Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.84 n.1 Pretoria Jan. 2013
CLINICAL COMMUNICATIONS
Canine spirocercosis-associated extraskeletal osteosarcoma with central nervous system metastasis
Paolo PazziI; Samantha TompkinsII; Robert M. KirbergerI
IDepartment of Companion Animal Clinical Studies, University of Pretoria, South Africa
IIDepartment of Paraclinical studies, University of Pretoria, South Africa
ABSTRACT
A five-year-old male Boerboel presented for examination, collapsed for an unknown period of time. On clinical examination, multifocal subcutaneous masses and enlarged prescapular lymph nodes as well as neurological deficits that suggested a multifocal neurological syndrome were found. Fine needle aspirates of the prescapular lymph nodes revealed cells suggestive of osteosarcoma. Radiographs showed foci of mineralisation within the soft tissue masses as well as diffuse pulmonary metastasis and a caudodorsal mediastinal mass believed to be a Spirocerca lupi nodule. Computed tomography imaging, necropsy and histopathology confirmed S. lupi oesophageal neoplastic transformation (extraskeletal osteosarcoma), believed to be the primary lesion, and the majority of secondary metastasis to the brain, spine, heart, multiple muscular groups and abdominal organs. This is the first known report of extraskeletal osteosarcoma metastasis to the brain and spinal cord in a dog.
Introduction
Spirocerca lupi is a nematode that occurs in tropical, subtropical and temperate climates, with dogs as the final host. After ingestion, the larvae penetrate the dog's gastric mucosa, migrate within the walls of the gastroepiploic arteries to the cranial abdominal aorta and continue cranially to the caudal thoracic aorta. Here maturation occurs within approximately three months, before the worms finally migrate through the mediastinum to the submucosa or muscular layer of the oesophageal wall. A fibrous nodule forms around the worms (Dvir, Clift & Williams 2010) and is visible three to nine months after larval ingestion (Bailey, Cabrera & Diamond 1963). Pathognomonic radiographic findings for spirocercosis includes, amongst others, caudal thoracic spondylitis together with a caudodorsal mediastinal soft tissue mass (Dvir, Kirberger & Malleczek 2001; Mazaki-Tovi et al. 2002).
Malignant oesophageal neoplasia in non-endemic spirocercosis areas is very rare (< 0.5% of all neoplasia cases) and does not typically include sarcoma (Ridgway & Suter 1979). Neoplastic transformation of the oesophageal nodule induced by S. lupi is a relatively common finding, with up to 26% of clinical cases becoming neoplastic (Dvir et al. 2001). Although extraskeletal osteosarcoma (ESO) is rare in dogs, oesophageal ESO is the most common type of neoplasia associated with spirocercosis; however, fibrosarcoma, undifferentiated sarcoma and chondrosarcoma also occur (Bailey et al. 1963; Lindsay, Kirberger & Williams 2010; Ranen et al. 2004; Wandera 1976). Sarcomas induced by S. lupi have been reported to metastasise to pulmonary, renal, gastric, adrenal and cardiac sites, as well as to the tongue and lymph nodes (Bailey 1972; Ranen et al. 2004).
Osteosarcomas are classified as skeletal or extraskeletal, with skeletal osteosarcoma metastasis to the central nervous system reported rarely in humans and dogs (Marina et al. 1993; McNeill et al. 2007; Spodnick et al. 1992; Stefanowicz et al. 2011). Primary sites of ESO in dogs, other than the oesophagus, include mammary tissue, subcutaneous tissue, the spleen, intestine, the liver, kidneys, testicles, the vagina, eyes, synovia, the omentum, adrenal glands and meninges (JiHyun et al. 2007; Kuntz et al. 1998; Misdorp et al. 1971; Patnaik 1990; Patnaik, Liu & Johnson 1976; Ringenberg, Neitzel & Zachary 2000; Salm & Mayes 1969; Schena et al. 1989; Turnwald, Smallwood & Helman 1979) but not the central nervous system to date. The most common sites of metastasis of ESO include the liver, lungs, local lymph nodes and the omentum, and occasionally the kidneys and heart (Kuntz et al. 1998; Patnaik 1990). In humans, metastasis of ESO to the brain has been reported only twice (Bindal et al. 1994; Salm 1959).
