Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the South African Veterinary Association
versão On-line ISSN 2224-9435
versão impressa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.84 no.1 Pretoria Jan. 2013
ORIGINAL RESEARCH
Fat absorption and deposition in Japanese quail (Coturnix coturnix japonica) fed a high fat diet
Mhlengi M. Magubane; Busisani W. Lembede; Kennedy H. Erlwanger; Eliton Chivandi; Janine Donaldson
School of Physiology, University of the Witwatersrand Medical School, South Africa
ABSTRACT
Dietary fat contributes significantly to the energy requirements of poultry. Not all species are able to increase their absorptive capacity for fats in response to a high fat diet. The effects of a high fat diet (10% canola oil) on the lipid absorption and deposition in the liver, breast and thigh muscles of male and female Japanese quail were investigated. Thirty-eight Japanese quail (Coturnix coturnix japonica) were randomly divided into a high fat diet (HFD) and a standard diet (STD) group. The birds were fed the diets for seven weeks after which half of the birds were subjected to oral fat loading tests (OFLT) with plant oils containing long-chain and medium-chain triglycerides. The remaining birds were included for the lipid deposition measurements. Thereafter the birds were euthanised, blood samples were collected and liver, breast and thigh muscle lipid deposition was determined. Female quail on both diets had significantly higher plasma triglyceride concentrations (p < 0.05) compared with their male counterparts. No significant differences in plasma triglyceride concentrations were observed after the OFLTs. Female quail had significantly heavier liver masses compared with the males but there was no significant difference in the liver lipid content per gram liver mass. Female quail on the HFD had higher lipid content (p < 0.05) in the breast muscle compared with their male counterparts whilst male quail on the HFD had higher lipid content (p < 0.05) in the thigh muscle in comparison with both males and females on the standard diet. Dietary supplementation with 10% canola oil did not alter gastrointestinal tract lipid absorption, but it caused differences between the sexes in muscle lipid accumulation, the physiological significance of which requires further investigation.
Introduction
Meat provides a variety of essential nutrients, particularly amino acids, which are not easily obtained from plants. Amongst the animal meat consumed by humans, broiler meat is regarded as a healthy meat type (Jiménez-Colmenero, Carballo & Cofrades 2001; Mutucumarana et al. 2010). Most commercial poultry farmers raise chickens and ducks (Williams 1999). The Japanese quail (Coturnix coturnix japonica), native to Asia and Europe (Minvielle 2004; National Research Council 1991), offers a number of advantages over the chicken, which include its small body size, its resistance to many poultry diseases that afflict chickens, its greater capacity to scavenge for food, early onset of laying, high reproduction rates, as well as low feed intake (National Research Council 1991; Santos et al. 2011). The Japanese quail is fast becoming recognised within the commercial poultry industry as a source of uniquely flavoured meat for consumers, at an affordable price compared with most poultry species (Minvielle 2004; Nuernburg et al. 2011; Wilson, Abbott & Abplanalp 1961).
Nutrition is the most studied aspect in poultry production because it plays a major role in determining meat quality and is a major cost component of poultry production (Malakian 2010; Mutucumarana et al. 2010; Wood et al. 2008). Meat quality is largely dependent on fat content (Chashnidel et al. 2010). Dietary fats influence meat tenderness and juiciness, reportedly as a result of the different melting points of different fatty acids (Shahriar, Toghyan & Lotfi 2010; Wood et al. 2004). In order to maximise profit in terms of poultry production, quail farmers need to be in a position to formulate quail-specific diets that meet their specific nutritional requirements and thus improve overall meat quality. Previous studies have shown that nutritive diets for broiler production are generally formulated using added fats and oils (Malakian 2010; Selvaraj & Purushothaman 2004). It is essential to include up to 10% fats and oils in broiler diets as they provide a good source of energy (Malakian 2010; Qureshi et al. 2004). Mateos, Sell and Eastwood (1982) reported that fat in broiler chicken diets improved the intestinal digestion and absorption of fats.
Dietary fat digestion and absorption occurs in the small intestine (Bickerstaffe, West & Annison 1970). Fat digestion and absorption are facilitated by phospholipids, bile salts and pancreatic lipase. The phospholipids and bile salts play an important role in the emulsification of dietary triglycerides and other fat-soluble nutrients (Krogdahl 1985), whereas the pancreatic lipase is essential for hydrolysing the triglycerides at the water-lipid interface (Bickerstaffe et al. 1970). Fat digestion and absorption depend largely on the physical properties of dietary fat (Jiang et al. 1993).
