Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.84 n.1 Pretoria Jan. 2013
CLINICAL COMMUNICATION
Schmallenberg virus - Is it present in South Africa?
Rhoda Leask; Albertha M. Botha; Gareth F. Bath
Department of Production Animal Studies, University of Pretoria, Onderstepoort campus, South Africa
ABSTRACT
In July 2006, a case of two out of three lambs born to one ewe in a flock of 45 had signs that, in retrospect, were possibly consistent with Schmallenberg virus infection. This occurred in the Onderstepoort area (Gauteng Province) but a definitive diagnosis was not made. Then, in May 2008, a farmer in the Delmas area (Mpumalanga Province) reported that deformed lambs had been born to several ewes in the flock. Six of the approximately 50 mated ewes gave birth to lambs showing varying degrees of arthrogryposis, torticollis, kyphosis, mandibular brachygnathia and hydrocephalus. Of these, only two were born alive but they died within a few hours. Blood was collected from the ewes with deformed lambs, a random sample of ewes that had given birth to normal lambs and a lamb that was normal but had a twin that was deformed. The samples were tested for Wesselsbron and Akabane antibodies using a complement fixation test and a haemagglutination/haemagglutination inhibition test that were available at that time. Bluetongue virus antibodies were also tested for using a commercial Enzyme-linked immunosorbent assay (ELISA) test. All samples showed negative results for all diseases tested. At the time Rift Valley fever virus had not been diagnosed in that region for many years and so it was not included in the testing. It is unlikely that this was the cause as no liver pathology was detected on postmortem examination of the lambs and no adult ewes had died. The farmer reported that another farm just a few kilometres away experienced the same deformities in some of their lambs but this farm was not investigated. During investigation it was thought that the cause was possibly a new strain of Akabane virus, although there was no way to confirm it. However, with the recent discovery of the Schmallenberg virus, it is possible that this virus has been present in South Africa for at least the last four years without being identified.
Introduction
The recently identified 'Schmallenberg virus' has gained much prominence in 2011 and subsequently (Animal Health and Veterinary Laboratory Agency [AHVLA] 2012; Bilk et al. 2012; Department for Environment, Food and Rural Affairs [DEFRA] 2012a, 2012b; Hoffmann et al. 2012; Lievaart-Peterson et al. 2012; Ministry for Primary Industries [MPI] 2012; Van den Brom et al. 2012; Veterinary Laboratories Agency [VLA] 2012; World Organisation for Animal Health [OIE] 2012). Unfortunately, little is known about the virus at this stage. The OIE technical factsheet (2012) identifies the causative agent as an enveloped, negative-sense, segmented, single-stranded RNA virus. The Friedrich-Loeffler-Institut (FLI) classified the virus as belonging to the Bunyaviridae family, Orthobunyavirus, related to Simbu serogroup viruses (Bilk et al. 2012; DEFRA 2012a; Lievaart-Peterson et al. 2012; OIE 2012; VLA 2012). However, Lievaart-Peterson et al. (2012) stated that classification of the virus has not yet been acknowledged by the International Committee on Taxonomy of Viruses. These viruses occur in Asia, Africa, Australia (Lievaart-Peterson et al. 2012; Parsonson, Della-Porta & Snowdon 1977; VLA 2012) and Israel (Parsonson et al. 1977) and include, especially, the Shamonda, Akabane (DEFRA 2012b; OIE 2012) and Aino (OIE 2012) viruses.
The Schmallenberg virus was first identified in Germany in November 2011 (Bilk et al. 2012; DEFRA 2012a, 2012b; Hoffmann et al. 2012; Lievaart-Peterson et al. 2012; OIE 2012; VLA 2012).
Subsequently, it has been identified in the Netherlands (Bilk et al. 2012; DEFRA 2012a, 2012b; OIE 2012; Van den Brom et al. 2012; VLA 2012), the United Kingdom (AHVLA 2012; Bilk et al. 2012; DEFRA 2012a, 2012b; OIE 2012; VLA 2012), France (Bilk et al. 2012; DEFRA 2012b; OIE 2012; VLA 2012), Luxembourg and Italy (OIE 2012; VLA 2012), Belgium (DEFRA 2012a, 2012b; VLA 2012) and Spain (Bilk et al. 2012).
