Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.83 n.1 Pretoria Jan. 2012
CLINICAL COMMUNICATIONS
Avian bornavirus genotype 4 recovered from naturally infected psittacine birds with proventricular dilatation disease in South Africa
Robert D. LastI; Herbert WeissenböckII; Nora NedorostII; H.L. ShivaprasadIII
IVetdiagnostix - Veterinary Pathology Services, Cascades, South Africa
IIDepartment of Pathobiology, University of Veterinary Medicine, Austria
IIISchool of Veterinary Medicine, University of California, United States of America
ABSTRACT
The occurrence of proventricular dilatation disease caused by avian bornavirus (ABV) in captive psittacine birds has long been suspected in South Africa. This report documents the first detection by polymerase chain reaction and gene sequence analyses of ABV from three clinical cases of proventricular dilatation disease (PDD) in captive bred blue and gold macaws (Araara rauna) resident in this country. Lymphoplasmacytic encephalitis, gastrointestinal myenteric gangioneuritis and leiomyositis were the most prominent histopathological changes and ABV genotype 4 was detected in tissues from all three birds. Immunohistochemical stains for ABV antigen revealed positive labelling of neurons and glial cells of the brain, myenteric ganglia and nerve fibres as well as smooth muscle cells of the gastrointestinal tract of all three birds. In one bird, positive labelling of the peripheral nerves was observed. The identical sequence of the analaysed genome fragment of all three samples, history that all of these birds had originated from the same breeding facility, and young age at presentation raise the question of possible vertical transmission.
Introduction
Avian bornaviruses are a recently identified genetically heterogeneous group of representatives of the Bornaviridae family which are currently divided into at least 7 genotypes (Hoppes et al. 2010; Payne et al. 2011). These viruses - it is still not finalised whether they will be classified as different species or different genotypes or genogroups of a single virus species - are associated with proventricular dilatation disease (PDD), predominantly but not exclusively in psittacine birds (Gancz, Clubb & Shivaprasad 2010; Gancz et al. 2009; Gray et al. 2010; Honkavuori et al. 2008; Hoppes et al. 2010; Kistler et al. 2008; Weissenbock et al. 2009a). To date at least seven genotypes of avian bornavirus are known to occur (Hoppes et al. 2010; Payne et al. 2011). Birds may present with gastrointestinal dysfunction (dysphagia, regurgitation, passage of undigested food), neurological signs (ataxia, abnormal gait, proprioreceptive defects) or both, with emaciation (Gancz et al. 2009, 2010; Hoppes et al. 2010; Kistler et al. 2008).
Proventricular dilatation disease is a usually fatal disease of birds with worldwide occurrence, which is characterised by lymphoplasmacytic cell infiltrates into the central, peripheral and autonomic nervous systems and in some cases various visceral organ sites (Gancz et al. 2010; Hoppes et al. 2010; Kistler et al. 2010; Lierz et al. 2009; Ouyang et al. 2009; Wunschmann et al. 2011). In clinically affected birds, the condition is characterised by gastrointestinal motility disorders and/or neurological deficits (Gancz et al. 2010; Ouyang et al. 2009; Payne et al. 2011). Severe disease with widespread cell tropism has been described in birds infected with certain genotypes (most notably genotypes 2 and 4), as well as carrier birds re-exposed to virulent strains of the virus (Payne et al. 2011; Wunschmann et al. 2011). Avian bornavirus has to date been documented in more than 80 species of psittacine birds as well as several other avian species (Delnatte et al. 2011; Gancz et al. 2010; Gray et al. 2010; Hoppes et al. 2010; Weissenbock et al. 2009b; Wunschmann et al. 2011). As some of the species of birds known to be infected are on the critically endangered list, avian bornavirus is considered a major threat to aviculture worldwide, as these species rely on captive breeding for their survival (Gancz et al. 2010; Gray et al. 2010).
Proventricular dilatation disease has been previously reported in South Africa, although the implicated avian bornavirus was not identified in any of these cases (Gancz et al. 2010). Diagnosis had been based on consistent clinical data, gross pathological features and characteristic histopathology, as the link to avian bornavirus had not yet been established. The association of avian bornavirus with PDD led to the development of specific immunohistochemical (IHC), molecular and serological diagnostic assays (De Kloet, Kerski & De Kloet 2011; Gancz et al. 2010; Hoppes et al. 2010; Weissenbock et al. 2009a). The first identification and gene sequencing of avian borrnavirus recovered from clinical cases of PDD in exotic birds from South Africa is described.
