Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the South African Veterinary Association
versión On-line ISSN 2224-9435
versión impresa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.84 no.1 Pretoria ene. 2013
ORIGINAL RESEARCH
Pharmacokinetics, urinary excretion and plasma protein binding of ofloxacin in water buffalo calves (Bubalus bubalis)
Ajay K. Ola; Harpal S. Sandhu; Vinod K. Dumka; Bibhuti Ranjan
Department of Pharmacology and Toxicology, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana-141004, India
ABSTRACT
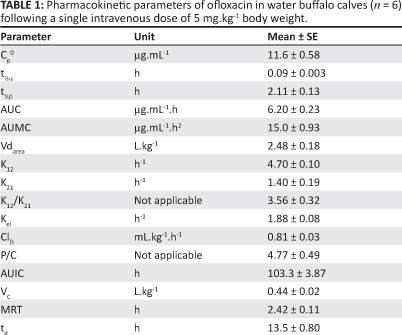
Pharmacokinetics and urinary excretion of an intravenous dose of 5 mg.kg-1 ofloxacin were investigated in water buffalo calves. Plasma concentrations of ofloxacin were determined by high-performance liquid chromatography. Ofloxacin was rapidly distributed from the central to the peripheral compartment as evidenced by a short distribution half-life (0.09 h ± 0.003 h) and high K12 (4.7 h-1 ± 0.1 h-1), and was detected in plasma for 8 h. The large volume of distribution (2.48 L.kg-1 ± 0.18 L.kg-1) obtained in this study indicated high distribution of ofloxacin in water buffalo calves. The elimination half-life, the area under the plasma drug concentration-time curve and total body clearance were 2.11 h ± 0.13 h, 6.20 mg.mL-1 ± 0.23 mg.mL-1.h and 0.81 mL.kg-1.h-1 ± 0.03 mL.kg-1.h-1, respectively. About 18.7% of administered drug was bound to plasma proteins and approximately 32.5% of the administered dose was recovered in urine within 48 h. The results of the study indicated a favourable pharmacokinetic profile of ofloxacin in water buffalo calves, which suggests that ofloxacin may be effective against urinary pathogens in this species.
Introduction
Fluoroquinolones have gained widespread acceptance in veterinary medicine thanks to their broad spectrum of activity against Gram-positive and Gram-negative aerobes, mycoplasma and rickettsia (Brown 1996). These antibacterials are currently used for the treatment of invasive and systemic bacterial infections, such as upper and lower respiratory infections, gonorrhoea, bacterial gastroenteritis, skin and soft tissue infections and urinary tract infections that occur in humans and animals (Duan & Yuan 2001). Ofloxacin, (±)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4 benzoxazine-6 carboxylic acid (Lode et al. 1987), a synthetic fluoroquinolone, was introduced in the late 1980s (Andersson & MacGowan 2003). It possesses a broad spectrum of activity against a variety of Gram-positive and Gram-negative bacteria, including some anaerobes. Pharmacokinetic studies of ofloxacin after intravenous administration have been conducted in humans (Bethell et al. 1996), dogs (Yoshida et al. 1998), swine (Son et al. 2000), goats (Baruah et al. 2004), cattle (Gaur et al. 2004), chicken (Kalaiselvi et al. 2006) and rabbits (Ahmad et al. 2008). However, there is little information available on the pharmacokinetics of ofloxacin in buffalo species. In view of the marked variation in the kinetic behaviour of antimicrobial drugs amongst species, the present study was undertaken to determine the pharmacokinetics, in vitro plasma protein binding and urinary excretion following a single intravenous administration of ofloxacin in water buffalo calves.
Materials and methods
The study was conducted in six healthy male water buffalo calves aged between 6 and 12 months and weighing between 75 kg and 120 kg. The animals were obtained from the university dairy farm and acclimatised in the departmental animal shed under standard conditions of management for two weeks prior to the commencement of the study. They were maintained on green fodder and water ad libitum. Ofloxacin was administered at a dose of 5 mg.kg-1 body weight into the left jugular vein.