The virus is believed to be transmitted by arthropod vectors, including midges (Bilk et al. 2012; DEFRA 2012a, 2012b; OIE 2012; VLA 2012), mosquitoes (Bilk et al. 2012; DEFRA 2012b) and ticks (DEFRA 2012a), although further studies are required. Vertical transmission to calves, lambs and kids has been proven (OIE 2012). Direct transmission is unlikely (OIE 2012). Hosts include cattle, sheep, goats (AHVLA 2012; DEFRA 2012a, 2012b; OIE 2012; VLA 2012) and bison (OIE 2012).
The disease is usually inapparent (OIE 2012), although, in the acute phase, may include the following clinical signs: fever (DEFRA 2012a; VLA 2012) greater than 40 °C (DEFRA 2012b; OIE 2012), impaired general condition or loss of body condition, anorexia or inappetence (OIE 2012; VLA 2012), decreased milk yield (DEFRA 2012a; OIE 2012), in some cases up to 50% (VLA 2012) and diarrhoea (DEFRA 2012a, 2012b; OIE 2012; VLA 2012). Recovery occurs within a few days (individual animals) and flock recovery within 2-3 weeks (OIE 2012; VLA 2012). Deformed and stillborn calves, lambs and kids show the following macroscopic lesions: deformity of the jaw (DEFRA 2012b), brachygnathia inferior, arthrogryposis, ankylosis, torticollis, scoliosis (Bilk et al. 2012; OIE 2012) hydrocephaly (OIE 2012), stiff neck (DEFRA 2012b). Live neonates also show some of the following signs: ataxia (DEFRA 2012b; VLA 2012), feeding difficulty, hyperexcitability (DEFRA 2012b) or fits (VLA 2012), exaggerated movements, flaccid paralysis (DEFRA 2012b) or recumbency (VLA 2012). Other, less common, deformities include blindness (DEFRA 2012b; VLA 2012), hydranencephaly (Bilk et al. 2012; DEFRA 2012b; VLA 2012), hypoplasia of the central nervous system, porencephaly, and subcutaneous oedema in calves (OIE 2012).
Differential diagnoses in adult ruminants include bluetongue virus (OIE 2012; Van der Sluijs et al. 2011), epizootic hemorrhagic disease (EHD) virus (OIE 2012), foot and mouth disease (FMD) virus, Wesselsbron disease (Merck Veterinary Manual 2012), bovine viral diarrhoea (BVD), Border disease and other pestiviruses, bovine herpes virus 1 and other herpes viruses, Rift Valley fever virus, bovine ephemeral fever virus, and toxic substances (OIE 2012). Differential diagnoses for the deformities in offspring include genetic factors (OIE 2012; Parsonson et al. 1977), Wesselsbron disease (Merck Veterinary Manual 2012), bluetongue virus (OIE 2012; Van der Sluijs et al. 2011), pestivirus, other viruses of the Simbu group (e.g. Akabane virus), and toxic substances (OIE 2012; Parsonson et al. 1977).
Identification of the infective agent is by real time polymerase chain reaction (RT-PCR) (Bilk et al. 2012; OIE 2012) developed by the FLI (Bilk et al. 2012; Lievaart-Peterson et al. 2012; VLA 2012), as well as by cell culture isolation of the virus. Serological tests on serum samples include indirect immunofluorescence, neutralisation test and ELISA (still being developed) (OIE 2012).
Schmallenberg virus is not yet a notifiable disease in the United Kingdom or the rest of Europe, but farmers and veterinarians are encouraged to report cases of stillborns, malformations and nervous signs (DEFRA 2012a, 2012b; MPI 2012; VLA 2012).
The following case histories from 2006 and 2008 describe incidences in retrospect of suspected Schmallenberg virus based on pathological and serological investigations in two neighbouring provinces in South Africa.
Case histories
Case 1
In July 2006, a Mutton Merino ewe from a small flock in the Onderstepoort area, Gauteng Province, gave birth to triplets (Figure 1).