To our knowledge, this is the first report describing ESO metastasis to the brain and spinal cord in canines and only the third reported in any species. This case also involved extensive metastasis to the heart, subcutaneous tissue, musculature, lungs, lymph nodes and multiple abdominal organs.
Case history
A five-year-old intact male Boerboel presented in a state of collapse, the duration of which was unclear. Clinical examination revealed normal vital parameters, multifocal thoracic subcutaneous masses, severely enlarged prescapular lymph nodes and firm, swollen left semimembranous and semitendinous muscles. Findings on neurological examination included tetraparesis, head tilt to the right, bilateral rotary nystagmus, mydriatic pupils, bilateral decreased facial sensation and hyporeflexia of all limbs except for the left patellar reflex, which was hyperreflexic. No deep pain sensation was present in the right limbs and only superficial pain was present in the left limbs. A multifocal neurological syndrome was suspected.
Haematology showed a mild normocytic, hypochromic and slightly regenerative anaemia (haematocrit = 0.26 L/L, reference range 0.37 L/L - 0.55 L/L; mean corpuscular haemoglobin content = 31 g/dL, reference range 32 g/dL - 36 g/dL), moderate left-shift neutrophilia (mature neutrophils = 30.7 χ 109/L, reference range 3.0 χ 109/L - 11.5 χ 109/L; immature neutrophils = 2.8 χ 107L, reference range 0.0 χ 109/L - 0.5 χ 107L) and mild thrombocytosis (532 χ 109/L, reference range 200 χ 109/L - 500 χ 107L). Biochemistry revealed mild hypoalbuminaemia (19 g/L, reference range 27 g/L - 35 g/L). Fine needle aspirates of both prescapular lymph nodes indicated cells consistent with osteocytes and osteoblasts, suggestive of osteosarcoma.
Thoracic radiographs (right and left lateral and ventrodorsal views) were taken whilst the patient was conscious. Radiological abnormalities included spondylitis of five caudal thoracic vertebrae and an extensive nodular lung pattern, with nodules ranging from 3 mm to 40 mm in diameter, as well as a 60 mm diameter caudodorsal mediastinal soft tissue mass. Subcutaneous soft tissue opacities containing amorphous bone bilaterally without visible underlying rib involvement were present on dorsoventral skyline views of the left and right ribs centred at the level of the fifth intercostal space. Shoulder radiographs allowed visualisation of bilaterally enlarged centrally mineralised prescapular lymph nodes and additional soft tissue opacities medial to the right scapula and caudolateral to the left mid-humerus, with the latter showing central mineralisation. A mediolateral left femoral view revealed severe soft tissue swelling involving the entire caudal and proximal-cranial aspect of the left femur and contained poorly and inhomogeneously marginated to well-marginated central amorphous new bone. The caudal mid-femoral diaphysis had a 60 mm long thick, brush-like periosteal reaction.
Owing to the poor prognosis the patient was euthanased. This was followed by a whole-body helical computed tomography (CT) scan (Siemens Emotion Duo, Siemens, Germany), primarily for academic purposes. The subcutaneous and thoracic nodules found clinically and on the radiographs were all identified on the CT scan, as well as some additional nodules (e.g. a left temporal subcutaneous nodule of 3 cm diameter [Figure 1]). Most of these nodules had varying degrees of central mineralisation. In the superficial area of the left temporal lobe there was a well-marginated, round, mildly hyperattenuating (Hounsfield units 56) nodule of 20 mm diameter (Figure 1). Additional thoracic and abdominal changes seen by CT included extensive thoracic and abdominal aortic mineralisation (Figure 2 and Figure 3), two mineralised nodules (6 mm and 12 mm in diameter, respectively) in the interventricular septum, mineralised 20 mm diameter nodules associated with the pylorus (Figure 3), and an 8 mm mineralised nodule in the caudal pole of the left kidney. A caudal oesophageal soft tissue nodule was extensively mineralised (Figure 3).