To conduct the pharmacokinetic study, the animals were kept in metabolic stalls of standard size. Blood samples (5 mL) were withdrawn from the contralateral jugular vein into heparinised glass centrifuge tubes before administration of the drug and again at 1 min, 2.5 min, 5 min, 7.5 min, 10 min, 15 min, 20 min, 30 min, 1 h, 2 h, 3 h, 4 h, 6 h, 8 h, 10 h, 12 h, 16 h and 24 h after administration of the drug. Plasma was separated by centrifugation at 1300 g for 15 min at room temperature and kept at -20 °C until analysis, usually on the day after collection. Urine samples were collected from each animal at predetermined time intervals (2 h, 4 h, 6 h, 8 h, 12 h, 16 h, 24 h, 36 h and 48 h) after administration of the drug. The metabolic stalls are designed in such a way that the total amount of urine excreted by the animals within a period is automatically collected without contamination or spillage in containers placed beneath the stalls. At the end of a given time interval, the total volume of urine collected in the container was measured for each animal and, after filtration, 2 mL samples were extracted for analysis.
In vitro binding of ofloxacin to plasma proteins was determined according to the equilibrium dialysis technique (Lode et al. 1987) to yield protein binding constants. Various concentrations of ofloxacin, ranging from 1 mg.mL-1 to 20 mg.mL-1, were prepared in pooled plasma sampled from untreated animals. Each dialysis bag (pore size = 4 Å), filled with 5 mL plasma containing a known concentration of ofloxacin, was then immersed in a separate tube containing 5 mL phosphate buffer (0.2 M; pH = 7.4). The tubes were incubated at 37 °C for 24 h, with occasional shaking. At the end of the incubation period the buffer and contents of the dialysis bags were analysed separately to determine the concentration of ofloxacin present. Dialysis of each prepared ofloxacin concentration was analysed in triplicate. The extent of in vitro plasma protein binding of ofloxacin was calculated according to:

where CP1 is the concentration of ofloxacin in plasma after incubation, CB is the concentration of ofloxacin in phosphate buffer after incubation and CP is the concentration of ofloxacin in plasma before incubation. Binding capacity of the plasma protein to ofloxacin (βi) and the dissociation rate constant of the protein drug complex (Kβ) were calculated according to the method of Pilloud (1973).
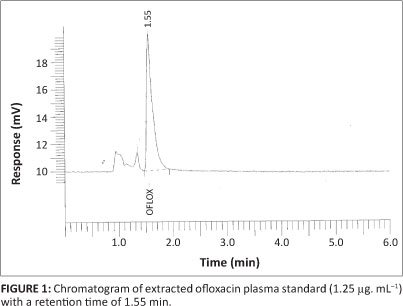
The concentration of ofloxacin was analysed using high-performance liquid chromatography (HPLC) (Kalaiselvi et al. 2006). Briefly, 300 µL of plasma was added to 440 µL of acetonitrile (1:1.5). The mixture was vortexed for 15 s and centrifuged for 10 min at 900 g. The supernatant was transferred to a 2 mL microcentrifuge tube. Double the volume of HPLC-grade water was added. The aliquot was filtered through a 0.2 µm nylon membrane filter, after which 20 µL filtrate was injected into the HPLC system (Perkin Elmer Series 200, Massachusetts, USA) equipped with an autosampler, diode array detector, 200 µL loop size and a reverse-phase C-18 column (particle size = 5 µm; column length = 150 mm χ 4.6 mm) and measured at 290 nm. The data were analysed with manufacturer-supplied software (TotalChrom Navigator version 6.3.1). The mobile phase comprised 0.1 M orthophosphoric acid (pH adjusted to 2.5 using 45% KOH) and acetonitrile, mixed in a ratio of 75:25. The flow rate of the mobile phase was 1.2 mL.min-1 and the retention time of ofloxacin was 1.55 min. The chromatogram of the ofloxacin standard prepared in plasma of untreated buffalo calves is shown in Figure 1. The validated limit of ofloxacin quantification for this method was 0.03 µg.mL-1. Extraction recoveries were greater than 94% and the coefficient of variation was less than 10%. Linear calibration curves were obtained for a concentration range of 0.03 µg.mL-1 - 10.0 µg.mL-1 (R2 > 0.9999). Intra-day precision was estimated from six replicates of three standard samples used for calibration curves (RSD < 10%). Inter-day precision was estimated from the analysis of standard samples on three separate days (RSD < 10%).