Fats with a chain length of between eight and twelve carbon atoms are classified as medium chain triglycerides (MCT), for example coconut oil (Simpson & Doxey 1983), whereas fats with a chain length of fourteen or more carbon atoms are classified as long chain triglycerides (LCT), for example olive, soybean or sunflower oil (Jiang et al. 1993; Simpson & Doxey 1983). The way in which MCT and LCT are digested and absorbed from the gastrointestinal tract is physiologically different amongst animals (Bach, Frey & Lutz 1989; Greenberger, Rodgers & Isselbacher 1966). An oral fat loading test is often used to determine the fat absorptive capacities in animals using both MCT and LCT oils (Simpson & Doxey 1983). Fat digestion and absorption are also influenced by the levels of triglycerides in the body (Fedde, Waibel & Burger 1959), which in turn can be influenced by sex. Generally, laying females have more fat stores compared with males of the same species due to the egg formation requirements (Hermier 1997; Jensen, Schumaier & Latshaw 1970; Potter & McCarthy 1985). The stored fat can originate from the diet or endogenously. The liver is the main site for lipid synthesis in birds (Didier et al. 1983; Katz & McGarry 1984). The liver also regulates lipid metabolism in birds (Greenberger et al. 1966), which is affected by many factors such as fasting and exogenous lipids (Didier et al. 1983; Greenberger et al. 1966; Serr, Suh & Lee 2009). During short-term fasting (12 h) more lipids are utilised as an energy source in the liver than in the muscles, thereby improving the meat quality of muscles as more lipids are stored (Colin et al. 2009; Lamošova, Máčajová & Zeman 2004).
Previous studies have shown that dietary supplementation with canola oil improves meat quality in broilers (Shahriar et al. 2007; Zannini et al. 2008). In some species, increasing dietary fat levels increases the lipid absorptive capacity of the gastrointestinal tract, but it is unclear whether the same occurs in quail. The purpose of the present study was to investigate the effects of a high fat (10% canola oil) diet on lipid absorption and lipid deposition in male and female Japanese quail.
Materials and methods
Ethical approval for the study
The study was approved by the Animal Ethics Screening Committee (AESC) of the University of the Witwatersrand, South Africa (AESC approval number: 2011/07/03).
Animals
The study took place in the Central Animal Service (CAS) unit at the University of the Witwatersrand, Johannesburg. A total of 38, male (n = 19) and female (n = 19), five-week-old Japanese quail, obtained from a commercial supplier (Pleysier Incubators c.c., Lanseria, South Africa), were used in the study. Nineteen of the birds (a mixed group of male and female) were used exclusively for the oral fat loading tests, whilst the remaining 19 birds were included for the lipid deposition measurements, as well as the plasma triglyceride and liver mass measurements made at euthanasia.
Feeding and housing
The birds were randomly divided into two dietary groups (mixed groups of both male and female). A standard diet (STD) group (n = 19) served as the control group and received a commercially supplied poultry diet ad libitum (The Perfect Balance, Epol®, South Africa). The second group (n = 19) received a high fat diet (HFD) ad libitum. The high fat diet was made by adding canola oil (Southern Oil Ltd, Swellendam, South Africa) to the commercially supplied feed at 10% of the mass of the feed. All of the birds had free access to water for the duration of the study. Both diets were supplemented with 1.5% limestone (Feed lime, Agrilime (Pty) Ltd, Protea Park, South Africa) in order to provide the extra calcium needed for egg shell formation (Rao & Roland 1990) for the quail that were laying. All birds were de-wormed with 90 mg/kg piperazine (Kyron Laboratories (Pty) Ltd, Benrose, South Africa) prior to commencing the study, to prevent infestation with endoparasites that could affect body mass gain (Robbins, Ye & Fletcher 2011). The birds were housed in deep litter pens with wood shavings as bedding. The primary flight feathers on the left wing were clipped to prevent flight. The birds were given a three-week adaptation period prior to commencing the study to enable them to familiarise themselves with the environment, which was enriched with perches in the form of logs. The birds were provided with supplementary heating by means of an infrared light for the duration of the study. Lighting was restricted to 12 h in each 24 h period; lights were switched on from 06:00.
Measurements and treatments
Body mass
The birds were weighed twice weekly; they were placed into a pre-weighed cage which was then placed onto the digital balance (ScoutTM Pro, Ohaus Corporation, Pine Brook, USA). The birds were handled for a couple of minutes several times before each weighing to accustom them to handling.
