Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the South African Veterinary Association
versão On-line ISSN 2224-9435
versão impressa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.84 no.1 Pretoria Jan. 2013
ORIGINAL RESEARCH
Molecular detection of Yaba monkey tumour virus from a vervet monkey
Helene BrettschneiderI; Johan H. VosterII; Emily P. LaneI; Erna van WilpeIII; Peter BidenIV; Desire L. DaltonI, V; Antoinette KotzeI, V
IResearch and Scientific Services, National Zoological Gardens of South Africa, South Africa
IIVetdiagnostix, Pietermaritzburg, South Africa
IIIFaculty of Veterinary Science, University of Pretoria, South Africa
IVScottburgh Veterinary Clinic, Scottburgh, South Africa
VDepartment of Genetics, University of the Free State, South Africa
ABSTRACT
Yaba monkey tumour virus (YMTV) was first diagnosed in a colony of captive rhesus monkeys (Macaca mulatta) in Yaba, Nigeria. It has been implicated as the cause of cutaneous nodules in wild baboons (Papio species), rhesus monkeys (Macaca mulatta) and cynomolgus monkeys (Macaca fascicularis). This article reports a case of cutaneous pox lesions caused by YMTV in a free-ranging adult female vervet monkey (Chlorocebus pygerythrus) from the Umkomaas coastal area in South Africa. The virus was identified by molecular sequencing from fragments of the insulin metalloprotease-like protein and intracellular mature virion membrane protein as well as the DNA polymerase genes. Phylogenetic analyses of these gene regions revealed a 99% similarity of the sample to YMTV. Although human disease caused by YMTV is normally mild, it is recommended that persons in contact with non-human primates in the area of Umkomaas who develop cutaneous lesions should inform their doctors of the possibility of this infection. The extent and significance of the virus to human and non-human primates in South Africa are not known. To the authors' knowledge, this is the first diagnosis of YMTV in South Africa and in vervet monkeys.
Introduction
Poxviruses are a large family of complex, highly epitheliotropic deoxyribonucleic acid (DNA) viruses that cause cutaneous and systemic diseases in humans, birds and both free-ranging and domestic mammals. Most members of the family Poxviridae cause mild, localised skin disease from which the term 'pox' derives. Severe systemic disease may be caused by some poxviruses including sheeppox virus, fowlpox virus, ectromelia virus, monkeypox virus and the now eradicated human smallpox virus (variola virus). Economically important pox-viral diseases include: lumpy skin disease, which causes severe epidemics in cattle in southern Africa; sheeppox and goatpox, which cause serious losses in Africa, but are absent in southern Africa; and orf, which is a significant disease in sheep and goats worldwide. A few poxviruses cause hyperplastic or neoplastic conditions such as molluscum contagiosum in humans and horses, and Shope's fibroma of rabbits. Whilst many poxviruses are host specific, some affect a wide range of species, including cowpox virus and vaccinia virus, whilst others are zoonotic pathogens, for example pseudocowpox virus and the rare tanapox virus (Coetzer & Tustin 2004; Fenner 2000; Ginn, Mansell & Rakich 2007; Stich et al. 2002).
Vertebrate pox viruses are classified into eight genera, namely: Orthopoxvirus (including camelpox virus, cowpox virus, monkeypox virus and vaccinia virus); Parapoxvirus (including bovine papular stomatitis virus, orf virus, parapox virus of red deer, pseudocowpox virus); Avipoxvirus; Capripoxvirus (including goatpox virus, lumpy skin disease virus); Leporipoxvirus; Suipoxvirus; Molluscipoxvirus; and Yatapoxvirus (including tanapox virus and Yaba monkey tumour virus) (Ginn et al. 2007).
Tanapox virus is a mild, human cutaneous disease reported from Kenya, for which free-ranging monkeys are thought to be the reservoir of the disease (Downie & Espana 1972). This virus caused outbreaks of cutaneous disease in monkey colonies and associated humans during 1965 and 1966 in the United States (Downie & Espana 1972).