Case history
Bird 1, a 3-month-old blue and gold macaw parrot (Araara rauna), was initially presented to the referring veterinarian in June 2010, experiencing difficulty lifting its wings. Five days later the bird started vomiting and was treated for a yeast overgrowth based on crop smears. Seven days later the bird was returned, emaciated, and cloacal swab smears suggested a bacterial overgrowth. Despite intensive antimicrobial therapy, the bird died three days later. At post-mortem examination, this bird was found to be emaciated, but no other significant gross abnormalities were observed.
A week later, bird 2, a 3-month-old blue and gold macaw parrot from the same bird fancier was submitted for postmortem examination. This bird had demonstrated wasting and gut stasis prior to death. The most prominent features on necropsy were emaciation, proventricular dilatation and the presence of undigested food throughout the entire gastrointestinal tract.
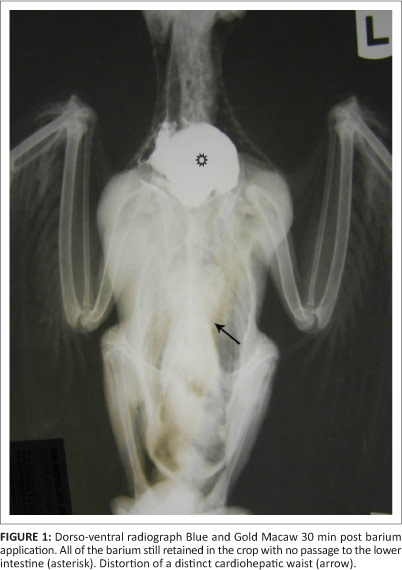
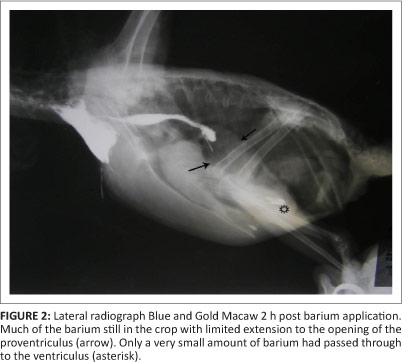
Bird 3, a 2-month-old blue and gold macaw from a different fancier, was examined in March 2011. This parrot had originated from the same breeding facility as birds 1 and 2. This particular bird presented with apparent ventricular obstruction. Radiographic studies revealed distortion of the shape of the cardiohepatic waist, and barium studies indicated very slow passage through to the ventriculus, with much of the barium remaining in the crop and eventually being regurgitated (Figures 1 and 2). A ventriculotomy was performed but no foreign object could be found. Despite supportive and antimicrobial therapy, the bird died two days later. A post-mortem examination was performed by the referring veterinarian and he described emaciation, haemorrhagic enteritis, nephrosis and pneumonia.
