Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.84 n.1 Pretoria Jan. 2013
ORIGINAL RESEARCH
Antimicrobial susceptibility profiles of Staphylococcus intermedius isolates from clinical cases of canine pyoderma in South Africa
Catherine A. BluntI; Moritz van VuurenII; Jacqueline PicardII
IDepartment Diagnostic Microbiology, Vetdiagnostix Veterinary Pathology Services, South Africa
IIDepartment of Veterinary Tropical Diseases, University of Pretoria, South Africa
ABSTRACT
Successful treatment of canine pyoderma has become compromised owing to the development of antimicrobial resistance with accompanying recurrence of infection. Canine skin samples submitted to a veterinary diagnostic laboratory for microbiological culture and sensitivity between January 2007 and June 2010, from which Staphylococcus intermedius was isolated, were selected for this investigation. Antimicrobial resistance of S. intermedius was most prevalent with reference to ampicillin followed by resistance to tetracycline and then potentiated sulphonamides. In general, antimicrobial resistance was low and very few methicillin-resistant isolates were detected. Temporal trends were not noted, except for ampicillin, with isolates becoming more susceptible, and potentiated sulphonamides (co-trimoxazole), with isolates becoming more resistant. In general, both the Kirby-Bauer disc diffusion and broth dilution minimum inhibitory concentration tests yielded similar results for the antimicrobial agents tested. The main difference was evident in the over-estimation of resistance by the Kirby-Bauer test for ampicillin, co-trimoxazole, penicillin and doxycycline. Knowledge of trends in bacterial resistance is important for veterinarians when presented with canine pyoderma. Analysis of antimicrobial susceptibility profiles of S. intermedius isolated from canine pyodermas will guide veterinarians' use of the most appropriate agent and encourage prudent use of antimicrobials in companion animals.
Introduction
Staphylococcus species are facultatively anaerobic, Gram-positive, coccal bacteria that belong to the family Micrococcaceae (Rich 2005). They are mostly harmless commensals of the skin and mucous membranes, but are potentially pathogenic to humans and many other animal species (Vanni et al. 2009). Nearly all cases of pyoderma in dogs are caused by Staphylococcus intermedius (DeBoer 2006). The name S. intermedius was proposed for isolates that differed from Staphylococcus aureus in various biochemical reactions and with regard to cell wall composition (Hajek 1976). The name Staphylococcus pseudintermedius is now given to the canine-specific strain of S. intermedius. For the purpose of this study, these canine-specific isolates will be referred to as S. intermedius. Staphylococcus intermedius is also an important cause of wound infections, otitis externa, cystitis and ocular and respiratory disease. Similar to S. aureus colonisation seen in humans, healthy dogs frequently carry S. intermedius as part of their normal microflora. It is a transient inhabitant of the skin and hair coat. Reservoir sites include the oral and nasal cavities as well as the perineum and anus (Hartmann et al. 2005). Although S. intermedius is not usually isolated from humans owing to its host-specificity for canine corneocytes, transmission between humans and their pets has been demonstrated (Fitzgerald 2009).
Since the introduction of antimicrobials, Staphylococcus species have shown rapid development and increased spread of resistance, particularly in nosocomial infections (Werckenthin et al. 2001). Antimicrobial resistance is of increasing concern in both veterinary and human medicine as it has led to treatment failures and hence increased morbidity, mortality and treatment costs (Pellerin et al. 1998). Owing to increased attention to small-animal welfare antimicrobial agents are increasingly prescribed for pets, including preparations formerly reserved for human use and the treatment of human infections (Guardabassi, Schwartz & Lloyd 2004b). Deep pyoderma associated with S. intermedius is possibly the most common reason for administration of antimicrobials in dogs (Guardabassi, Loeber & Jacobson 2004a). The choice of an antimicrobial is usually empirical. This approach (i.e. prescribing drugs without the use of microbiological culturing and sensitivity testing) is probably a major contributor to the emergence of resistant staphylococci strains (Lilenbaum et al. 2000). Antimicrobial resistance is, however, complex and involves various bacterial species, resistance mechanisms, transfer mechanisms and reservoirs (Guardabassi et al. 2004b).
Awareness and monitoring of antimicrobial resistance in veterinary staphylococcal isolates is required as the development of resistance in animal pathogens can result in treatment failure in individual patients and resultant zoonotic risk to pet owners. Moreover, increases in resistance to antimicrobial classes that are important in human medicine may result in the withdrawal of previously available antibacterial agents from veterinary use (Loeffler et al. 2007).