Pharmacokinetic parameters were calculated manually using regression analysis (Gibaldi & Perrier 1982). The means of pharmacokinetic variables were obtained by averaging the variables calculated for drug disposition after intravenous drug administration to each animal.
Ethical considerations
The experimental protocol followed the ethical guidelines on the proper care and use of animals and was approved by the institutional animal ethics committee (IAEC Reg. no. No.497/01/a/CPCSEA dt. 18.2.2002 vide approval no. VPS/2008/874-885 Dt.15.5.08).
Results
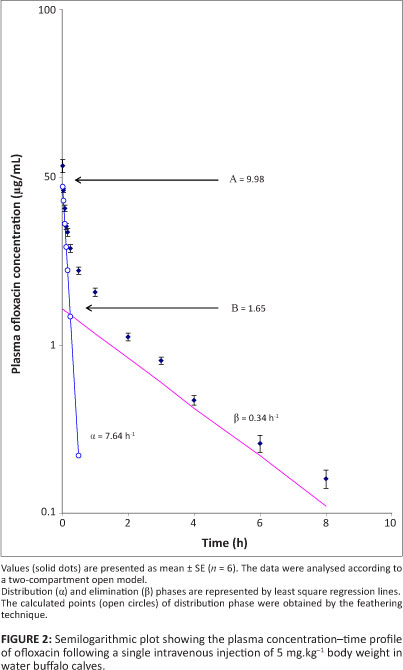
The plasma concentration-time profile of ofloxacin is presented in Figure 2. At 1 min, the mean plasma concentration was 11.7 µg.mL-1 ± 1.03 mg.µL-1. The drug was detected in plasma for up to 8 h after dosing (0.16 µg.mL-1 ± 0.02 µg.mL-1). Evaluation of the results revealed that plasma disposition of ofloxacin followed a two-compartment open model, which is adequately described by the bi-exponential equation:

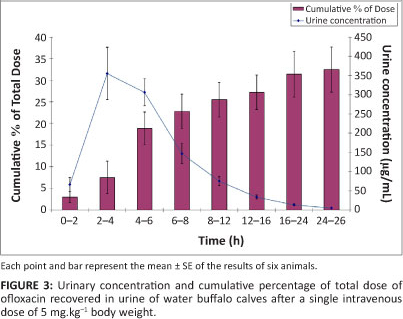
where Cp is the plasma level of ofloxacin at time t and e represents the base of the natural logarithm. A and B are the extrapolated zero-time intercepts of the distribution and elimination phases, respectively. The distribution and elimination rate constants are represented by α and β, respectively. The pharmacoldnetit: parameters that describe the disposition pattern of ofloxacin in water buffalo calves are presented in Table 1. A graphical representation of the mean urinary concentration of ofloxacin and the cumulative percentage of drug excreted in urine at different time intervals is shown in Figure 3. Table 2 shows the extent and kinetic constants of in vitro binding of different concentrations of ofloxacin to plasma proteins in water buffalo calves.