Oral fat loading tests
The birds (n = 19) were fasted overnight (12 h) prior to the fat loading tests (Williams 1999). Both the STD and HFD groups were further divided into two subgroups. STD group I (n = 5) and HFD group I (n = 4) received coconut oil (Absolute Organix, Johannesburg, South Africa), which is rich in medium-chain triglycerides (MCT). STD group II (n = 5) and HFD group II (n = 5) received olive oil (R.M. S.p.A, Lucca, Italy), which is rich in long-chain triglycerides (LCT). The oils were administered at a dose of 10 mL/kg body mass by oral intubation of the crop with a silastic gavage tube. Blood samples (0.1 mL) were then collected for the determination of triglyceride levels using syringes (1 mL), following a pinprick to the wing vein with sterile hypodermic needles (25 g). The blood samples were collected at fixed time points, specifically, immediately before (time 0) and then 30 min, 60 min, 90 min, 120 min, 240 min, 360 min and 480 min after administration of the fat load. The triglyceride concentrations were measured using a GCT meter (Accutrend® Plus GCTL-mmol/L, Roche, Manheim, Germany).
After a seven-day recovery period following the first intervention, the treatments were swapped amongst subgroups and the oral fat loading tests were performed again (as described above).
Terminal procedure
The birds were given a seven-day recovery period following the second oral fat loading test. Nineteen of the birds were fasted overnight with free access to water and the remaining 19 birds had free access to both feed and water. The birds were then euthanised by an anaesthetic overdose of sodium pentobarbital (Eutha-naze, Centaur Labs, South Africa), at 100 mg/kg, infused intravenously into the wing vein. The liver, breast and thigh muscles were carefully removed, weighed and frozen (-5 °C) until further analysis (lipid content).
Liver and muscle lipid content
Lipids were extracted from the liver, breast and thigh muscle samples using procedures as described by Bligh and Dyer (1959). Briefly, liver samples (approximately 5 g) and muscle samples (15 g) were placed into a chloroform-methanol (2:1) solution overnight at 4 °C. The samples were then filtered through filter paper (Albert®, Pore 7-11, Size 185 mm) and 30 mL, 0.9% saline added, mixed, and allowed to stand overnight at 4 °C to allow separation into two phases. The bottom (chloroform) phase was collected and reduced to dryness under vacuum at 37 °C using a water bath (Labex®, Krugersdorp, South Africa) and then made up to 20 mL with chloroform. Aliquots of 2 mL of the extracts were placed in dried, pre-weighed vials and re-dried at 50 °C for 30 min, cooled and then reweighed to determine the lipid content. The livers were also dried and their dry matter content determined.
Data analysis
All data are expressed as mean ± s.d. A two-way analysis of variance (ANOVA) was used to compare plasma triglyceride concentrations, liver mass, and lipid percentage in the liver, thigh muscle and breast muscle for the STD group compared with the HFD group, as well as for the comparisons between male and female quail within each diet group. A repeated-measures ANOVA was used to assess differences between male and female quail in weekly body mass and plasma triglyceride concentrations at specific time points during the oral fat loading tests. A Bonferroni post-hoc test was used as a correction test. The level of significance was set at p < 0.05. All statistical analyses were performed using GraphPad Prism 5 (Graph-pad Software Inc., San Diego, USA).
Results
Body mass
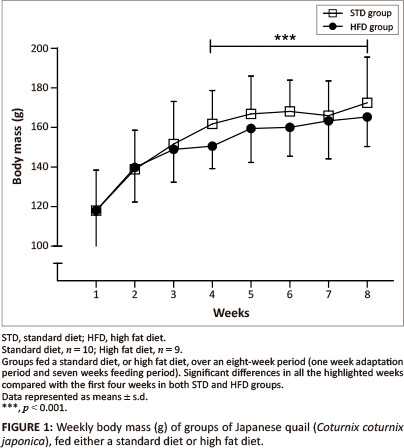
The initial body mass was similar for both the STD and HFD groups (118 g ± 20.4 g) (Figure 1). There were no significant differences in body mass observed between the STD and HFD groups throughout the duration of the study. However, body mass was significantly higher (p < 0.001) in the last four weeks compared with the first four weeks of the study and remained relatively stable within both the STD and HFD groups.
Oral fat loading tests
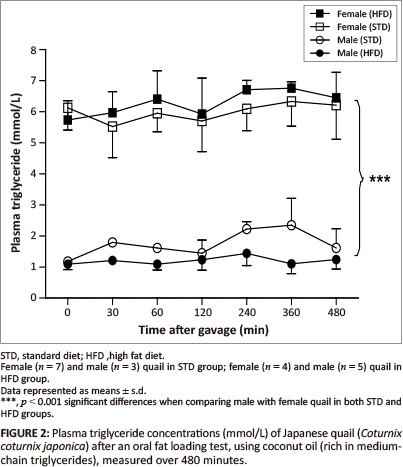
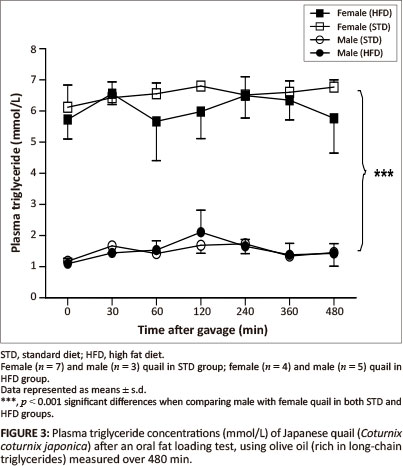
The GCT meter used to determine the plasma triglyceride concentrations had a detection range of between 0.8 mmol/L and 6.86 mmol/L. Basal triglyceride concentrations were significantly higher in the female birds compared with the male birds. Following gavage with the coconut oil or olive oil, the plasma triglyceride concentrations were not significantly different between the STD and HFD groups over the 480 min (Figure 2 and Figure 3).