The in vitro susceptibility of a pathogen to an antimicrobial agent can be assessed by disc diffusion or by measuring the minimum inhibitory concentration (MIC), both of which indicate the lowest drug concentration capable of inhibiting the growth of the bacterium under investigation. Susceptibility testing is controlled with regard to medium, atmosphere and temperature conditions and incubation duration (Blondeau 2009) to facilitate suitable incubation conditions.
The Kirby-Bauer disc diffusion method is a flexible and relatively inexpensive technique that is commonly used in diagnostic laboratories. Dilution methods offer flexibility in the sense that standard media used to test for frequently encountered organisms can be supplemented or replaced with alternative media to allow for accurate testing of fastidious bacteria, which may otherwise not be reliably surveyed by disc diffusion. Dilution methods can also be adapted for automation. The flexibility of dilution testing is also evident in the reporting formats that may be used. Results can be reported quantitatively (µg/mL) or categorically (susceptible, intermediate and resistant), although the two approaches can also be used simultaneously (Murray et al. 1999).
Materials and methods
Sampling
Canine skin samples submitted to Vetdiagnostix Veterinary Pathology Services for microbiological culture and sensitivity testing and from which S. intermedius was isolated were selected for this investigation. A total of 319 samples from male and female dogs of various ages and breeds from across South Africa were included in this study. Duplicates were not excluded. Samples included skin swabs, skin biopsies, skin abscess and pustule swabs, and fine needle aspirates.
Identification of Staphylococcus intermedius
Following growth on 5% sheep blood agar after 24 h incubation at 37 °C, S. intermedius was identified on the basis of colony characteristics, catalase production, Gram's stain, lack of pigment production, delayed acid production from D-mannitol, slow or weak maltose production and positive Deoxyribonuclease (DNase) reaction on DNase agar (Quinn et al. 1994). All media used were quality controlled using S. aureus ATCC 25923. The canine-specific strain of S. intermedius (now officially known as S. pseudintermedius) can be determined accurately only by DNA sequencing. The isolates found in this study will therefore be referred to as S. intermedius, as phenotypic typing cannot reliably distinguish the canine-specific species from others.
Antimicrobial susceptibility tests
Bacteria identified as S. intermedius in this study were tested for antimicrobial susceptibility by the disc diffusion method on Mueller Hinton agar. A suspension of the test organism in sterile saline (0.5 McFarland) was evenly spread onto the Mueller Hinton agar plates, after which the plates were disked and incubated at 37 °C for 18 h -24 h (CLSI 2008).
All isolates were tested against the following antimicrobial agents: ampicillin (10 µg), cephalothin (30 pg), chloramphenicol (30 µg), amoxicillin-clavulanic acid (30 µg), enrofloxacin (5 µg), gentamicin (10 µg), neomycin (30 µg), oxacillin (1 IU), tetracycline (30 µg) and trimethoprim-sulphamethoxazole (co-trimoxazole) (25 µg). The isolates collected between June 2009 and June 2010 were tested against the following additional antimicrobial agents: amikacin (30 µg), ceftiofur (30 µg), clindamycin (2 µg), doxycycline (30 µg), erythromycin (15 µg), imipenem (10 µg), marbofloxacin (5 µg), penicillin (10 IU), rifampicin (5 µg) and ticarcillin (75 µg). After measuring the zones of inhibition, the strains were classified as sensitive or resistant to the drugs tested according to standards and criteria set out by the Clinical and Laboratory Standards Institute (CLSI 2008). The strains showing intermediate results were classified as sensitive or resistant depending on whether the reading was closer to the sensitive or the resistant cut-off.
In addition, the MIC results for the aforementioned antimicrobial drugs were determined for the S. intermedius isolates collected between June 2009 and June 2010. The laboratory does not routinely make use of the MIC method and therefore retrospective data were not available for isolates. The broth microdilution method was used and commercial COMPAN1F Sensititre MIC plates were obtained for this purpose (Trek Diagnostics). A 0.5-McFarland suspension of the test organism was prepared in sterile saline and 10 µL of this suspension was added to 990 µL of cation-adjusted Mueller Hinton broth. Chilled calcium and magnesium ion stock solutions were added at 0.1 mL per litre for each desired increment of 1 mg/L in the final concentration in the adjusted Mueller Hinton broth (CLSI 2008). An aliquot of 100 µL of this inoculated broth suspension was then pipetted into each of the 96 wells on the commercial microtitre plate, which was incubated at 37 °C for 18 h - 24 h.
Data analysis
Microsoft Excel was used to calculate, compare and graph the resultant parameters. The statistical analysis is descriptive, using numerator or denominator data.