Yabapox (also known as Yaba monkey tumour virus [YMTV]) was first diagnosed in the 1950s in a colony of captive rhesus monkeys (Macaca mulatta) and a baboon (Papio papio) in Yaba, Nigeria (Bearcroft & Jamieson 1958; Whittaker & Glaister 1985). Yabapox has been implicated as the cause of cutaneous nodules in wild, recently imported baboons (Papio spp.) into the United Kingdom and United States, as well as Asian rhesus monkeys and cynomolgus monkeys (Macaca fascicularis) in the United States (Buller & Palumbo 1991; Schielke et al. 2002; Whittaker & Glaister 1985). This article reports a case of cutaneous pox lesions caused by YMTV in a free-ranging vervet monkey (Chlorocebus pygerythrus). To the authors' knowledge, this is the first diagnosis of YMTV in South Africa and in vervet monkeys. The significance of the case is discussed briefly.
Materials and methods
Animals
A clinical examination was conducted and skin biopsies were taken from a recently trapped, adult female vervet monkey, which was housed at a rescue centre in Umkomaas, KwaZulu Natal, South Africa. The monkey was placed in isolation from other captive monkeys at the shelter pending a diagnosis. Biosecurity measures, such as dedicated cleaning utensils, shoes and gloves, were put in place. However, no practical way could be found to prevent free-ranging monkeys from interacting with the monkeys in the enclosure. Two other adult females in the troop were seen to have similar lesions and in all cases, the lesions resolved spontaneously within about 6 weeks. A skin biopsy from one of these animals was taken for laboratory diagnosis. The territory of the troop overlaps with a primary school, where a feeding table for the monkeys has been erected by the staff for educational purposes. Therefore, it was especially important to indentify the viruses. In addition, one adult male from an adjacent territory was observed to have an ulcerated lateral canthus of the eye and a raised yellow nodule on the medial canthus. This monkey was not trapped for further evaluation.
Histopathology and electron microscopy
After routine processing and haematoxylin and eosin staining, two surgically removed nodules in formalin were submitted for histopathological examination by light microscope. Formalin-fixed paraffin embedded tissues were retrieved from the wax block, infiltrated with 1% osmium tetroxide in xylene and a xylene-epoxy resin mixture before being embedded in epoxy resin (Van den Bergh Weerman & Dingemans 1984). Ultra thin resin sections were examined with a Philips CM10 transmission electron microscope (FEI, Eindhoven, Netherlands) operated at 80 kV.
Molecular detection
Two skin samples frozen at -20 °C were transported to the Molecular Genetics Unit of the National Zoological Gardens of South Africa in Pretoria for molecular characterisation. A subsample of the biopsy was halved and DNA was extracted in duplicate using the Zymo Research (ZR) Genomic DNATM Tissue MiniPrep kit (Zymo Research, California, USA) following the manufacturer's protocol. Molecular analysis of the pox genome from both isolates was achieved by amplification of a 220 base pair (bp) region of the insulin metalloprotease-like protein, and intracellular mature virion (IMV) membrane protein, genes (Li et al. 2012) as well as a 1082 bp fragment of the DNA polymerase gene (POL). Primers for amplification of the DNA polymerase gene region were designed by alignment (MEGA5, Tamura et al. 2011) and comparison of the complete DNA polymerase gene of various members of the chordopoxvirinae, which were obtained from the National Centre for Biotechnology Information (NCBI, Genbank); these included YMTV (NC_005179), monkey pox (DQ011153-57, HQ857562-63), yaba-like disease virus (NC_002642, AJ293568), tanapox (EF420156-57) and lumpy skin disease (AF325528, AF409137).
Primers were evaluated by considering specificity through the National Centre for Biotechnology Information (NCBI) through the BLAST function. Annealing temperature and dimer formation were tested through the IDT OligoAnalyser (www.idtdna.com). The selected forward primer was POX_ POL_6F 5' TTG AAC CAA ACA TTC TTT SAA A 3' and the reverse primer was POX_POL_1052R 5' GAG KCT TCY AGT TCA ACT GAA AAG GC 3'. An internal reverse primer for sequencing purposes was designed as POX_POL_620R 5' AAA TAG AAT TTG ARG CGG TWT A 3'.