Discussion
In accordance with these findings, a two-compartment open model for disposition of ofloxacin has also been reported in calves (Gaur et al. 2004; Parry et al. 2007). Minimum inhibitory concentration (MIC90) values of ofloxacin against some of the common isolates of veterinary importance, including Salmonella, are reported to be as low as 0.06 µ&ηΛ-1 (Greene & Budsberg 1993; Parry et al. 2007). Therefore, for purposes of this discussion the MIC90 of 0.06 mg.mL-1 of ofloxacin has been taken into consideration. At 1 min after injection, the plasma level (11.7 mg.mL-1 ± 1.03 mg.mL-1) was approximately 195-fold higher than the MIC of ofloxacin. The drug was detected above the MIC level for up to 8 h after administration. Comparable plasma levels of ofloxacin (14.76 µg.mL-1) have been reported 1 min after a single intravenous injection in goats (Baruah et al. 2004), but low initial plasma levels (4.26 µg.mL-1) were reported in calves (Gaur et al. 2004). Ofloxacin was rapidly transferred from the central to the peripheral compartment in water buffalo calves, as is evident from the short distribution half-life (0.09 h ± 0.003 h) and high values of K12 (4.7 h-1 ± 0.1 h-1) and the K12/K21 ratio (3.56 ± 0.32). Low values of t½α in calves (0.12 h) and rabbits (0.7 h) and a high K12/K21 ratio (1.15) in calves have also been reported after intravenous administration of ofloxacin (Ahmad et al. 2008; Mohan & Garg 2006).
The values of the area under the plasma drug concentration-time curve (AUC) and the area under the first moment of plasma drug concentration-time curve (AUMC) were 6.2 ìg.mL-1.h ± 0.23 µg.mL-1.h and 15.0 µg.mL-1.h2 ± 0.93 µg.mL-1. h2, respectively. In contrast, high values have been reported for AUC of ofloxacin in calves (26.5 µg.mL-1.h) and goats (58.9 µg.mL-1.h) following a dose of 5mg.kg-1 (Baruah et al. 2004; Gaur et al. 2004). The large Vdarea(2.48 L.kg-1 ± 0.18 L.kg-1) measured in these buffalo calves indicated extensive distribution of ofloxacin. In accordance with this observation, large volumes of distribution have been reported for ofloxacin in calves (1.4 L.kg-1), goats (2.18 L.kg-1), swine (2.06 L.kg-1), rabbits (4.47 L.kg-1 ± 0.11 L.kg-1) and poultry (1.76 L.kg-1) after intravenous injection (Ahmad et al. 2008; Baruah et al. 2004; Kalaiselvi et al. 2006; Mohan & Garg 2006; Son et al. 2000). Furthermore, a high P/C ratio (4.77 ± 0.49) observed in the present study indicated good penetration of the drug. Similar values have also been reported for ofloxacin in calves (4.32) after intravenous administration (Gaur et al. 2004).
Total body clearance of ofloxacin, which represents the sum of the metabolic and excretory processes, was 810 mL.kg-1.h-1 ± 3.0 mL.kg-1.h-1 in the examined buffalo calves. Rapid clearance of ofloxacin, as indicated by high values of ClB, has also been shown in calves (430 mL.kg-1.h-1) goats (135.6 mL.kg-1.h-1) and poultry (317.6 mL.kg-1.h-1.) after intravenous administration (Baruah et al. 2004; Kalaiselvi et al. 2006; Mohan & Garg 2006).
The elimination half-life of ofloxacin in the present study was 2.11 h ± 0.13 h, which is comparable to that measured in calves (2.21 h), rabbits (1.77 h) and in broilers (4.46 h) (Ahmad et al. 2008; Kalaiselvi et al. 2006; Mohan & Garg 2006). However, in contrast to the present findings, a very long elimination half-life for ofloxacin has been reported in goats (15.58 h) after intravenous administration (Baruah et al. 2004). The value of MRT of ofloxacin was 2.42 h ± 0.11 h in buffalo calves. In support of the present finding, high MRT values have been reported for ofloxacin in rabbits (2.18 h) and chicken (5.48 h) after intravenous administration (Ahmad et al. 2008; Kalaiselvi et al. 2006).
The concentration of ofloxacin in the urine of the water buffalo calves was very high (more than 85 times the MIC90), even 48 h after administration and approximately 32.5% of the administered drug was recovered in the urine. Similar results have been reported in bovines after intravenous administration of levofloxacin, with 38.4% of the total administrated dose being recovered in urine of calves within 24 h (Dumka et al. 2008 2008). These findings suggest that ofloxacin may be an appropriate drug for treating urinary tract infections in bovines. However, contrary to the aformentioned findings, high urinary recovery (57.1% - 74.2%) has been reported after intravenous administration of ofloxacin in swine (Son et al. 2000).
