic laryngeal paralysis: Results of treatment by bilateral vocal fold resection in 40 dogs', Journal of the American Animal Hospital Association 30, 389-395. [ Links ]
Jackson, A.M., Tobias, K., Long, C., Bartges, J. & Harvey, R., 2004, 'Effects of various anesthetic agents on laryngeal motion during laryngoscopy in normal dogs', Veterinary Surgery 33, 102-106. http://dx.doi.org/10.1111/j.1532-950x.2004.04016.x, PMid:15027970 [ Links ]
Jaggy, A., Oliver, J.E., Ferguson, D.C., Mahaffrey, E.A. & Jun, T.G., 1994, 'Neurological manifestations of hypothyroidism: A retropsective study of 29 dogs', Journal of Veterinary Medicine 8(5), 328-336. [ Links ]
Jeffery, N.D., Talbot, C.E., Smith, P.M. & Bacon, N.J., 2006, 'Acquired idiopathic laryngeal paralysis as a prominent feature of generalised neuromuscular disease in 39 dogs', Veterinary Record 158, 17-21. http://dx.doi.org/10.1136/vr.158.L17, PMid:16400098 [ Links ]
Kvitko-White, H., Balog, K., Scott-Moncrieff, J.C., Johnson, A. & Lantz, G.C., 2012, 'Acquired bilateral laryngeal paralysis associated with systemic lupus erythematosus in a dog', Journal of the American Animal Hospital Association 48(1), 60-65. [ Links ]
Kwon, T.K., Jeong, W.J., Sung, M.W. & Kim, K.H., 2007, 'Development of endoscopic arytenoid adduction using cricoid implant', Annals of Otology, Rhinology and Laryngology 116, 770-778. PMid:17987783 [ Links ]
LaHue, T.R., 1989, 'Treatment of laryngeal paralysis in dogs by unilateral cricoarytenoid laryngoplasty', Journal of the American Animal Hospital Association 25, 317-324. [ Links ]
MacPhail, C.M. & Monnet, E., 2001, 'Outcome of and postoperative complications in dogs undergoing surgical treatment of laryngeal paralysis: 140 cases (19851998)', Journal of the American Veterinary Medical Association 218, 1949-1956. http://dx.doi.org/10.2460/javma.2001.218.1949, PMid:11417740 [ Links ]
MacPhail, C.M. & Monnet, E., 2008, 'Laryngeal paralysis', in J.D. Bonagura & R.W. Kirk (eds.), Kirk's current veterinary therapy, pp. 627-630, Elsevier Saunders, Philadelphia. [ Links ]
Mahony, O.H., Knowles, K.E., Braund, K.G., Averill, D.R. & Frimberger, A.E., 1998, 'Laryngeal paralysis-polyneuropathy complex in young Rottweilers', Journal of Veterinary Internal Medicine 12, 330-337. http://dx.doi.org/10.1111/j.1939-1676.1998.tb02131.x, PMid:9773408 [ Links ]
Mazzaferro, E.M., 2009, 'Oxygen therapy', in D.C. Silverstein & K. Hopper (eds.), Small animal critical care medicine, pp. 78-81, Elsevier Saunders, St. Louis. http://dx.doi.org/10.1016/B978-1-4160-2591-7.10019-0 [ Links ]
Michael, P., 2002, 'Inflammatory myopathies', Veterinary Clinics of North America: Small Animal Practice 32, 147-167. http://dx.doi.org/10.1016/S0195-5616(03)00083-4 [ Links ]
Millard, R.P. & Tobias, K.M., 2009, 'Laryngeal paralysis in dogs', Compendium on Continuing Education for the Practicing Veterinarian 31, 212-219. [ Links ]
Monnet, E. & Tobias, K.M. 2012, 'Larynx', in K.M. Tobias & S.A. Johnston (eds.), Veterinary surgery small animal, vol. 2, pp. 1718-1733, Elsevier Saunders, St. Louis. [ Links ]