The first lamb was born naturally and appeared to be normal. The ewe was then identified as having dystocia and the second and third lambs were delivered by caesarian section. The second lamb had torticollis and mandibular brachygnathia and died shortly after delivery. The third lamb showed kyphosis in the thoracic region, a less severe torticollis, mandibular brachygnathia and meconium staining, and was stillborn. This was the only ewe to give birth to deformed lambs in the flock of approximately 45 ewes and the case was not investigated further as vaccination records were up to date and there had been no sign of disease in the ewes during pregnancy. Parsonson et al. (1977) reported that a ewe that was infected with Akabane virus gave birth to twins, one normal and one deformed. Infection with an Akabane virus could therefore have been responsible in this case, although it was not confirmed.
Case 2
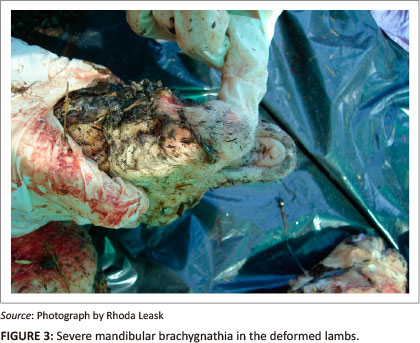
Two years later, in May 2008, a farmer in the Delmas region of the Mpumalanga Province reported that there was a lamb born with deformities. The deformities described by the farmer included a 'severely overshot jaw, bent stiff legs, and a bent neck'. The vaccination protocol of the farmer was well known and these ewes had been mated outside the normal breeding seasons. The farmer was questioned about bluetongue virus vaccinations and admitted having forgotten to vaccinate against bluetongue virus and so assumed this was the cause of the deformities. Two of the ewes had to be treated with antibiotics a few months prior to parturition and one of them gave birth to a deformed lamb. Approximately one week later, two more deformed lambs were born into the same flock and the farmer requested a postmortem examination at the Pathology Section, Faculty of Veterinary Science, University of Pretoria. These lambs had torticollis, arthrogryposis (one had kyphosis) (Figure 2), mandibular brachygnathia (Figure 3) and hydrocephalus. No other abnormalities were detected on macroscopic necropsy.

Microscopic examinations were not performed as they were unlikely to provide more valuable information. The possibility that anthelmintics or antimicrobials were responsible for the deformities was ruled out through investigation of history and records. During the course of the investigation, more lambs were born, two with deformities and one normal that died shortly after birth. From a flock of 350 ewes, of which 50 were pregnant, a total of six lambs were born with deformities. One of the ewes gave birth to twins, one normal and one deformed. Four of the six deformed lambs were stillborn and two died shortly after birth.
Blood samples were collected from five ewes that were identified by the farmer as having given birth to deformed lambs, eight ewes that were randomly selected (having given birth to normal lambs), and the surviving twin of a deformed lamb. One of these ewes was identified according to farm records as having received oxytetracycline (dosage and drug specifics not recorded) and had given birth to a deformed lamb. The blood samples were sent for detection of virus-specific antibodies against Akabane, Wesselsbron (using the complement fixation test [CFT] and haemagglutination/ haemagglutination inhibition [HA/HI]) and bluetongue (using a commercial ELISA test). Serum samples were not tested for Rift Valley fever antibodies as there was no liver (or other) pathology observed in dead lambs that indicated this was the cause of death and no adult sheep had shown clinical signs of the disease or died. Two years later, this farm had an outbreak of Rift Valley fever that was easily diagnosed on clinical signs and postmortem examinations. All the sera were negative for Wesselsbron, bluetongue and Akabane virus-specific antibodies.