Liver, breast and thigh muscle lipid content
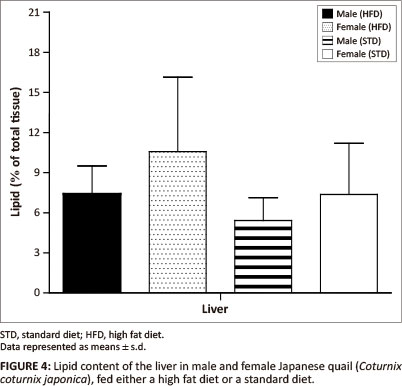
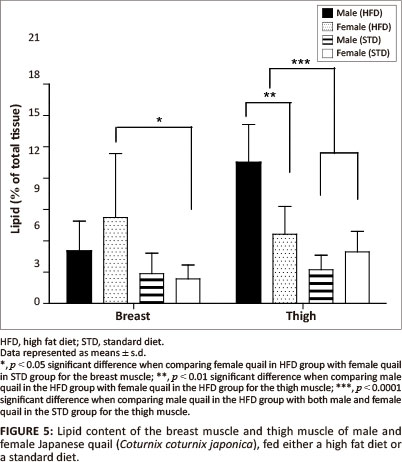
The liver lipid content was not significantly different between female and male birds of both the STD and HFD groups (Figure 4). The breast muscle lipid content was significantly higher (p < 0.05) in female birds of the HFD group than female birds of the STD group, but not significantly different from the male birds of both the HFD and STD groups (Figure 5). The thigh muscle lipid content was significantly higher (p < 0.01) in male birds of the HFD group than female birds of the HFD group and both male and female birds of the STD group (Figure 5).
Effect of fasting on plasma triglyceride concentrations
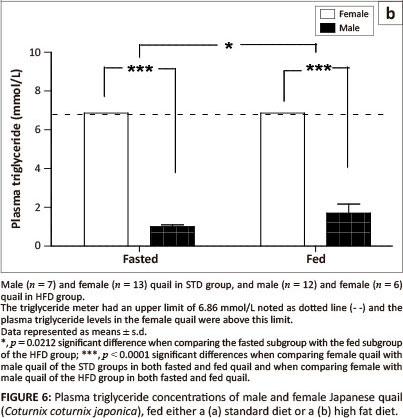
Plasma triglyceride concentrations in both the fed and fasted subgroups were significantly higher (p <0.0001) in female birds compared with male birds of both the STD group and the HFD group (Figure 6a and Figure 6b). There were no significant differences in plasma triglyceride concentrations between fasted and fed male birds in the STD group (Figure 6a). Plasma triglyceride concentrations were significantly higher (p = 0.0212) in the birds fed prior to euthanasia than those fasted in the HFD group (Figure 6b). The plasma triglyceride concentrations were above the maximum detectable value (> 6.86 mmol/L) of the GCT meter for the HFD group.
Liver mass (fasted vs. fed)
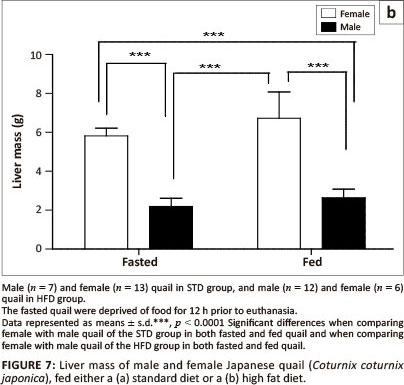
With respect to the liver mass in the STD group, liver mass of fed female birds did not differ significantly from fasted female birds, but was significantly higher (p < 0.0001) than both fed and fasted male birds (Figure 7a). No differences were observed between male birds. Furthermore, the liver mass of both fed and fasted female birds in the HFD group was significantly higher than that of both the fed and fasted male birds (Figure 7b). No differences were observed between female or male birds in either of the groups.