Polymerase chain reaction (PCR) was carried out using a 25 µL reaction volume and was conducted with Thermo Scientifics' DreamTaq™ Green PCR master mix, which has a 2 x DreamTaq Green buffer containing 0.4 mM of each 2'deoxynucleotide triphosphate (dNTP) and 4 mM magnesium chloride (MgCL2). The final reaction conditions were as follows:
Two X PCR buffer, 5 pmol of each of the forward and reverse primer and 50 ng genomic DNA template. PCR cycling conditions were as follows: Initial denaturation at 94 °C for 5 min; five cycles of denaturation at 94 °C for 30 s, annealing at 48 °C for 50 s and extension at 72 °C for 1 min; 10 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 50 s and extension at 72 °C for 1 min; and 15 cycles of denaturation at 94 °C for 30 s, annealing at 52 °C for 50 s and extension at 72 °C for 1 min. A final extension step of 72 °C for 5 min concluded the cycling.
Contamination was minimised by the inclusion of a negative control for each gene region and by doing PCR setup in a DNA-free hood. After the initial PCR amplification, products were purified according to the Exo/Sap amplicon purification method. Cycle sequencing of the PCR products obtained was performed with the BigDye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems, California, USA) according to the manufacturer's protocol. Sequencing products were purified with the ZR DNA Sequencing CleanUp Kit (Zymo Research, California, USA) and sequenced on an ABI 3130 genetic analyser in forward and reverse. The raw sequence data were analysed using the ABI Prism DNA Sequencer software version 3.4.1.
Phylogenetic analyses
For initial identification, all sequences were blasted against the NCBI database using the blastn search option. Sequences were then aligned with reference data using the ClustalX function (Thompson et al. 1997) incorporated in MEGA5 (Tamura et al. 2011) and phylogenetically analysed using distance (Neighbor-Joining [NJ]) in MEGA5, and Bayesian inference methods (BI) in MrBayes version 3.1 (Huelsenbeck & Ronquist 2001). The best-fit model of sequence evolution was selected under the Akaike information criterion (AIC) in jModeltest (Posada 2008). All sequences generated in this study have been submitted to Genbank under accession numbers (KC218441-444).
Results
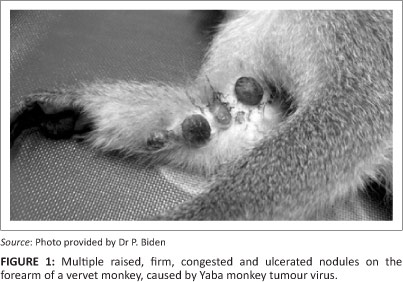
Macroscopically, both female vervet monkeys had multiple large, raised, congested, ulcerated and firm oval nodules (1.0 cm - 1.5 cm in diameter) on the forearm and on the skin between the fingers (Figure 1). One finger was missing in one animal. Other lesions ranged from yellow nodules containing caseous material, smaller nodules with black dry scabs and round non-sunken cutaneous ulcers.
Histologically, large poorly circumscribed, unencapsulated, expansile to infiltrative inflammatory cellular infiltrates were mostly confined to the superficial to deep dermis (Figure 2). The inflammatory cells were closely packed in diffuse sheets, with swirling patterns and supported by scant stroma. These cells were highly pleomorphic with two distinct cell types, namely dominant large macrophage-like cells and a smaller number of elongated spindle cells. The large macrophage-like cells varied in size and shape with mostly ill-defined and abundant amounts of amphophilic cytoplasm. Some of these cells had prominent vacuolar cytoplasm varying from finely vacuolar to having larger, more distinct and confluent vacuoles, giving them a foamy, or soap bubble appearance. Their nuclei were variable in size with many elongated, indented and heterochromatic nuclei with indistinct nucleoli. A small number of these cells were multinucleated. Some cells contained one or more distinct round and basophilic staining nucleoli. Small numbers of these cells had indistinct basophilic intracytoplasmic inclusions. The spindle cells had less cytoplasm, which was also ill-defined, and their nuclei were more hyperchromatic, elongated and without distinct nucleoli. Few multifocal small areas of mild necrosis were seen, associated with cells showing karyolysis and karyorrhexis and scant, suspect necrotic neutrophils. Smaller lymphocytic nodules were seen in a multifocal pattern, mostly towards the periphery. Scant neutrophils were present throughout. In some areas the stromal tissues were oedematous with associated mild congestion and small multifocal haemorrhages.