Histopathology and immunohistochemistry
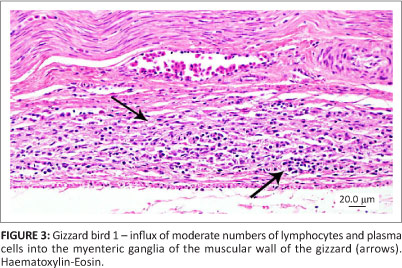
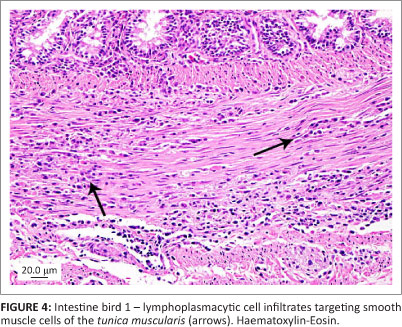
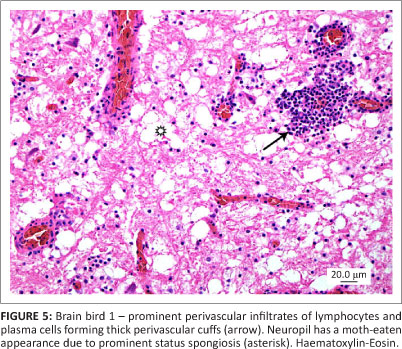
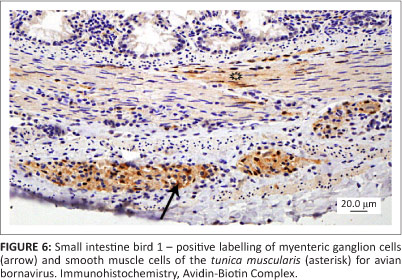
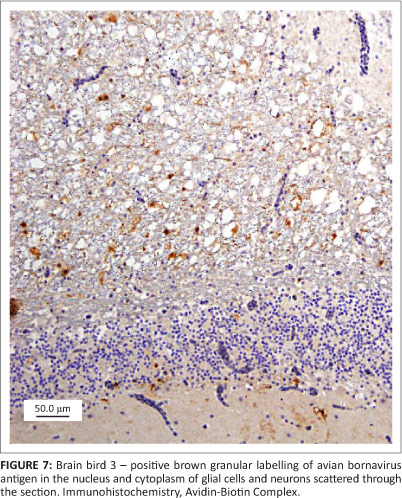
All three birds exhibited similar histological changes, with lymphoplasmacytic encephalitis plus myenteric ganglioneuritis and leiomyositis in the proventriculi, gizzards and intestine (Figures 3 and 4). The central nervous system changes were pronounced in bird 1 and characterised by lymphoplasmacytic perivascular cuffing and prominent status spongiosis of the neuropil (Figure 5). Immunohistochemical stains for avian bornavirus based on a previously published protocol (Weissenbock et al. 2009a) revealed positive viral antigen staining in neurons and glial cells of the brain, myenteric ganglion cells and smooth muscle cells of the gastrointestinal tract in all three birds, and in bird 2 one of the peripheral nerves labelled for avian bornavirus, but there was no cellular inflammatory response (Figures 6 and 7).
Polymerase chain reaction and sequencing
Multiple 10 µm recuts from the paraffin-embedded tissue blocks of all three birds were subjected to ribonucleic acid (RNA) extraction, followed by reverse transcription polymerase chain reaction (RT-PCR) and sequencing of the putative M gene region according to a previously published protocol (Weissenbock et al. 2009a). This primer pair amplified a 352 base-pair sequence, which corresponded to nucleotide positions 1908 to 2259 of the complete genome of avian bornavirus (ABV) strain 'bil' (GeneBank accession no. EU781967). Avian bornavirus sequences were recovered from all three birds and sequence analysis revealed that all three isolates were virtually identical. A basic local alignment search tool (BLAST) search against GenBank sequences confirmed these sequences as ABV genotype 4 which were a 100% - 99% identical to sequences derived from different psittacine species from Israel and Canada (GenBank accession numbers: FJ002341, GQ496351, FJ0023331, FJ002330, FJ002340,FJ002342).
Discussion
The nature of the pathology, intralesional demonstration of ABV antigen in histological sections with IHC stains and recovery of ABV genetic material from tissues of these birds confirm the occurrence of PDD in psittacines in South Africa. Specific avian bornavirus IHC stains, molecular techniques and serological assays are not yet routinely available in South Africa, and this has complicated the diagnostic work-up of suspect cases in this country. The extent of the distribution of ABV in different avian species in South Africa is currently unknown, but with the advent of specific pathogen detection procedures and serological techniques a more informed assessment of the extent of infection and risk of ABV to the avian populations in South Africa should be possible (De Kloet et al. 2011; Lierz et al. 2009). The new molecular techniques are also extremely useful in the ante-mortal diagnosis of PDD, complementing and enhancing diagnostic imaging techniques and largely replacing the previous 'gold standard' but highly invasive diagnostic procedure of crop biopsy for histopathology and IHC (Gancz et al. 2010).