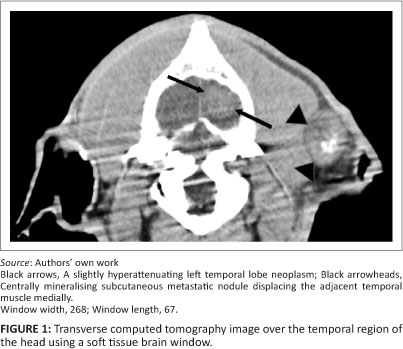

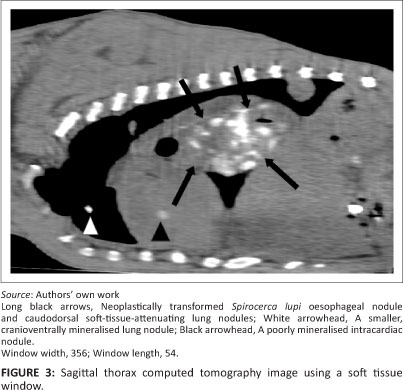
A complete necropsy was performed and showed a cauliflower-like neoplastic caudal oesophageal S. lupi nodule, which was believed to be the primary site of neoplasia, and a smaller, smooth and slightly more caudal nodule that contained live S. lupi worms. A well-circumscribed expansile mass that was almost indistinguishable from the compressed cortical tissue was seen macroscopically within the brain. Suspected sites of central nervous system metastasis, in addition to those seen on the CT scan, included four firm, well-circumscribed nodules (3 mm - 10 mm in diameter) in the spinal cord. Sites of suspected metastasis outside the central nervous system correlated with those seen on the CT scan and included the lungs, bilateral ribs, the myocardium, the gastrointestinal tract, kidneys, the pancreas, the left and right triceps and femoral muscles, peripheral lymph nodes and multiple subcutaneous sites.
Histopathology of all the nodules confirmed diffuse multifocal metastatic osteosarcomas, with the primary lesion believed to have originated from the oesophageal S. lupi nodule. Within the temporal lobe, several small foci of malacic brain tissue were all that remained of the neural tissue in the area. The origin of the tumour appeared to be associated with blood vessels. It subsequently grew within the brain parenchyma and was characterised by loosely associated yet densely packed spindle-shaped osteoblasts, together with multi-nucleated osteoclasts. Osteoid was a prominent feature, with several small spicules of mineralised osteoid forming bone. Histopathology of a representative sample of a spinal nodule at the junction of the grey and white matter showed a well-circumscribed metastatic focus, characterised by typical large, spindle-shaped osteoblasts, with round to oval vesicular nuclei and prominent single nucleoli. Homogenous eosinophilic osteoid was prominent between the cells.
Discussion
The pathognomonic radiographic findings of a caudodorsal mediastinal soft tissue mass, caudal thoracic vertebral spondylitis in conjunction with multifocal soft tissues opacities within the lung parenchyma and extrapulmonary mineralised masses (diffuse osteosarcomas based on cytology) led to the clinical diagnosis of metastatic osteosarcoma secondary to spirocercosis. Other differentials considered included pulmonary abscessation and granulomatous or fungal disease, but these seemed unlikely. The CT and necropsy findings explained the clinical status of the dog, confirmed the diagnosis and supported the multifocal neurological syndrome described. Essential to clinical diagnosis were the thoracic radiographs and cytology of the prescapular lymph nodes.
The frequency of oesophageal neoplastic transformation induced by S. lupi, together with confirmation of the S. lupi nodule as an osteosarcoma, supports but does not confirm the suspicion of the S. lupi nodule as the primary neoplastic site. In this case, some degree of mineralisation was visualised radiographically in a number of metastatic soft tissue nodules, but the absence of radiographically visible mineralisation does not exclude the possibility of osteosarcoma (Kuntz et al. 1998). Extraskeletal osteosarcomas are generally more aggressive than skeletal osteosarcomas and may explain the widespread metastasis.