Nelissen, P. & White, R.A., 2011, 'Arytenoid lateralization for management of combined laryngeal paralysis and laryngeal collapse in small dogs', Veterinary Surgery 41, 261-265. PMid:22103399 [ Links ]
O'Brien, J.A. & Hendriks, J., 1986, 'Inherited laryngeal paralysis. Analysis in the husky cross', Veterinary Qauterly 8, 301-302. http://dx.doi.org/10.1080/01652176.1986.9694059, PMid:3798712 [ Links ]
Olivieri, M., Voghera, S.G. & Fossum, T.W., 2009, 'Video-assisted left partial arytenoidectomy by diode laser photoablation for treatment of canine laryngeal paralysis', Veterinary Surgery 38, 439-444. http://dx.doi.org/10.1111/j.1532-950X.2009.00546.x, PMid:19538663 [ Links ]
Paniello, R.C., West, S.E. & Lee, P., 2001, 'Laryngeal reinnervation with the hypoglossal nerve. I. Physiology, histochemistry, electromyography, and retrograde labeling in a canine model', Annals of Otology, Rhinology and Laryngology 110, 532-542. PMid:11407844 [ Links ]
Parnell, N.K., 2010, 'Diseases of the throat', in S.J. Ettinger & E.C. Feldman (eds.), Textbook of veterinary internal medicine: Diseases of the dog and the cat, 7th edn., vol. 1, pp. 1040-1047, Elsevier Saunders, St. Louis. [ Links ]
Polizopoulou, Z.S., Koutinas, A.F., Papadopoulos, G.C. & Saridomichelakis, M.N., 2003, 'Juvenile laryngeal paralysis in three Siberian husky x Alaskan malamute puppies', Veterinary Record 153, 624-627. http://dx.doi.org/10.1136/vr.153.20.624, PMid:14653342 [ Links ]
Radlinsky, M.G., Mason, D.E. & Hodgson, D., 2004, 'Transnasal laryngoscopy for the diagnosis of laryngeal paralysis in dogs', Journal of the American Animal Hospital Association 40, 211-215. PMid:15131101 [ Links ]
Radlinsky, M.G, Williams, J., Frank, P.M. & Cooper, T.C., 2009, 'Comparison of three clinical techniques for the diagnosis of laryngeal paralysis in dogs', Veterinary Surgery 38, 434-438. http://dx.doi.org/10.1111/j.1532-950X.2009.00506.x, PMid:19538662 [ Links ]
Rice, D.H., 1982, 'Laryngeal reinnervation', Laryngoscope 92, 1049-1059. http://dx.doi.org/10.1288/00005537-198209000-00016, PMid:7121159 [ Links ]
Ridyard, A.E., Corcoran, B.M., Tasker, S., Willis, R., Welsh, E.M., Demetriou, J.L. et al., 2000, 'Spontaneous laryngeal paralysis in four white-coated German shepherd dogs', Journal of Small Animal Practice 41, 558-561. http://dx.doi.org/10.1111/j.1748-5827.2000.tb03153.x, PMid:11138855 [ Links ]
Ross, J.T., Matthiesen, D.T., Noone, K.E. & Scavelli, T.A., 1991, 'Complications and long-term results after partial laryngectomy for the treatment of idiopathic laryngeal paralysis in 45 dogs', Veterinary Surgery 20, 169-173. http://dx.doi.org/10.1111/j.1532-950X.1991.tb00330.x, PMid:1853548 [ Links ]
Rudorf, H., Barr, F.J. & Lane, J.G., 2001, 'The role of ultrasound in the assessment of laryngeal paralysis in the dog', Veterinary Radiology and Ultrasound 42, 338-343. http://dx.doi.org/10.1111/j.1740-8261.2001.tb00949.x, PMid:11499709 [ Links ]
Schofield, D.M., Norris, J. & Sadanaga, K.K., 2007, 'Bilateral thyroarytenoid cartilage lateralization and vocal fold excision with mucosoplasty for treatment of idiopathic laryngeal paralysis: 67 dogs (1998-2005)', Veterinary Surgery 36(6), 519-525. http://dx.doi.org/10.1111%2Fj.1532-950X.2007.00302.x [ Links ]