Binding of drugs to plasma proteins plays a major role in their distribution, elimination and therapeutic effectiveness. The extent of protein binding has been reported to affect the therapeutic efficacy of drugs directly (Craig & Kunin 1976). Within a plasma concentration range of 1 mg.mL-1 - 20 mg.mL-1, the extent of plasma protein binding of ofloxacin ranged from 10.76% to 30.4%, with an overall mean of 18.67% ± 1.94%. Protein binding of ofloxacin in buffalo calves was concentration independent. The association and dissociation rate constant (Kp) ranges were measured as 5.7 x 10-6 mol.g-1 - 7.9 x 10-6 mol.g-1 and 8.1 x 10-6 mol.g-1 - 8.3 x 10-6 mol.g-1, respectively. The weak and low extent of binding, as evidenced by the lower association rate constant compared with the dissociation rate constant, indicated that ofloxacin bound to plasma proteins only to a moderate extent in water buffalo calves. In accordance with these findings, low plasma protein binding (15.28%) has also been reported in goats (Baruah et al. 2004).
Conclusion
The results of the study indicated a favourable pharmacokinetic profile of ofloxacin in water buffalo calves, which suggests that ofloxacin may be effective against urinary pathogens. The pharmacokinetic parameters such as mean elimination half-life, volume of distribution and body clearance need to be verified further in a larger population of this species.
Competing interests
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Authors' contributions
H.S.S. (Guru Angad Dev Veterinary and Animal Sciences University) was the project leader, V.K.D. (Guru Angad Dev Veterinary and Animal Sciences University) was responsible for experimental design, A.K.O. (Guru Angad Dev Veterinary and Animal Sciences University) performed most of the experiments and B.R. (Guru Angad Dev Veterinary and Animal Sciences University) was responsible for HPLC analysis of the samples. V.K.D. wrote the manuscript.
Acknowledgements
We duly acknowledge the funding and facilities provided by the Guru Angad Dev Veterinary and Animal Sciences University for conducting this study.
References
Ahmad, M., Raza, H., Murtaza, G. & Katar, N., 2008, 'Pharmacokinetic variations of ofloxacin in normal and febrile rabbits', Pakistan Veterinary Journal 28, 181-185. [ Links ]
Andersson, M.I. & MacGowan, A.P., 2003, 'Development of the quinolones', Journal of Antimicrobial Chemotherapy 51(Suppl 1), 1-11. http://dx.doi.org/10.1093/jac/dkg212, PMid:12702698 [ Links ]
Baruah, H., Roy, D.C., Roy, R.K. & Khonikor, H.N., 2004, 'Pharmacokinetics, tissue residue and plasma protein binding of ofloxacin in goats', Journal of Veterinary Science 5, 97-101. PMid:15192335 [ Links ]
Bethell, D.B., Day, N.P.J., Dung, N.M., McMullin, C., Loan, H.T., Tam, D.T.H. et al., 1996, 'Pharmacokinetics of oral and intravenous ofloxacin in children with multidrug-resistant typhoid fever', Antimicrobial Agents and Chemotherapy 40, 21672172. PMid:8878600 [ Links ]
Brown, S.A., 1996, 'Fluoroquinolones in animal health', Journal of Veterinary Pharmacology and Therapeutics 19, 1-14. http://dx.doi.org/10.1111/j.1365-2885.1996.tb00001.x, PMid:8992019 [ Links ]
Craig, W.A. & Kunin, C.M., 1976, 'Significance of serum protein and tissue binding of antimicrobial agents', Annual Review of Medicine 27, 287-300. http://dx.doi.org/10.1146/annurev.me.27.020176.001443, PMid:180874 [ Links ]