Discussion
The present study investigated the effects of dietary supplementation with canola oil (10% of the diet) on body mass, lipid absorption and lipid deposition in the liver and the breast and thigh muscles of the Japanese quail. After seven weeks of supplementation with 10% canola oil there was no significant difference in body mass between quail in the HFD and STD groups. All birds had a significant body mass gain (p < 0.001) during the first four weeks of feeding and then maintained a stable body mass thereafter, irrespective of the diet type. This suggests that the weekly body mass gain observed in the Japanese quail was not influenced by the diet type, but by the growing stage in attaining matured body mass. Wilson et al. (1961) and Ricklefs (1979) reported that quail attain a mature body mass at eight weeks of age, which is in agreement with the results of the current study. The plasma triglyceride concentrations were affected by the HFD both in the fed and fasted quail, in both sexes, particularly in the female quail. Although female quail presented with higher plasma triglyceride concentrations than male quail, female quail in the HFD group had plasma triglyceride concentrations that were above the maximum detectable value of the GCT meter (> 6.86 mmol/L) compared with female quail in the STD group (Figure 6a and Figure 6b). The higher plasma triglyceride concentrations in female quail both for the STD and HFD groups could be attributed in part to the increased lipid synthesis that occurs during the laying period (Hermier 1997), as they were laying a lot of eggs during the feeding period.
MCTs are rapidly hydrolysed and absorbed across the intestinal wall and transported directly into the liver whereby, with efficient oxidation, the elevation of triglyceride concentrations can be prevented in the peripheral circulation (Hill 1972). On the other hand, LCTs are not quickly absorbed across the intestinal wall; they are hydrolysed and then transported into the peripheral circulation via the lymphatic system (Bartley 1980). Therefore, MCTs are expected to be rapidly available in the peripheral circulation compared with LCTs. After administration of both the coconut oil (MCT) and the olive oil (LCT) there were no significant alterations in plasma triglyceride concentrations for either sex in both the HFD and STD groups. The most likely reason for the unaltered plasma triglyceride concentrations following administration of the coconut oil could be the efficient oxidation of this fat by the liver as mentioned above, thus increased levels of MCTs in the peripheral circulation could be prevented (Hill 1972; Isselbacher 1966; Jiang et al. 1993). This differs from studies with pigs and dogs, in which administration of either LCTs or MCTs resulted in elevated plasma levels of triglycerides (Williams 1999). It is recommended that future studies should consider measuring triglyceride concentrations in the portal vein samples to avoid the effects of hepatic metabolism. The unaltered plasma triglyceride concentrations following administration of the olive oil could be as a result of the duration of the plasma triglyceride measurements after administration of the oil, as LCTs take longer to be absorbed (Williams 1999). It is therefore recommended that future studies should consider increasing the duration for measurements during fat loading tests, allowing for a more accurate representation of the absorption of LCTs. Significantly higher plasma triglyceride concentrations (p < 0.001) were observed in the female quail compared with the male quail throughout the duration of the fat loading tests, with both oils and in both the STD and HFD groups. This was expected since laying birds generally present with higher lipid levels required for egg formation (Hermier 1997; Jensen et al. 1970; Potter & McCarthy 1985).
During egg-laying, oestrogen increases lipid formation by the liver in order to meet the demand for yolk deposition (Hermier 1997).
In terms of the lipid deposition, it was found that the HFD (10% canola oil) influenced the lipid deposition of the muscle tissues for these birds. There were no significant differences in the liver lipid content (as a percentage of the total tissue) amongst the male and female birds of either of the diet groups. However, the female birds had significantly heavier livers than the male birds, with a resultant greater total amount of stored lipids. The diets did not affect the hepatic lipid stores in the birds. When being fed a high fat diet, the liver is required to store excess lipids, thus the liver lipid content is expected to be higher in comparison with birds consuming a low-fat diet (Mossab et al. 2002). Female quail fed a HFD deposited significantly more lipids in the breast muscle compared with female quail in the STD group (p < 0.05), but not when compared with male quail of both diet groups. However, the lipid content of the muscles was not significantly different between male quail on the different diets. These results are in agreement with those presented by Miller, Menge and Denton (1962), who showed that chickens fed a high fat diet deposited more lipids in the breast muscle than chickens fed a low-fat diet. Furthermore, it was found that male birds fed a HFD deposited significantly more lipids in the thigh muscle than the STD group (p < 0.01 and p < 0.001). This could be attributed in part to increased muscular activity in male quail, which were observed to walk around the pens more than the female quail. It is possible that there was a greater transfer of fatty acids and glucose to the active muscle tissue, resulting in an increased amount of fatty acids being deposited as well as an increased conversion of glucose into fatty acids and their subsequent deposition in the muscle (Simpson & Doxey 1983).