Extraskeletal osteosarcomas are rare in humans and animals, but in areas where spirocercosis is endemic neoplastic transformation of S. lupi nodules to an osteosarcoma is found frequently (Dvir et al. 2008; Van der Merwe et al. 2008). The pathogenesis of S. lupi neoplastic transformation is unclear, but two leading hypotheses to explain the infection-associated neoplastic transformations exist. These include (1) uncontrolled local inflammation leading to genetic instabilities and malignant transformations (Vennervald & Polman 2009) and (2) that the transformations are caused by the parasite itself, most likely in conjunction with the inflammatory response it produces (Kaewpitoon et al. 2008; Mulvenna et al. 2010; Smout et al. 2009). The most common site of ESO metastasis induced by S. lupi is the lungs (Ranen et al. 2004), whilst uncommonly reported sites of metastasis include the kidneys, regional lymph nodes, the stomach, the spleen, the pancreas, adrenal glands, the heart and the tongue (Dvir et al. 2008; Ranen et al. 2004; Ranen et al. 2008).
Neurological signs previously associated with aberrant migration of S. lupi larvae or metastatic neoplastic transformation include paraparesis, seizures, unilateral hindlimb lameness and hindlimb paralysis mimicking intervertebral disk disease (Du Plessis, Keller & Millward 2007; Dvir et al. 2001; Lindsay et al. 2010; Mazaki-Tovi et al. 2002). Metastasis to the spinal cord from S. lupi neoplastic transformation has been reported only as a result of a low-grade chondrosarcoma (Lindsay et al. 2010). The neurological findings presented here indicate that in endemic areas spirocercosis should be on the list of differential diagnoses in dogs displaying focal or multifocal neurological syndrome.
Conclusion
This is the first report of an extraskeletal osteosarcoma associated with S. lupi, with severe diffuse metastasis to soft tissues, including the brain. Other than two cases described in humans (Bindal et al. 1994; Salm 1959), metastasis to the brain has now been described for the first time in another species.
Acknowledgements
Competing interests
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Authors' contributions
P.P. (University of Pretoria) was the veterinary clinician responsible for this clinical case and wrote the case report. R.M.K. (University of Pretoria) provided intellectual and practical contributions regarding the imaging study and writing of the description. S.T. (University of Pretoria) was responsible for pathology descriptions and review of the article content.
References
Bailey, W.S., 1972, 'Spirocerca lupi: a continuing inquiry', Journal of Parasitology 58, 3-22. http://dx.doi.org/10.2307/3278233, PMid:5012526 [ Links ]
Bailey, W.S., Cabrera, D.J. & Diamond, D.L., 1963, 'Beetles of the family Scarabaeidae as intermediate hosts for Spirocerca lupi', Journal of Parasitology 49, 485-488. http://dx.doi.org/10.2307/3275823, PMid:13969028 [ Links ]
Bindal, R.K., Sawaya, R.E., Leavens, M.E., Taylor, S.H. & Guinee, V.F., 1994, 'Sarcoma metastatic to the brain: results of surgical treatment', Neurosurgery 35, 185-190. http://dx.doi.org/10.1227/00006123-199408000-00002, PMid:7969824 [ Links ]
Du Plessis, C.J., Keller, N. & Millward, I.R., 2007, 'Aberrant extradural spinal migration of Spirocerca lupi: four dogs', Journal of Small Animal Practice 48, 275-278. http://dx.doi.org/10.1111/j.1748-5827.2006.00262.x, PMid:17425698 [ Links ]