The overlying epidermis was severely ulcerative over almost the entire length of these biopsies. Small portions of intact epidermis, mostly at the periphery of the ulcerative lesions, were mildly hyperplastic with mild associated orthokeratotic hyperkeratosis. In the ulcerative areas, moderate necrosis of the superficial exposed tissues was seen covered by thick layers of necrotic crusts consisting of necrotic material, admixed with abundant pale eosinophilic proteinaceous material (interpreted as a mixture of serum and fibrin). Abundant basophilic coccoid bacterial colonies were seen.
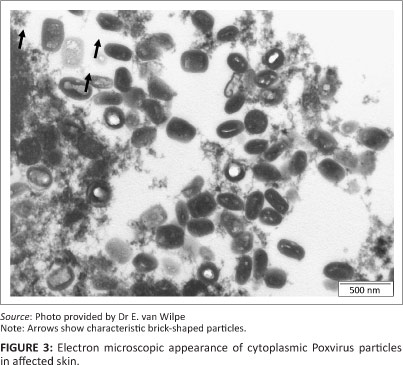
Ultrastructurally, numerous cytoplasmic poxvirus particles (measuring 288 nm x 211 nm) were present. In the longitudinal sectioning plane, some of the particles displayed the characteristic brick shape with rounded edges (Figure 3).
PCR amplification and sequencing of the of the IMV and POL gene regions were used to assign unequivocal YMTV identity to the isolate. All the fragments produced readable sequence of 190 bp (IMV) and 1020 bp (DNApol), and were 213 bp (IMV) and 1005 bp (DNApol) in length following trimming and consensus sequence formation. Phylogenetic analyses were performed on a 16-taxon IMV gene dataset (two sample isolates and 14 NCBI Pox reference strains) and an 18-taxon DNA polymerase dataset (two sample isolates and 16 NCBI Pox reference strains). The TPM3uf+G model of sequence evolution with gamma parameter = 0.261 was chosen as the best fit model in jModeltest under the AIC for the IMV-dataset, whilst the 010023+G+F model of sequence evolution with gamma parameter = 0.5010 was chosen for the DNA polymerase gene dataset.
Results from Neighbor-joining (NJ) (10 000 bootstrap replicates) and Bayesian Inference (BI) (10 million generations, 25% burnin) recovered the same tree topology and the bootstrap and support indices are summarised on the branches of the NJ trees displayed in Figure 4 (a) (IMV) and (b) (DNA pol). Bootstrap and support indices are indicated for NJ (above) and BI (below). Samples from this study are indicated by 'POX_2012_RSA'/ whilst Genbank-acquired sequences are prefixed with the appropriate accession number.
Phylogenetic analyses of both gene regions support the initial sample identification with good bootstrap and posterior probability support. Both isolates from this sample clustered closely with reference samples isolated from YMTV with a 99.0% (IMV) and 99.2% - 99.4% (DNA pol) genetic similarity. Samples were genetically distinct from tanapox, lumpy skin disease and monkeypox with a 76.0% - 86.0% (IMV) and 69.0% - 83.0% (DNA pol) genetic similarity. On the basis of the molecular results, a diagnosis of YMTV infection was made and it was recommended that the quarantine be lifted since this is a disease endemic to tropical African non-human primates.
Discussion
The macroscopic, histological and electron microscopic appearance are consistent with poxviral lesions. NCBI blast results as well as phylogenetic analyses of two gene regions reveal the poxviruses from the vervet monkey to be most closely related to Yaba monkey tumour virus. Both the IMV and DNA polymerase gene regions have been used widely in viral taxonomic and identification studies due to the conserved nature of the central genomic region where these genes are found (Li et al. 2012). Here, this association is with good bootstrap and posterior probability support (98% - 100%) for both distance and Bayesian analyses, with a percentage similarity (99%) of these gene regions falling within the reported intraspecific range for poxviruses (Gubser et al. 2004). Phylogenetic analyses in this study also reveal that this virus is genetically distinct from tanapox, lumpy skin disease as well as monkeypox, excluding the possibility of this known zoonotic pox.