In this study the female quail had significantly heavier livers compared with the male quail that were fed either the STD or HFD. Short-term fasting results in the depletion of lipids and glycogen in the liver in order to maintain high blood glucose levels, thus reducing the liver mass (Boswell, Li & Takeuchi 2002; Colin et al. 2009; Didier et al. 1983; Lamošova et al. 2004). In the current study, fasting resulted in a significant reduction in the liver mass (p < 0.05) in female quail fed a standard diet, but not in female quail fed a high fat diet. The quail continued to lay despite being fasted and hence the reduction in liver mass could be due to depletion of glycogen from the liver.
In conclusion, addition of canola oil at 10% of the diet did not alter body mass and did not increase blood levels of triglycerides following fat loading tests in the Japanese quail. However, it caused increased lipid deposition in the thigh muscle of the male and breast muscle of the female Japanese quail. Fasting the birds depleted lipids in the liver but not in the muscle tissues of these birds. The significance of the findings for meat quality and regulatory mechanisms in the birds needs further investigation.
Acknowledgements
The authors extend their thanks to the staff at the Central Animal Services at the University of the Witwatersrand for ensuring animal welfare; Ms Zinhle Gasa and Ms Rachael Dangarembizi for assisting with data collection. Funding for this project was provided by the School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg and the National Research Foundation of South Africa.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this article.
Authors' contributions
J.D. (University of the Witwatersrand) and E.M.C. (University of the Witwatersrand) were the project leaders and J.D. and K.H.E. (University of the Witwatersrand) were responsible for experimental and project design. M.M.M and B.W.L performed most of the experiments. M.M.M. was responsible for the data analysis and wrote the draft manuscript. Subsequent editing of the manuscript was performed by J.D, E.M.C and K.H.E. All authors are affiliated to the School of Physiology, Faculty of Health Sciences, University of the Witwatersrand.
References
Bach, A.C., Frey, A. & Lutz, O., 1989, 'Clinical and experimental effects of medium-chain-triglyceride-based fat emulsions - A review', Clinical Nutrition 8, 223-235. http://dx.doi.org/10.1016/0261-5614(89)90032-0 [ Links ]
Bartley, J.C., 1980, 'Lipid metabolism and its diseases', in J.J. Kaneko (ed.), Clinical biochemistry of domestic animals, pp. 53-95, Academic Press, New York. [ Links ]
Bickerstaffe, R., West, C.E. & Annison, E.F., 1970, 'Lipid metabolism in the perfused chicken liver', Journal of Biochemistry 118, 427-431. [ Links ]
Bligh, E.G. & Dyer, W.J., 1959, 'A rapid method of total lipid extraction and purification', Canadian Journal of Biochemistry and Physiology 37, 911-917. http://dx.doi.org/10.1139/o59-099, PMid:13671378 [ Links ]
Boswell, T., Li, Q. & Takeuchi, S., 2002, 'Neurons expressing neuropeptide Y mRNA in infundibular hypothalamus of Japanese quail are activated by fasting and co-express agouti-related protein mRNA', Molecular Brain Research 100, 31-42. http://dx.doi.org/10.1016/S0169-328X(02)00145-6 [ Links ]
Chashnidel, Y., Moravej, H., Towhidi, A., Asadi, F. & Zeinodini, S., 2010, 'Influence of different levels of n-3 supplemented (fish oil) diet on performance, carcass quality and fat status in broilers', African Journal of Biotechnology 9, 687-691. [ Links ]
Colin, A., Swennen, Q., Skiba-Cassy, S., Buyse, J., Chartrin, P., Le Bihan-Duval, E. et al., 2009, 'Regulation of fatty acid oxidation in chicken (Gollus gollus): Interactions between genotype and diet composition', Comparative Biochemistry and Physiology Part B: Biochemistry & Molecular Biology 153, 171-177. http://dx.doi.org/10.1016/j.cbpb.2009.02.012, PMid:19258045 [ Links ]
Didier, R., Remesy, C. & Demigne, C., 1983, 'Changes in glucose and lipid metabolism in starved or starved-refed Japanese quail (Coturnix coturnix joponico) in relation to fine structure of liver cells', Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 74, 839-848. http://dx.doi.org/10.1016/0300-9629(83)90356-0 [ Links ]