Dvir, E., Clift, S.J. & Williams, M.C., 2010, 'Proposed histological progression of the Spirocerca lupi-induced oesophageal lesion in dogs', Veterinary Parasitology 168, 71-77. http://dx.doi.org/10.1016/jJ.vetpar.2009.10.023, PMid:19963322 [ Links ]
Dvir, E., Kirberger, R.M. & Malleczek, D., 2001, 'Radiographic and computed tomographic changes and clinical presentation of spirocercosis in the dog', Veterinary Radiology and Ultrasound 42, 119-129. http://dx.doi.org/10.1111/j.1740-8261.2001.tb00914.x, PMid:11327359 [ Links ]
Dvir, E., Kirberger, R.M., Mukorera, V., Van der Merwe, L.L. & Clift, S.J., 2008, 'Clinical differentiation between dogs with benign and malignant spirocercosis', Veterinary Parasitology 155, 80-88. http://dx.doi.org/10.1016/j.vetpar.2008.04.006, PMid:18534758 [ Links ]
JiHyun, H., YoungHwan, G., BoKyung, B., MiHyeon, Y., EulSoo, C., DaeYong, K. et al., 2007, 'Extraskeletal osteosarcoma of the mammary gland in a dog', Journal of Veterinary Clinics 24, 663-666. [ Links ]
Kaewpitoon, N., Kaewpitoon, S.J., Pengsaa, P. & Sripa, B., 2008, 'Opisthorchis viverrini: the carcinogenic human liver fluke', World Journal of Gastroenterology 14, 666 674. http://dx.doi.org/10.3748/wjg.14.666, PMid:18205254 [ Links ]
Kuntz, C.A., Dernell, W.S., Powers, B.E. & Withrow, S., 1998, 'Extraskeletal osteosarcomas in dogs: 14 cases', Journal of the American Animal Hospital Association 34, 26-30. PMid:9527426 [ Links ]
Lindsay, N., Kirberger, R.M. & Williams, M., 2010, 'Imaging diagnosis - spinal cord chondrosarcoma associated with spirocercosis in a dog', Veterinary Radiology and Ultrasound 51, 614-616. http://dx.doi.org/10.1111/j.1740-8261.2010.01718.x, PMid:21158232 [ Links ]
Marina, N.M., Pratt, C.B., Shema, S.J., Brooks, T., Rao, B. & Meyer, W.H., 1993, 'Brain metastases in osteosarcoma. Report of a long-term survivor and review of the St. Jude Children's Research Hospital experience', Cancer 71, 3656-3660. http://dx.doi.org/10.1002/1097-0142(19930601)71:11<3656:AID-CNCR2820711130> 3.0.CO;2-L [ Links ]
Mazaki-Tovi, M., Baneth, G., Aroch, I., Harrus, S., Kass, P.H., Ben-Ari, T. et al., 2002, 'Canine spirocercosis: clinical, diagnostic, pathologic, and epidemiologic characteristics', Veterinary Parasitology 107, 235-250. http://dx.doi.org/10.1016/S0304-4017(02)00118-8 [ Links ]
McNeill, C.J., Overley, B., Shofer, F.S., Kent, M.S., Clifford, C.A., Samluk, M. et al., 2007, 'Characterization of the biological behaviour of appendicular osteosarcoma in Rottweilers and a comparison with other breeds: a review of 258 dogs', Veterinary and Comparative Oncology 5, 90-98. http://dx.doi.org/10.1111/j.1476-5829.2006.00116.x, PMid:19754792 [ Links ]
Misdorp, W., Cotchin, E., Hampe, J.F., Jabara, A.G. & Von Sandersleben, J., 1971, 'Canine malignant mammary tumours', Veterinary Pathology 8, 99-117. PMid:4367432 [ Links ]
Mulvenna, J., Sripa, B., Brindley, P.J., Gorman, J., Jones, M.K., Colgrave, M.L. et al., 2010, 'The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini', Proteomics 10, 1063-1078. PMid:20049860 [ Links ]
Patnaik, A.K., 1990, 'Canine extraskeletal osteosarcoma and chondrosarcoma: a clinicopathologic study of 14 cases', Veterinary Pathology 27, 46-55. http://dx.doi.org/10.1177/030098589002700107, PMid:2309381 [ Links ]
Patnaik, A.K., Liu, S. & Johnson, G.F., 1976, 'Extraskeletal osteosarcoma of the liver in a dog', Journal of Small Animal Practice 17, 365-370. http://dx.doi.org/10.1111/j.1748-5827.1976.tb06972.x, PMid:1065784 [ Links ]