Yaba monkey tumour virus is a naturally occurring zoonotic disease of baboons (Schielke et al. 2002). Cases in other monkey species may result from the housing of wild-caught stressed rhesus and cynomolgus monkeys with vervet monkeys and baboons. Transmission is thought to occur in humans and non-human primates by trauma and/or insect bites (Schielke et al. 2002). Virus replication in most poxviral infections results in transient epidermal and endothelial cell necrosis and the typical vesicular lesions (Ginn et al. 2007). In yaba virus infections, instead of cell death, more persistent infection of fibroblastic and/or macrophage cells occurs, resulting in cell transformation that likely accounts for the neoplastic-like lesions seen (Buller & Palumbo 1991). Infections may be recurrent or resolve with lasting immunity (Schielke et al. 2002).
The ecological and behavioural factors that resulted in this transient outbreak in this troop of vervet monkeys were not investigated, but crowding, social stress, malnutrition and altered vector population dynamics (perhaps due to climate change) may have played a role. The location of some of the lesions on hairless areas of the face and limbs, in keeping with reported cases (Whittaker & Glaister 1985), supports the possibility of an insect vector in this case. Immune suppression and/or stress may facilitate disease, since many of the reported cases occurred in non-human primates recently transported to colonies in the United Kingdom and the United States (Downie & Espana 1972; Schielke et al. 2002; Whittaker & Glaister 1985). Similarily, tanapox virus infections occurred in humans during a time of flooding in Africa, when humans and both domestic and wild animals were crowded onto islands of dry ground (Downie & Espana 1972). Although disease is thought to be mild and self-limiting in humans, its course may be altered in immune-suppressed individuals.
Non-human primates may transmit other life-threatening zoonotic diseases including tuberculosis, shigellosis, salmonellosis, Marburg disease, measles and rabies (Martin 1986). This case emphasises the importance of observing protective measures by members of the veterinary and para-veterinary professions, conservation officials and members of the public when in contact with, or handling non-human primates. In addition, human cases of the related tanapox virus have been attributed to insect bites (Stich et al. 2002). Therefore, persons in contact with non-human primates in the area of Umkomaas who develop cutaneous lesions should inform their medical doctors of the possibility of this infection. To the authors' knowledge, YMTV has not been previously diagnosed in vervet monkeys, nor in South Africa. The extent and significance of the virus to human and non-human primates in South Africa are not known.
Conclusion
This study reports the first known case of Yaba monkey tumour virus in a free-ranging adult female vervet monkey from South Africa. The virus was positively identified by molecular sequencing from two gene fragments, where subsequent phylogenetic analyses of these revealed a 99% similarity of the sample to reference strains of Yaba monkey tumour virus. Although the cutaneous lesions caused by this disease are spontaneously resolved in these monkeys, it can infect humans with only mild symptoms. The extent and significance of the virus to human and non-human primates in South Africa are not known and the authors recommend that persons in contact with non-human primates in the area of Umkomaas who develop cutaneous lesions should inform their doctors of the possibility of this infection.
Acknowledgements
The authors wish to thank the South African National Research Foundation (NRF) for funding allocated to AK for the molecular work in this study. We also acknowledge Ms Thando Radebe for assistance with molecular work. Publication of this article was sponsored by the Wildlife Group (http://www.vets4wildlife.co.za) of the South African Veterinary Association.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this article.
Authors' contributions
H.B. (National Zoological Gardens of South Africa) was the molecular biologist responsible for all molecular work; J.H.V. (Vetdiagnostix) made the initial diagnosis and histopathological identification; E.P.L. (National Zoological Gardens of South Africa) confirmed the histopathological findings and facilitated collaboration; E.W. (University of Pretoria) was responsible for all electron microscopy work; P.B. (Scottburgh Veterinary Clinic) was responsible for the tumour isolation; D.L. (National Zoological Gardens of South Africa) and A.K. (National Zoological Gardens of South Africa) facilitated the molecular and pathological work. All authors contributed to the write-up and review of this article.