Classically, the pathology of clinical cases of PDD has largely been limited to inflammation of neuroectodermal cells of the autonomic system of the gastrointestinal tract, central nervous system and adrenals (Gancz et al. 2010; Hoppes et al. 2010; Kistler et al. 2008; Lierz et al. 2009; Ouyang et al. 2009; Wunschmann et al. 2011). More recently, pancytotropic viral distribution involving epithelial cells of various organs in addition to neuroectodermal cells has been described in acute symptomatic infections involving ABV gentotypes 2 and 4 (Kistler et al. 2010; Payne et al. 2011; Wunschmann et al. 2011). The nature of the pathology in the birds described in this report, in conjunction with the pancytotropic tissue distribution of ABV antigen, would be consistent with that previously described for certain ABV genotype 4 isolates. Central nervous system lesions in these cases are also in line with the more pronounced central nervous system (CNS) pathology documented in recent reports (Hoppes et al. 2010; Keller et al. 2010; Ouyang et al. 2009; Payne et al. 2011; Wunschmann et al. 2011).
Sequence analysis of a part of the matrix protein gene produced an identical result all three cases. The sequence could be clearly assigned to the genotype 4. It cannot be stated with certainty that the identity of the three samples is due to an infection with the same genotype. The resolution at this particular rather conserved genome area is too small, showing only between one to seven nucleotide differences, and there is a chance that even slightly distinct genotypes show an identical sequence at this particular region. This information, in conjunction with the pathology described (especially CNS pathology), viral distribution, the fact that all had originated from the same breeding facility plus the young age at clinical presentation, raises the question of possible vertical transmission in these cases (Kistler et al. 2010; Lierz et al. 2011; Weissenbock et al. 2009a; Wunschmann et al. 2011).
Acknowledgements
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this paper.
Authors' contributions
R.D.L. (Veterinary Pathology Services), H.W. (University of Veterinary Medicine), N.N. (University of Veterinary Medicine), H.L.S. (University of California) contributed to this article.
References
De Kloet, A.H., Kerski, A. & De Kloet, S.R., 2011, 'Diagnosis of avian bornavirus infection in psittaciformes by serum antibody detection and reverse transcription polymerase chain reaction assay using feather calami', Journal of Veterinary Diagnostic Investigation 23, 421-429. http://dx.doi.org/10.1177/1040638711403406, PMid:21908270 [ Links ]
Delnatte, P., Berkvens, C., Kummrow, M., Smith, D.A., Campbell, D., Crawshaw, G. et al., 2011, 'New genotype of avian bornavirus in wild geese and trumpeter swans in Canada', Veterinary Record 169, 108. http://dx.doi.org/10.1136/vr.d4620,PMid:21784813 [ Links ]
Gancz, A.Y., Clubb, S. & Shivaprasad, H.L., 2010, 'Advanced diagnostic approaches and current management of proventricular dilatation disease', Veterinary Clinics of North America: Exotic Animal Practice 13, 471-494. http://dx.doi.org/10.1016/j.cvex.2010.05.004, PMid:20682431 [ Links ]
Gancz, A.Y., Kistler, A.L., Greninger, A.L., Farnoushi, Y., Mechani, S., Perl., S. et al., 2009, 'Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenates containing avian bornavirus 4', Virology Journal 6, 100-111. http://dx.doi.org/10.1186/1743-422X-6-100, PMid:19589169 [ Links ]
Gray, P., Hoppes, S., Suchodolski, P., Mirhosseini, N., Payne, S., Villanueva, I. et al., 2010, 'Use of avian bornavirus isolates to induce proventricular dilatation disease in conures', Emerging Infectious Diseases 16, 473-479. http://dx.doi.org/10.3201/eid1603.091257, PMid:20202423 [ Links ]
Honkavuori, K.S., Shivaprasad, H.L., Williams, B.L., Quan, P., Hornig, M., Street, C. et al., 2008, 'Novel bornavirus in psittacine birds with proventricular dilatation disease', Emerging Infectious Diseases 14, 1883-1886. http://dx.doi.org/10.3201/eid1412.080984, PMid:19046511 [ Links ]