Ranen, E., Dank, G., Lavy, E., Perl, S., Lahav, D. & Orgad, U., 2008, 'Oesophageal sarcomas in dogs: histological and clinical evaluation', Veterinary Journal 178, 78-84. http://dx.doi.org/10.1016/j.tvjl.2007.06.024, PMid:17804268 [ Links ]
Ranen, E., Lavy, E., Aizenberg, I., Perl, S. & Harrus, S., 2004, 'Spirocercosis-associated esophageal sarcomas in dogs: A retrospective study of 17 cases (1997 2003)', Veterinary Parasitology 119, 209-221. http://dx.doi.org/10.1016/j.vetpar.2003.10.023, PMid:14746980 [ Links ]
Ridgway, R.L. & Suter, P.F., 1979, 'Clinical and radiographic signs in primary and metastatic esophageal neoplasms of the dog', Journal of the American Veterinary Medical Association 174, 700-704. PMid:429231 [ Links ]
Ringenberg, M.A., Neitzel, L.E. & Zachary, J.F., 2000, 'Meningeal osteosarcoma in a dog', Veterinary Pathology 37, 653-655. http://dx.doi.org/10.1354/vp.37-6-653, PMid:11105956 [ Links ]
Salm, R., 1959, 'A case of primary osteogenic sarcoma of extraskeletal soft tissues', British Journal of Cancer 13, 614-617. http://dx.doi.org/10.1038/bjc.1959.66, PMid:14441040 [ Links ]
Salm, R. & Mayes, S.E., 1969, 'Retroperitoneal osteosarcoma in a dog', Veterinary Record 85, 651-653. PMid:5261205 [ Links ]
Schena, C.J., Stickle, R.L., Dunstan, R.W., Trapp, A.L., Reimann, K.A., White, J.V. et al., 1989, 'Extraskeletal osteosarcoma in two dogs', Journal of the American Veterinary Medical Association 194, 1452-1456. PMid:2722641 [ Links ]
Smout, M.J., Laha, T., Mulvenna, J., Sripa, B., Suttiprapa, S., Jones, A. et al., 2009, 'A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells', PLoS Pathogens 5, e1000611, viewed 18 February 2012, from http://www.plospathogens.org/article/info%3Adoi%2F10.1371%2Fjournal.ppat.1000611 [ Links ]
Spodnick, G.J., Berg, J., Rand, W.M., Schelling, S.H., Couto, G., Harvey, H.J. et al., 1992, 'Prognosis for dogs with appendicular osteosarcoma treated by amputation alone - 162 cases (1978-1988)', Journal of the American Veterinary Medical Association 200, 995-999. PMid:1577656 [ Links ]
Stefanowicz, J., Izycka-Swieszewska, E., Szurowska, E., Bien, E., Szarszewski, A., Liberek, A. et al., 2011, 'Brain metastases in paediatric patients - characteristics of a patient series and review of the literature', Folia Neuropathologica 49, 271-281. PMid:22212917 [ Links ]
Turnwald, G.H., Smallwood, J.E. & Helman, R.G., 1979, 'Esophageal osteosarcoma in a dog', Journal of the American Veterinary Medical Association174, 1009-1011. PMid:285065 [ Links ]
Van der Merwe, L.L., Kirberger, R.M., Clift, S., Williams, M., Keller, N. & Naidoo, V., 2008, 'Spirocerca lupi infection in the dog: a review', Veterinary Journal 176, 294309. http://dx.doi.org/10.1016/j.tvjl.2007.02.032, PMid:17512766 [ Links ]
Vennervald, B.J. & Polman, K., 2009, 'Helminths and malignancy', Parasite Immunology 31, 686-696. http://dx.doi.org/10.1111/j.1365-3024.2009.01163.x, PMid:19825108 [ Links ]
Wandera, J.G., 1976, 'Further observations on canine spirocercosis in Kenya', Veterinary Record 99, 348-351. http://dx.doi.org/10.1136/vr.99.18.348, PMid:1069399 [ Links ]
 Correspondence:
Correspondence:
Paolo Pazzi
Postal address: Private Bag X04
Onderstepoort 0110, South Africa
Email: paolo.pazzi@up.ac.za
Received: 19 Apr. 2012
Accepted: 26 Nov. 2012
Published: 24 Apr. 2013