Results
Susceptibility testing
Staphylococcus intermedius isolates showed greatest antimicrobial resistance to ampicillin, followed by tetracycline and then potentiated sulphonamides (Table 1 and Figure 1). In general, demonstrable antimicrobial resistance was low. Very few methicillin-resistant isolates were detected. Temporal trends were not noted, except for ampicillin, with isolates becoming more susceptible, and potentiated sulphonamides (co-trimoxazole), with isolates becoming more resistant.
Kirby-Bauer and minimum inhibitory concentration data for the period 2007-2010
In general, both the Kirby-Bauer and broth dilution MIC tests yielded similar results for the antimicrobial agents tested (Table 2 and Figure 2).
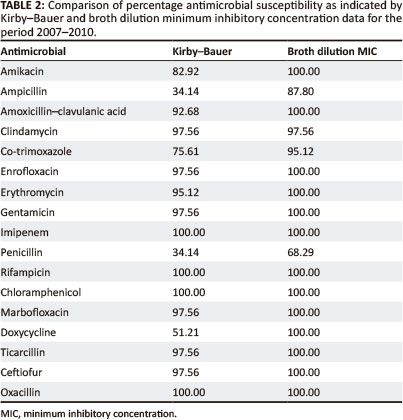
Table 3 shows the percentage MIC distribution of isolates for each dilution as well as the MIC50 (median) and MIC90 values. The percentage resistance represented in the table was based on published breakpoints (CLSI 2008). Using the MIC method, all of the tested isolates were found to be completely sensitive to ticarcillin, oxacillin, amoxicillin-clavulanic acid, imipenem, ceftiofur, chloramphenicol, doxycycline, gentamicin, amikacin and co-trimoxazole. Of the isolates tested, 2% - 40% showed some level of resistance to erythromycin, penicillin, ampicillin, enrofloxacin, clindamycin and marbofloxacin. The highest level of resistance was against erythromycin.
Discussion
The antimicrobial resistance predicament in human medicine has brought to light various aspects of the use of these substances in animals. There is, however, little useful information on antimicrobial resistance and hence optimal usage in companion animals (Prescott et al. 2002).
Susceptibility testing
Antimicrobial resistance of S. intermedins was generally low, with the greatest resistance recorded against ampicillin, tetracycline and potentiated sulphonamides. Very few methicillin-resistant isolates were detected. Temporal trends were not noted, except for isolates becoming more susceptible to ampicillin and more resistant to potentiated sulphonamides (co-trimoxazole). Pellerin et al. (1998) and Hartmann et al. (2005) similarly showed that resistance was most commonly observed to penicillin, tetracycline and sulphamethoxazoletrimethoprim (co-trimoxazole). These drugs should not be used without prior susceptibility testing (Aarestrup 2006). S. intermedins is less resistant to erythromycin, clindamycin, cephalexin, oxacillin, amoxicillin-clavulanic acid, enrofloxacin, marbofloxacin and gentamicin, with most strains being susceptible to these drugs (Pellerin et al. 1998). Werckenthin et al. (2001) found that resistance to penicillin and tetracycline is common in S. intermedins and is on the increase. Resistance to most other antimicrobials, particularly newer-generation antimicrobial agents such as the fluoroquinolones is still comparatively low.
Kirby-Bauer and minimum inhibitory concentration data
In general, both the Kirby-Bauer and broth dilution MIC tests yielded similar results for the antimicrobial agents tested. The main difference between the two tests was evident in the over-estimation of resistance by the Kirby-Bauer test for ampicillin, co-trimoxazole, penicillin and doxycycline. This could be related to the instability of these particular drugs in vitro. Inoculum densities may also have played a role, with denser inocula producing smaller zone sizes for the drugs tested.
The Kirby-Bauer method remains a convenient, low-cost means of conducting antimicrobial susceptibility tests and is widely used in veterinary laboratories. The test provides qualitative results that categorise isolates as susceptible, intermediate or resistant. Almost all veterinary-specific agents are available in the antimicrobial-impregnated discs. However, low-volume veterinary-specific agents may be available only from the pharmaceutical manufacturer. Smaller veterinary laboratories may experience difficulties standardising the inoculum used in this method; however, commercial systems are available for this purpose (Aarestrup 2006).