References
Buller, R.M.L. & Palumbo, G.L., 1991, 'Poxvirus pathogenesis', Microbiological Reviews 55, 80-122. PMid:1851533, PMCid:PMC372802 [ Links ]
Bearcroft, W.G.C. & Jamieson, M.F., 1958, 'An outbreak of subcutaneous tumours in rhesus monkeys', Nature 182, 195-196. http://dx.doi.org/10.1038/182195a0, PMid:13566242 [ Links ]
Coetzer, J.A. & Tustin, R.C., 2004, 'Poxviridae', in J.A.W. Coetzer & R.C. Tustin (eds.), Infectious diseases of livestock, 2nd edn., vol 2, pp. 1265-1267, Oxford University Press, Cape Town. [ Links ]
Downie, A.W. & Espana, C., 1972, 'Comparison of tanapox virus and yaba-like viruses causing epidemic disease in monkeys', Journal of Hygiene 70, 23-32. http://dx.doi.org/10.1017/S0022172400022051 [ Links ]
Fenner, F., 2000, 'Adventures of pox viruses in vertebrates', FEMS Microbiology Reviews 24, 123-133. http://dx.doi.org/10.1111/j.1574-6976.2000.tb00536.x, PMid:10717311 [ Links ]
Ginn, P.E., Mansell, J.E.K.L. & Rakich, P.M., 2007, 'Skin and appendages', in M.G. Maxie (ed.), Jubb, Kennedy, and Palmer's pathology of domestic animals, 5th edn., vol. 1, pp. 664-674, Elsevier Ltd, Edinburgh. [ Links ]
Gubser, C., Hue, S., Kellam, P. & Smith, G.L., 2004, 'Poxvirus genomes: A phylogenetic analysis', Journal of General Virology 85, 105-117. http://dx.doi.org/10.1099/vir.0.19565-0, PMid:14718625 [ Links ]
Huelsenbeck, J.P. & Ronquist, F., 2001, 'MrBayes: Bayesian inference of phylogenetic trees', Bioinformatics 17, 754-755. http://dx.doi.org/10.1099/vir.0.19565-0, PMid:14718625 [ Links ]
Li, Y., Meyer, H., Zhao, H. & Damon, I.K., 2010, 'GC Content-Based Pan-Pox Universal PCR assays for poxvirus detection', Journal of Clinical Microbiology 48, 268-276. http://dx.doi.org/10.1128/JCM.01697-09, PMid:19906902, PMCid:PMC2812294 [ Links ]
Martin, D.P., 2008, 'Infectious diseases', in M.E. Fowler (ed.), Zoo and wild animal medicine 2nd edn., 669-673, W.B. Saunders Co. London. PMid:18440544 [ Links ]
Posada, D., 2008, 'jModelTest: Phylogenetic model averaging', Molecular Biology and Evolution 25, 1253-1256. http://dx.doi.org/10.1093/molbev/msn083, PMid:18397919 [ Links ]
Schielke, J.E., Kalishman, J., Liggitt, D. & Bielefeldt-Ohmann, H., 2002, 'What is Your Diagnosis?: Multifocal Subcutaneous Tumours in a Young Male Baboon', Contemporary Topics 46, 26-29. [ Links ]
Stich, A., Meyer, H., Kohler, B. & Fleisher, K., 2002, 'Tanapox: First report in a European traveller and identification by PCR', Transactions of the Royal Society of Tropical Medicine and Hygiene 96, 178-179. http://dx.doi.org/10.1016/S0035-9203(02)90295-6 [ Links ]
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S., 2011, 'MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods', Molecular Biology and Evolution 28, 2731-2739. http://dx.doi.org/10.1093/molbev/msr121, PMid:21546353, PMCid:PMC3203626 [ Links ]
Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F. & Higgins, D.G., 1997, 'The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools', Nucleic Acids Research 25, 4876-4882. http://dx.doi.org/10.1093/nar/25.24.4876, PMid:9396791, PMCid:PMC147148 [ Links ]
Van den Bergh Weerman, M.A. & Dingemans, K.P., 1984, 'Rapid deparaffinization for electron microscopy', Ultrastructural Pathology 7, 55-57. http://dx.doi.org/10.3109/01913128409141854, PMid:6393475 [ Links ]
Whittaker, D. & Glaister, J.R., 1985, 'A yaba-like condition in a young baboon (Papio anubis)', Laboratory Animals 19, 177-179. PMid:2993739 [ Links ]
 Correspondence:
Correspondence:
Helene Brettschneider
PO Box 754
Pretoria, South Africa
Email: helene@nzg.ac.za
Received: 09 Jan. 2013
Accepted: 12 Apr. 2013
Published: 20 Sept. 2013
