Duan, J. & Yuan, Z., 2001, 'Development of an indirect competitive ELISA for ciprofloxacin residues in food animal edible tissues', Journal of Agricultural Food Chemistry 49, 1087-1089. http://dx.doi.org/10.1021/jf000091j, PMid:11312816 [ Links ]
Dumka, V.K., Singh, H. & Srivastava, A.K., 2008, 'Disposition kinetics and urinary excretion of levofloxacin on concomitant administration with meloxicam in cross bred calves', Environmental Toxicology and Pharmacology 26, 56-60. http://dx.doi.org/10.1016/j.etap.2008.01.007, PMid:21783888 [ Links ]
Gaur, A., Saini, S.P.S., Garg, S.K., Chaudhary, R.K. & Srivastava, A.K., 2004, 'Pharmacokinetics of ofloxacin after a single intravenous bolus dose in neonatal calves', Journal of Veterinary Pharmacology and Therapeutics 27, 115-117. http://dx.doi.org/10.1111/j.1365-2885.2004.00493.x, PMid:15096110 [ Links ]
Gibaldi, M. & Perrier, D., 1982, Pharmacokinetics, Marcel Dekker, New York. [ Links ]
Greene, C.E. & Budsberg, S.C., 1993, Quinolone antimicrobial agents, Marcel Dekker, New York. [ Links ]
Kalaiselvi, L., Sriranjani, D., Ramesh, S., Sriram, P. & Mathuram, L.N., 2006, 'Pharmacokinetics of ofloxacin in broiler chicken', Journal of Veterinary Pharmacology and Therapeutics 29, 185-189. http://dx.doi.org/10.1111/j.1365-2885.2006.00729.x, PMid:16669862 [ Links ]
Lode, H., Höffken, G., Olschewski, P., Sievers, B., Kirch, A., Borner, K. et al., 1987, 'Pharmacokinetics of ofloxacin after parenteral and oral administration', Antimicrobial Agents and Chemotherapy 31, 1338-1342. http://dx.doi.org/10.1128/AAC.31.9.1338, PMid:3479046 [ Links ]
Mohan, A. & Garg, S.K., 2006, 'Pharmacokinetics of single intravenous bolus dose of ofloxacin in calves', Indian Journal of Pharmacology 38, 368-369. http://dx.doi.org/10.4103/0253-7613.27712 [ Links ]
Parry, C.M., Ho, V.A., Phuong, L.T., Bay, P.V.B., Lanh, M.N., Tung, L.T. et al., 2007, 'Randomized controlled comparison of ofloxacin, azithromycin, and an ofloxacin-azithromycin combination for treatment of multidrug-resistant and nalidixic acid-resistant typhoid fever', Antimicrobial Agents and Chemotherapy 51, 819-825. http://dx.doi.org/10.1128/AAC.00447-06, PMid:17145784 [ Links ]
Pilloud, M., 1973, 'Pharmacokinetics, plasma protein binding and dosage of oxytetracyoline in cattle and horses', Research in Veterinary Science 15, 224-230. [ Links ]
Son, D.S., Ikenoue, N., Tagawa, Y., Shimoda, M. & Kokue, E., 2000, 'Non-linear pharmacokinetics of ofloxacin after a single intravenous bolus dose in pigs', Journal of Veterinary Pharmacology and Therapeutics 23, 311-315. http://dx.doi.org/10.1046/j.1365-2885.2000.00289.x, PMid:11107005 [ Links ]
Yoshida, K., Yatre, K., Nishida, S. & Yamamoto, N., 1998, 'Pharmacokinetic disposition and arthropathic potential of oral ofloxacin in dogs', Journal of Veterinary Pharmacology and Therapeutics 21, 128-132. http://dx.doi.org/10.1046/j.1365-2885.1998.00114.x, PMid:9597650 [ Links ]
 Correspondence:
Correspondence:
Vinod Dumka
Postal address: Department of Pharmacology and Toxicology
Guru Angad Dev Veterinary and Animal Sciences University
Ludhiana-141004, India
Email: vkdumka@yahoo.com
Received: 14 May 2012
Accepted: 16 Feb. 2013
Published: 18 Apr. 2013