Based on the results of the MIC method, all of the isolates tested were found to be completely sensitive to ticarcillin, oxacillin, amoxicillin-clavulanic acid, imipenem, ceftiofur, chloramphenicol, doxycycline, gentamicin, amikacin and co-trimoxazole. Of the isolates tested, 2% - 40% showed some level of resistance to the following antimicrobials: erythromycin, penicillin, ampicillin, enrofloxacin, clindamycin and marbofloxacin. The highest level of resistance was shown to erythromycin. The genes that confer erythromycin resistance in canine staphylococci are almost exclusively ermB genes. The increase in resistance to the lincosamides, lincomycin, clindamycin and erythromycin may be attributed to the increased use of these drugs in the last decade(Pellerin et al. 1998).
The MIC method may be performed in a variety of ways. This method can be used either to provide a quantitative result or to categorise the organism as susceptible or resistant. Standardised methods for testing more fastidious organisms such as anaerobes and Campylobacter species have been developed. The MIC method is preferred for use in surveillance or epidemiological investigations as it allows for calculation of summary statistics. Of the various MIC formats used, the broth microdilution method is most widely used and is available in a variety of commercial systems as either dry or frozen panels. It permits testing of a wide range of antimicrobials on a small scale. However, these MIC panels can be inflexible and using custom panels could incur additional cost for a laboratory. Furthermore, not all veterinary-specific antimicrobial agents are available on all panels. Laboratories involved in surveillance programmes or epidemiological studies usually prefer to test a smaller number of antimicrobial agents for an extended number of dilutions. Many diagnostic laboratories choose to use a breakpoint panel. Breakpoint panels allow the laboratory to test a larger number of compounds with dilution ranges spanning the interpretive criteria or breakpoints for each agent (Aarestrup 2006).
Limitations of the study
Recommendations
Bacteria that exhibit antimicrobial resistance will continuously evolve. The bacterial resistance dilemma in human medicine has highlighted the rapid emergence of community-acquired resistance. Data on antimicrobial resistance and trends in antimicrobial use in companion animal practice are still relatively limited. Advice to veterinarians on antimicrobial use needs to be agreed on and continuously monitored and revised. Active and effective infection control programmes need to be implemented in veterinary hospitals to minimise the spread of resistant organisms or their resistance genes. Owing to the limited data available it is imperative that veterinary clinical microbiologists agree on standards for reporting and monitoring resistance and the relationship with trends in antimicrobial use.
Conclusion
Antimicrobial resistance patterns largely reflect evolving patterns in the use of antimicrobial drugs. Laboratory reports of resistance often result from treatment failures rather than treatment successes, as animals that have been treated previously will be more likely to yield resistant bacteria than those that have not. Antimicrobial resistance in canine bacterial pathogens is possibly of less concern than in human pathogens as pets are exposed to antimicrobial agents for shorter periods and less frequently. Pets are also less likely to be hospitalised. Euthanasia is often a preferred option in chronically ill pets and financial constraints restrict the use of agents such as imipenem (Prescott et al. 2002). S. intermedins isolates from dogs have developed increased resistance to some drugs but decreased resistance to others. These changes reflect the evolving patterns in antimicrobial use over time. It is the opinion of the authors that antimicrobial resistance is not yet at a critical stage but should be monitored carefully. More information is needed on antimicrobial resistance and its molecular basis in canine medicine.
Acknowledgements
This study was conducted at the Vetdiagnostix Veterinary Pathology Services laboratory under the supervision of Dr J. Picard and Prof. M. van Vuuren of the University of Pretoria.
Competing interests
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Authors' contributions
J.P. (University of Pretoria) and M.v.V. (University of Pretoria) were the supervisors of the project. C.A.B. (Vetdiagnostix) was responsible for conducting the research, including all experimental procedures, and writing the article.