Discussion
Deformities in lambs are not uncommon in South Africa (Aitkin 2007; Bath & De Wet 2001; Coetzer, Thomson & Tustin 1994; Radostits et al. 2000; Sherman & Smith 1994). Some differential diagnoses for the teratology seen include: bluetongue virus (particularly because of vaccination with modified-live vaccines), Wesselsbron disease, Rift Valley fever, Akabane, Middelburg virus and now Schmallenberg virus. Rift Valley fever was ruled out as a cause as there were no other pathological lesions, abortions or deaths to indicate that this could be the cause. Middelburg virus was also ruled out as a cause of disease in Case 2 as it is very rare and a group of 22 veterinarians and veterinary specialists in the sheep field from across the country have never seen or diagnosed it in small ruminants; of the group, only one pathologist had diagnosed it but only in horses. The blood samples were tested for Wesselsbron, bluetongue and Akabane. The CFT and HA/HI tests were the only serological assays available at that time to test for Wesselsbron and Akabane infections. Both these tests measure predominantly IgM (acute infection) and not IgG antibodies which would be present in transplacental infection in early pregnancy (Dr Truuske Gerdes, pers. comm., 09 May 2012). The CFT and HA/HI tests might have shown trace antibodies if present. The negative results of these tests do not therefore prove conclusively that the infection was absent. However, the absence of any other pathological lesions observed with Wesselsbron disease and absence of antibodies against bluetongue virus excludes these viruses as the likely cause.
At the time that these cases were investigated, it was presumed that the infectious agent was a new strain of Akabane virus because of the similarities in deformities, as well as the birth of the twins (one deformed and one normal), as reported previously by Parsonson et al. (1977). As some viruses, infections or toxic agents that cause teratology inflict damage some months before lambs are born, it is often difficult to determine the cause, especially for private practitioners, the more so when new pathogens are emerging that current diagnostic tests cannot identify. Unfortunately in these cases (having occurred 4-6 years ago), the sample material is no longer available to test whether or not it was the Schmallenberg virus. Currently, there are limited research data available on the Schmallenberg virus (Bilk et al. 2012; Hoffmann et al. 2012; OIE 2012) and most of the available information at this stage is electronic, anecdotal or in the form of media reports. There is a strong possibility, based on the history of this disease, with rapid detection in various European countries and the United Kingdom, that it could have been misdiagnosed or is undetected thus far in South Africa. As other diseases that are closely related to the Schmallenberg virus are present in South Africa, such as Akabane (OIE 2012), it is possible that it is already here and may have been present for some time, but cases and outbreaks have been wrongly attributed to the Akabane virus. Testing for the Schmallenberg virus is unfortunately not currently available in South Africa.
Conclusion
Identifying the cause of abortion or congenital malformations in livestock, especially viral agents, is notoriously difficult because the pathogen has usually disappeared at the time of birth or abortion. The presence of antibodies is also not definitive because infection could be incidental and precede or follow the pathological event, leading to malformations or abortions. Nevertheless, the current tests available for a range of viral agents make it possible to either effectively rule out or confirm the likelihood that they could be involved in the aetiology. These tests now include RT-PCR. Blood samples in ethylene-diamine-tetra-acetic acid (EDTA) and serum samples can be collected and submitted for laboratory testing. These tests should be used in investigations, as results could lead to actions appropriate to the virus identified being taken in time. If veterinarians use these tests consistently, we will have a better idea of the relative importance of viruses and whether more research is needed. This applies particularly to previously undiagnosed aetiological agents such as the Schmallenberg virus.
Acknowledgements
The authors would like to acknowledge Dr Truuske Gerdes for her contributions to the initial investigation on the Delmas farm in May 2008.
Competing interests
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this article.
Authors' contributions
A.M.B. (University of Pretoria) gathered information and references on the Schmallenberg virus. G.F.B. (University of Pretoria) assisted with editing and providing some references. R.L. (University of Pretoria) wrote the manuscript and contributed to gathering information and references.