Fedde, M.R., Waibel, P.E. & Burger, R.E., 1959, 'Factors affecting the absorbability of certain dietary fats in the chick', Journal of Nutrition 70, 447-452. [ Links ]
Greenberger, N.J., Rodgers, J.B. & Isselbacher, K.J., 1966, 'Absorption of medium and long chain triglycerides: Factors influencing their hydrolysis and transport', Journal of Clinical Investigation 45, 217-227. http://dx.doi.org/10.1172/JCI105334, PMid:5901507, PMCid:292686 [ Links ]
Hermier, D., 1997, 'Lipoprotein metabolism and fattening in poultry', Journal of Nutrition 127, 805-808. [ Links ]
Hill, F.W.G., 1972, 'Malabsorption syndrome in the dog: A study of thirty-eight cases', Journal of Small Animal Practice 13, 575-594. http://dx.doi.org/10.1111/ j.1748-5827.1972.tb06802.x, PMid:4666919 [ Links ]
Isselbacher, K.J., 1966, 'Biochemical aspects of fat absorption', Gastroenterology 50, 78-82. PMid:5900958 [ Links ]
Jensen, L.S., Schumaier, G.W. & Latshaw, J.D., 1970, 'Extra caloric effect of dietary fat for developing turkeys as influenced by calorie: protein ratio', Poultry Science 49, 1697-1704. http://dx.doi.org/10.3382/ps.0491697, PMid:5501098 [ Links ]
Jiang, Z.M., Zhang, S., Wang, X., Yang, N., Zhu, Y. & Wilmore, D., 1993, 'A comparison of medium-chain and long-chain triglycerides in surgical patients', Annals of Surgery 217, 175-184. http://dx.doi.org/10.1097/00000658-199302000-00012, PMid:8439215, PMCid:1242757 [ Links ]
Jiménez-Colmenero, F., Carballo, J. & Cofrades, S., 2001, 'Healthier meat and meat products: Their role as functional foods', Meat Science 59, 5-13. http://dx.doi.org/10.1016/S0309-1740(01)00053-5 [ Links ]
Katz, J. & McGarry, J.D., 1984, 'The glucose paradox. Is glucose a substrate for liver metabolism?', Journal of Clinical Investigation 74, 1901-1909. http://dx.doi.org/10.1172/JCI111610, PMid:6392338 PMCid:425376 [ Links ]
Krogdahl, A., 1985, 'Digestion and absorption of lipids in poultry', Journal of Nutrition 115, 675-685. PMid:3889239 [ Links ]
Lamosová, D., Máčajová, M. & Zeman, M., 2004, 'Effects of short-term fasting on selected physiological functions in adult male and female Japanese quail', Acta Veterinaria Brna 73, 9-16. [ Links ]
Malakian, M., 2010, 'The effect of different levels of full fat sunflower seed on performance of broiler chickens', Agricultural Journal 5, 190-195. http://dx.doi.org/10.3923/aj.2010.190.195 [ Links ]
Mateos, G.G., Sell, J.L. & Eastwood, J.A., 1982, 'Rate of food passage (transit time): As influenced by level of supplemental fat', Poultry Science 61, 94-100. http://dx.doi.org/10.3382/ps.0610094, PMid:7088787 [ Links ]
Miller, E.C., Menge, H. & Denton, C.A., 1962, 'Effect of dietary fat on tissue fat and plasma cholesterol level in broilers', Poultry Science 4, 970-983. http://dx.doi.org/10.3382/ps.0410970 [ Links ]
Minvielle, F., 2004, 'The future of Japanese quail for research and production', World's Poultry Science Journal 60, 500-507. http://dx.doi.org/10.1079/WPS200433 [ Links ]
Mossab, A., Lessire, M., Guillaumin, S., Kouba, M., Mourot, J., Peiniau, P. et al., 2002, 'Effect of dietary fats on hepatic lipid metabolism in the growing turkey', Comparative Biochemistry and Physiology Part B: Biochemistry & Molecular Biology 132, 473-483. http://dx.doi.org/10.1016/S1096-4959(02)00059-3 [ Links ]
Mutucumarana, R.K., Samarasinghe, K., Ranjith, G.W.H.A.A., Wijeratne, A.W. & Wickramanayake, D.D., 2010, 'Poultry offal meal as a substitute to dietary soybean meal for Japanese quails (Coturnix coturnix joponico): Assessing the maximum inclusion level and the effect of supplemental enzymes', Tropical Agricultural Research 21, 293-307. [ Links ]
National Research Council, 1991, Microlivestock: Little-known small animals with a promising economic future, pp. 147-156, National Academy Press, Washington, D.C. [ Links ]
Nuernberg, K., Slamecka, J., Mojto, J., Gasparik, J. & Nuernberg, G., 2011, 'Muscle fat composition of pheasants (Phasionus colchicus), wild ducks (Anas platyrhynchos) and black coots (Fulica otro)', European Journal of Wildlife Research 57, 795-803. http://dx.doi.org/10.1007/s10344-010-0489-3 [ Links ]