References
Aarestrup, F.M., 2006, Antimicrobial resistance in bacteria of animal origin. ASM Press, Washington DC. [ Links ]
Blondeau, J.M., 2009, 'New concepts in antimicrobial susceptibility testing: the mutant prevention concentration and mutant selection window approach', Veterinary Dermatology 20, 383-396. http://dx.doi.org/10.1111/j.1365-3164.2009.00856.x, PMid:20178475 [ Links ]
CLSI. See Clinical and Laboratory Standards Institute Clinical and Laboratory Standards Institute, 2008, Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: approved standard, 3rd edn., (CLSI document M31 - A3), Clinical and Laboratory Standards Institute, Philadelphia. [ Links ]
DeBoer, D.J., 2006, 'Canine staphylococcal pyoderma', US Companion Animal Health, 2006, pp. 26-28, viewed 28 April 2009, from www.touchbriefings.com/pdf/2397/deboer.pdf. [ Links ]
Fitzgerald, J.R., 2009. 'The Staphylococcus intermedius group of bacterial pathogens: species re-classification, pathogenesis and the emergence of methicillin resistance', Veterinary Dermatology 20, 490-495. http://dx.doi.org/10.1111/jM365-3164. 2009.00828.x, PMid:20178486 [ Links ]
Guardabassi, L., Loeber, M.E. & Jacobson, A., 2004a, 'Transmission of multiple antimicrobial-resistant Staphylococcus intermedius between dogs affected by deep pyoderma and their owners', Veterinary Microbiology 98, 23-27. http://dx.doi.org/10.1016/j.vetmic.2003.09.021, PMid:14738778 [ Links ]
Guardabassi, L., Schwartz, S. & Lloyd, D.H., 2004b, 'Pet animals as reservoirs of antimicrobial-resistant bacteria', Journal of Antimicrobial Chemotherapy 54, 321-332. http://dx.doi.org/10.1093/jac/dkh332, PMid:15254022 [ Links ]
Hajek, V., 1976, 'Staphylococcus intermedius, a new species isolated from animals', International Journal of Systematic Bacteriology 26, 401-408. http://dx.doi.org/10.1099/00207713-26-4-401 [ Links ]
Hartmann, F.A., White, D.G., West, S.E.H., Walker, R.D. & DeBoer, D.J., 2005, 'Molecular characterisation of Staphylococcus intermedius carriage by healthy dogs and comparison of antimicrobial susceptibility patterns to isolates from dogs with pyoderma', Veterinary Microbiology 108, 119-131. http://dx.doi.org/10.1016/j.vetmic.2005.03.006, PMid:15917140 [ Links ]
Lilenbaum, W., Veras, M., Blum, E. & Souza, G.N., 2000, 'Antimicrobial susceptibility of staphylococci isolated from otitis externa in dogs', Letters in Applied Microbiology 31,42-45. http://dx.doi.org/10.1046/j.1472-765x.2000.00759.x, PMid:10886613 [ Links ]
Loeffler, A., Linek, M., Moodley, A., Guardabassi, L., Sung, J.M.L., Winkler, M. et al., 2007, 'First report of multiresistant mecA-positive Staphylococcus intermedius in Europe: 12 cases from a veterinary dermatology referral clinic in Germany', Veterinary Dermatology 18, 412-419. http://dx.doi.org/10.1111/j.1365-3164.2007.00635.x, PMid:17991158 [ Links ]
Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C. & Yolken, R.H., 1999, Manual of clinical microbiology, ASM Press, Washington DC. [ Links ]
Pellerin, J.L., Bourdeau, P., Sebbag, H. & Person, J.M., 1998, 'Epidemiosurveillance of antimicrobial compound resistance of Staphylococcus intermedius clinical isolates from canine pyodermas', Comparative Immunology, Microbiology and Infectious Diseases 21, 115-133. http://dx.doi.org/10.1016/S0147-9571(97)00026-X [ Links ]
Prescott, J.F., Hanna, W.J.B., Reid-Smith, R. & Drast, K., 2002, 'Antimicrobial drug use and resistance in dogs', Canine Veterinary Journal 43, 107-116. PMid:11842592 [ Links ]
Quinn, P.J., Carter, M.E., Markey, B. & Carter, G.R., 1994, Clinical veterinary microbiology, Wolfe Publishing, London. [ Links ]
Rich, M., 2005, 'Staphylococci in animals: prevalence, identification and antimicrobial susceptibility, with an emphasis on methicillin-resistant Staphylococcus aureus', British Journal of Biomedical Science 62, 98-104. PMid:15997888 [ Links ]
Vanni, M., Tognetti, R., Pretti, C., Crema, F., Soldani, G., Meucci, V. et al., 2009, 'Antimicrobial susceptibility of Staphylococcus intermedius and Staphylococcus schleiferi isolated from dogs', Research in Veterinary Science 87, 192-195. http://dx.doi.org/10.1016/j.rvsc.2009.01.011, PMid:19268332 [ Links ]
Werckenthin, C., Cardoso, M., Martel, J.L. & Schwartz, S., 2001, 'Antimicrobial resistance in staphylococci from animals with particular reference to bovine Staphylococcus aureus, porcine Staphylococcus hyicus and canine Staphylococcus intermedius', Veterinary Research 32, 341-362. http://dx.doi.org/10.1051/vetres:2001129, PMid:11432424 [ Links ]
 Correspondence:
Correspondence:
Catherine Blunt
PO Box 13624
Cascades 3202, South Africa
catherineblunt@hotmail.com
Received: 30 May 2012
Accepted: 08 Mar. 2013
Published: 16 May 2013