References
Aitkin, I.D., 2007, Diseases of sheep, 4th edn., Blackwell, Oxford. [ Links ]
Animal Health and Veterinary Laboratory Agency (AHVLA), 2012, Archive for 'Schmallenberg', viewed 19 April 2012, from http://www.defra.gov.uk/ahvla/tag/Schmallenberg [ Links ]
Bath, G.F. & De Wet, J., 2001, Sheep and goat diseases, Tafelberg, Cape Town. [ Links ]
Bilk, S., Schulze, C., Fischer, M., Beer, M., Hlinak, A. & Hoffmann, B., 2012, 'Organ distribution of Schmallenberg virus RNA in malformed newborns', Veterinary Microbiology 159, 236-238. http://dx.doi.org/10.1016/j.vetmic.2012.03.035 [ Links ]
Coetzer, J.A.W., Thomson, G.R. & Tustin, R.C., 1994, Infectious diseases of livestock with special reference to southern Africa, Oxford University Press, Cape Town. [ Links ]
Department for Environment, Food and Rural Affairs (DEFRA), 2012a, Schmallenberg virus, viewed 19 April 2012, from http://www.defra.gov.uk/animal-diseases/a-z/schmallenberg-virus/ [ Links ]
Department for Environment, Food and Rural Affairs (DEFRA), 2012b, An update on Schmallenberg virus in northern Europe, Veterinary & science policy advice: International disease monitoring, VITT/1200 Schmallenberg virus in North Europe, 05 January 2012, viewed 19 April 2012, from http://www.defra.gov.uk/animal-diseases/files/poa-schmallenburg-update-120105.pdf [ Links ]
Hoffmann, B., Scheuch, M., Hoper, D., Jungblut, R., Holsteg, M., Schirrmeier, H. et al., 2012, 'Novel Orthobunyavirus in cattle, Europe, 2011', Emerging Infectious Diseases 18, 469-472. [ Links ]
Lievaart-Peterson, K., Luttikholt, S.J.M., Van den Brom, R. & Vellema, P., 2012, 'Schmallenberg virus infection in small ruminants - First review of the situation and prospects in northern Europe', Small Ruminant Research 106, 71-76. [ Links ]
Merck Veterinary Manual, 2012, Wesselsbron disease: Introduction, viewed 08 May 2012, from http://www.merckvetmanual.com/mvm/htm/bc/56600.htm [ Links ]
Ministry for Primary Industries (MPI), 2012, MAF monitoring Schmallenberg virus, viewed 08 May 2012, from http://www.mpi.govt.nz/news-resources/news/maf-monitoring-schmallenberg-virus [ Links ]
Parsonson, I.M., Della-Porta, A.J. & Snowdon, W.A., 1977, 'Congenital abnormalities in newborn lambs after infection of pregnant sheep with Akabane virus', Infection and Immunity 15, 254-262. [ Links ]
Radostits, O.M., Gay, C.C., Blood, D.C. & Hinchcliff, K.W., 2000, Veterinary medicine. A textbook of the diseases of cattle, sheep, pigs, goats and horses, 9th edn., Bailliere Tindall, London. [ Links ]
Sherman, D.M. & Smith, M.C., 1994, Goat medicine, Lea and Febiger, Philadelphia. [ Links ]
Van den Brom, R., Luttikholt, S.J.M., Lievaart-Peterson, K., Peperkamp, N.H.M.T., Mars, M.H., Van der Poel, W.H.M. et al., 2012, 'Epizootic of ovine congenital malformations associated with Schmallenberg virus infection', Tijdschrift voor Diergneeskunde 137, 106-111. [ Links ]
Van der Sluijs, M., Timmermans, M., Moulin, V., Vonk Noordegraaf, C., Vrijenhoek, M., Debyser, I. et al., 2011, 'Transplacental transmission of bluetongue virus serotype 8 in ewes in early and mid gestation', Veterinary Microbiology 149, 113-125. [ Links ]
Veterinary Laboratories Agency (VLA), 2012, Schmallenberg virus, viewed 10 April 2012, from http://vla.defra.gov.uk/science/sci_schmallenberg.htm [ Links ]
World Organisation for Animal Health (OIE), 2012, Technical factsheet: Schmallenberg virus, viewed 27 February 2012, from http://www.oie.int/fileadmin/Home/fr/Our_scientific_expertise/docs/pdf/ A_Schmallenberg_virus.pdf [ Links ]
 Correspondence to:
Correspondence to:
Rhoda Leask
Postal address: Private Bag X04
Onderstepoort 0010, South Africa
Email: rhoda.leask@up.ac.za
Received: 04 July 2012
Accepted: 30 Oct. 2012
Published: 12 Feb. 2013