Potter, L.M. & McCarthy, J.P., 1985, 'Varying fat and protein in diets of growing large white turkeys', Poultry Science 64, 1941-1949. http://dx.doi.org/10.3382/ps.0641941, PMid:4070128 [ Links ]
Qureshi, I.A., Khan, S.A., Chaudhry, Z.I., Mian, N.A., Tipu, M.Y. & Rai, M.F., 2004, 'Effects of high dietary fat on serum cholesterol and fatty liver syndrome in broilers', Pakistan Veterinary Journal 24, 153-154. [ Links ]
Rao, K.S. & Roland, D.A., 1990, 'in vivo limestone solubilization in commercial leghorns: Role of dietary calcium level, limestone particle size, in vitro limestone solubility rate, and the calcium status of the hen', Poultry Science 69, 2170-2176. http://dx.doi.org/10.3382/ps.0692170, PMid:2084675 [ Links ]
Ricklefs, R.E., 1979, 'Patterns of growth in birds. V. A comparative study of development in the starling, common tern, and Japanese quail', The Auk 96, 10-30. [ Links ]
Robbins, K.M., Ye, W. & Fletcher, O.J., 2011, 'Identification of Ascoridio numidoe in guinea fowl (Numida meleagris) and association with elevated mortality', Avian Diseases 55, 151-154. http://dx.doi.org/10.1637/9587-102110-Case.1, PMid:21500654 [ Links ]
Santos, T.C., Murakami, A.E., Fanhani, J.C. & Oliveira, C.A.L., 2011, 'Production and reproduction of egg- and meat-type quails reared in different group sizes', Brazilian Journal of Poultry Science 13, 9-14. [ Links ]
Selvaraj, R.K. & Purushothaman, M.R., 2004, 'Nutritive value of full-fat sunflower seeds in broiler diets', Poultry Science 83, 441-446. PMid:15049498 [ Links ]
Serr, J., Suh, Y. & Lee, K., 2009, 'Regulation of adipose triglyceride lipase by fasting and refeeding in avian species', Poultry Science 88, 2585-2591. http://dx.doi.org/10.3382/ps.2009-00265, PMid:19903957 [ Links ]
Shahriar, H.A., Rezaei, A., Lak, A. & Ahmadzadeh, L.A., 2007, 'Effect of dietary fat sources on blood and tissue biochemical factors of broiler', Journal of Animal and Veterinary Advances 6, 1304-1307. [ Links ]
Shahriar, H.A., Toghyan, M. & Lotfi, A., 2010, 'Effects of different type and levels of fat on fatty acids profile, cholesterol and triglyceride in thigh meat of broiler chicks', 2nd International Conference on Chemical, Biological and Environmental Engineering, Cairo, Egypt, November 2-4, 2010, pp. 341-342. http://dx.doi.org/10.1109/ICBEE.2010.5651206 [ Links ]
Simpson, J.W. & Doxey, D.L., 1983, 'Quantification assessment of fat absorption and its diagnostic value in exocrine pancreatic insufficiency', Research in Veterinary Science 35, 249-251. PMid:6635347 [ Links ]
Williams, R.B., 1999, 'A compartmentalised model for the estimation of the cost of coccidiosis to the world's chicken production industry', International Journal for Parasitology 29, 1209-1229. http://dx.doi.org/10.1016/S0020-7519(99)00086-7 [ Links ]
Wilson, W.O., Abbott, U.K. & Abplanalp, H., 1961, 'Evaluation of Coturnix (Japanese quail) as a pilot animal for poultry', Poultry Science 40, 651-657. http://dx.doi.org/10.3382/ps.0400651 [ Links ]
Wood, J.D., Enser, M., Fisher, A.V., Nute, G.R., Sheard, P.R., Richardson, R.I. et al., 2008, 'Fat deposition, fatty acid composition and meat quality: A review', Meat Science 78, 343-358. http://dx.doi.org/10.1016/j.meatsci.2007.07.019, PMid:22062452 [ Links ]
Wood, J.D., Richardson, R.I., Nute, G.R., Fisher, A.V., Campo, M.M., Kasapidou, E. et al., 2004, 'Effects of fatty acids on meat quality: A review', Meat Science 66, 21-32. http://dx.doi.org/10.1016/S0309-1740(03)00022-6 [ Links ]
Zannini, S.F., Vicente, E., Colnago, G.L., Pessotti, B.M.S. & Silva, M.A., 2008, 'Manipulation of the fatty acids composition of poultry meat and giblets by dietary inclusion of two oil sources and conjugated linoleic acid', Arquivo Brosileiro de Medicina Veterinária e Zootecnia 60, 1388-1398. http://dx.doi.org/10.1590/S0102-09352008000600013 [ Links ]
 Correspondence:
Correspondence:
Janine Donaldson
7 York Road
Johannesburg 2193, South Africa
janine.donaldson@wits.ac.za
Received: 12 June 2012
Accepted: 01 Feb. 2013
Published: 17 May 2013