Hoppes, S., Gray, P.L., Payne, S., Shivaprasad, H.L. & Tizard, I., 2010, 'The isolation, pathogenesis, diagnosis, transmission and control of avian bornavirus and proventricular dilatation disease', Veterinary Clinics of North America: Exotic Animal Practice 13, 495-508. http://dx.doi.org/10.1016/j.cvex.2010.05.014, PMid:20682432 [ Links ]
Keller, D.L., Honkavuori, K.S., Briese, T., Lipkin, W.I., Muthuswamy, A., Steinberg, H. et al., 2010, 'Proventricular dilatation disease associated with avian bornavirus in a scarlet macaw (Arama cao)', Journal of Veterinary Diagnostic Investigation 22, 961-965. http://dx.doi.org/10.1177/104063871002200619, PMid:21088184 [ Links ]
Kistler, A.L., Gancz, A., Clubb, S., Skewes-Cox, P., Fischer, K., Sorber, K. et al., 2008, 'Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: Identification of a candidate etiologic agent', Virology Journal 5, 88-103. http://dx.doi.org/10.1186/1743-422X-5-88, PMid:18671869 [ Links ]
Kistler, A.L., Smith, J.M., Greninger, A.L., DeRisi, J.L. & Ganem, D., 2010, 'Analysis of naturally occurring avian bornavirus infection and transmission during an outbreak of proventricular dilatation disease among captive psittacine birds', Journal of Virology 84, 2176-2179. http://dx.doi.org/10.1128/JVI.02191-09, PMid:19955301 [ Links ]
Lierz, M., Hafez, H.M., Honkavuori, K.S., Gruber, A.D., Olias, P., Abdelwhab, E.M. et al., 2009, 'Anatomical distribution of avian bornavirus in parrots, its occurrence in clinically healthy birds and ABV-antibody detection', Avian Pathology 38, 491496. http://dx.doi.org/10.1080/03079450903349238, PMid:19937538 [ Links ]
Lierz, M., Herden, C., Oberháuser, K., Heffels-Redmann, U. & Enderlein, D., 2011, 'Vertical transmission of avian bornavirus in psittacines', Emerging Infectious Diseases 17, 2390. http://dx.doi.org/10.3201/eid1712.111317, PMid:22172160 [ Links ]
Ouyang, N., Storts, R., Tian, Y., Wigle, W., Villanueva, I., Mirhosseini, N. et al., 2009, 'Histopathology and the detection of avian bornavirus in the nervous system of birds diagnosed with proventricular dilatation disease', Avian Pathology 38, 393401. http://dx.doi.org/10.1080/03079450903191036, PMid:19937526 [ Links ]
Payne, S., Shivaprasad, H.L., Mirhosseini, N., Gray, P., Hoppes, S., Weissenbock, H. et al., 2011, 'Unusual and severe lesions of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) acting as healthy carriers of avian bornavirus (ABV) and subsequently infected with a virulent strain of ABV', Avian Pathology 40, 15-22. http://dx.doi.org/10.1080/03079457.2010.536978, PMid:21331944 [ Links ]
Weissenbock, H., Bakonyi, T., Sekulin, K., Ehrensperger, F., Doneley, R.J.T., Durrwald, R. et al., 2009a, 'Avian bornaviruses in psittacine birds from Europe and Australia with proventricular dilatation disease', Emerging Infectious Diseases 15, 14531459. http://dx.doi.org/10.3201/eid1509.090353, PMid:19788814 [ Links ]
Weissenbock, H., Sekulin, K., Bakonyi, T., Hogler, S. & Nowotny, N., 2009b, 'Novel avian bornavirus in a nonpsittacine species (canary; Serinus canaria) with enteric ganglioneuritis and encephalitis', Journal of Virology 83, 11367-11371. http://dx.doi.org/10.1128/JVI.01343-09, PMid:19706702 [ Links ]
Wunschmann, A., Honkavuori, K., Briese, T., Lipkin, W.I., Shivers, J. & Armien, A.G., 2011, 'Antigen tissue distribution of avian bornavirus (ABV) in psittacine birds with natural spontaneous proventricular dilatation disease and ABV genotype 1 infection', Journal of Veterinary Diagnostic Investigation 23, 716-726. http://dx.doi.org/10.1177/1040638711408279, PMid:21908314 [ Links ]
 Correspondence to:
Correspondence to:
Robert Last
PO Box 13624
Cascades 3202, South Africa
vetdiagnostix@futurenet.
Received: 24 Nov. 2011
Accepted: 18 Sept. 2012
Published: 13 Dec. 2012