Shelton, G.D., 2002, 'Myasthenia gravis and disorders of neuromuscular transmission', Veterinary Clinics of North America: Small Animal Practice 32(1), 189-206. [ Links ]
Smith, M.M., 2000, 'Diagnosing laryngeal paralysis', Journal of the American Animal Hospital Association 36, 383-384. PMid:10997511 [ Links ]
Snelling, S.R. & Edwards, G.A., 2003, 'A retrospective study of unilateral arytenoid lateralisation in the treatment of laryngeal paralysis in 100 dogs (1992-2000)', Australian Veterinary Jouranl 81, 464-468. http://dx.doi.org/10.1111/j.1751-0813.2003.tb13361.x, PMid:15086080 [ Links ]
Stanley, B.J., Hauptman, J.G., Fritz, M.C., Rosenstein, D.S. & Kinns, J., 2010, 'Esophageal dysfunction in dogs with idiopathic laryngeal paralysis: A controlled cohort study', Veterinary Surgery 39, 139-149. http://dx.doi.org/10.1111/j.1532-950X.2009.00626.x, PMid:20210960 [ Links ]
Tobias, K.M., Jackson, A.M. & Harvey, R.C., 2004, 'Effects of doxapram HCl on laryngeal function of normal dogs and dogs with naturally occurring laryngeal paralysis', Veterinary Anaesthesia and Analgesia 31, 258-263. http://dx.doi.org/10.1111/j.1467-2995.2004.00168.x, PMid:15509290 [ Links ]
Trout, N.J., Harpster, N.K., Berg, J. & Carpenter, J., 1994, 'Long-term results of uliateral ventriculocordectomy and partial arytenoidectomy for the treatment of laryngeal paralysis in 60 dogs', Journal of the American Animal Hospital Association 30, 401-407. [ Links ]
Turner, D.M. & Ilkiw, J.E., 1990, 'Cardiovascular and respiratory effects of three rapidly acting barbiturates in dogs', American Journal of Veterinary Research 51, 598604. PMid:2327623 [ Links ]
Venker-van Haagen, A.J., 1982, 'Laryngeal paralysis in bouviers Belge des Flandres and breeding advice to prevent this condition', Tijdschrift voor Diergeneeskunde 107, 21-22. PMid:7054920 [ Links ]
Weinstein, J. & Weisman, D., 2010, 'Intraoperative evaluation of the larynx following unilateral arytenoid lateralization for acquired idiopathic laryngeal paralysis in dogs', Journal of the American Animal Hospital Association 46, 241-248. PMid:20610696 [ Links ]
White, R.A.S., 1989, 'Unilateral arytenoid lateralisation: An assessment of technique and long term results in 62 dogs with laryngeal paralysis', Journal of Small Animal Practice 30, 543-549. http://dx.doi.org/10.1111/j.1748-5827.1989.tb01469.x [ Links ]
Yeon, S.C., Lee, H.C., Chang, H.H. & Lee, H.J., 2005, 'Sound signature for identification of tracheal collapse and laryngeal paralysis in dogs', Journal of Veterinary Medical Science 67, 91-95. http://dx.doi.org/10.1292/jvms.67.91, PMid:15699602 [ Links ]
Zikes, C. & McCarthy, T., 2012, 'Bilateral ventriculocordectomy via ventral laryngotomy for idiopathic laryngeal paralysis in 88 dogs', Journal of the Amercian Animal Hospital Association 48(4), 234-244. http://dx.doi.org/10.5326%2FJAAHA-MS-5751 [ Links ]
 Correspondence:
Correspondence:
Adriaan Kitshoff
Postal address: 133 Salisbury Avenue
Merelbeke, Ghent 9820, Belgium
Email: adriaan.kitshoff@ugent.be
Received: 24 July 2012
Accepted: 18 Dec. 2012
Published: 05 Apr. 2013














