Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.83 n.1 Pretoria Jan. 2012
ORIGINAL RESEARCH
An investigation to determine the cause of haemorrhagic enteritis in commercial pig grower units in the northern parts of South Africa
Annemarie LabuscagneI; B. Tom SpencerII; Jackie A. PicardIII; Mark C. WilliamsIV
ICS Vet Consultancy, Waterkloof, South Africa
IIDepartment of Production Animal Studies, University of Pretoria, Onderstepoort campus, South Africa
IIIDepartment of Paraclinical Sciences, University of Pretoria, Onderstepoort campus, South Africa
IVDiscipline of Microbiology and Immunology, James Cook University, Australia
ABSTRACT
Necropsies were performed on 36 grower pigs that died peracutely on farms in the northern parts of South Africa. All these pigs were suffering from haemorrhagic enteritis and suspected toxaemia. Samples of the duodenum, jejunum and ileum were taken for histopathological examination and a section of ileum was collected for microbiological examination from each animal. Histological lesions characteristic of enterotoxigenic Clostridium infection were found. Large, Gram-positive bacilli were sometimes abundant in sections and mucosal smears of the intestine. However, only 40% of the cultures were positive for Clostridium perfringens.
Introduction
Over the past years, pig farmers in South Africa with good management systems have reported to consulting veterinarians that some of their grower pigs die acutely without prodromal signs. South African pig producers refer to this syndrome as either haemorrhagic bowel syndrome (HBS) or 'red gut', as a result of the intense red discolouration of the intestinal tract. Although the mortality does not spread within a group of animals, it tends to affect the best grown pigs close to market age, making it an expensive disease.
Even though the clinical and pathological picture was not typical, South African veterinarians thought that Lawsonia intracellularis infection was at least in part responsible for this disease syndrome. This belief stemmed from the fact that L. intracellularis infections are known to be endemic on the affected farms and are known to cause acute mortalities in this age group (Labuscagne 2008). Endemicity was borne out by a serological study conducted in 2007 in association with Boeringher Ingelheim, South Africa, which showed that most pigs sero-convert at six weeks of age. Bigger herds with fully slatted floors and wet or wet-dry feeding systems tended to be more prone to L. intracellularis infections (Labuscagne 2008).
Materials and methods
Necropsies
Pig farmers were asked to contact one of the authors if grower pigs died acutely and the following criteria were met:
• Those pigs weighed between 50 kg and 110 kg.
• Severe abdominal distension was present shortly after death.
Veterinarians employed as pig consultants attempted to perform necropsies within 4 h of death. A gross post-mortem examination was conducted on the carcasses available. Only those carcasses that had marked intestinal congestion, with red contents, were selected for sampling. Furthermore, there had to be no evidence of gastric or intestinal displacement, that is, intussusception, volvulus or gastric ulceration.
A 1 cm thick slice of intestine was collected from the following areas for histopathology:
• the ileum, 30 cm proximal to the ileo-caecal valve
• a mid-section of the jejunum
• the duodenum, 10 cm distal to the stomach.
Each sample was put individually into a 30 mL sample bottle filled with 10% buffered formalin in order to fix the tissue.
For bacteriology, a 6 cm piece of ileum, taken just cranial to the histopathology sample, was removed and tied off at both ends. It was then sealed in a plastic specimen jar and stored at 4 °C in a polystyrene holder. These specimens reached the laboratory within 24 h of collection. Each sample was uniquely identified.
Bacteriology
Smear
Once the contents had been removed at the laboratory, a mucosal scraping was made with a glass microscope slide and smeared over a clean slide. The slide was then stained using the Gram's staining method (Quinn et al. 1994) and examined under a microscope. Bacteria were classified as Gram-positive Clostridium-like organisms, Gram-positive Lactobacillus-like organisms, Gram-positive cocci, Gram-negative Campylobacter'-like organisms, Gram-negative rods and Gram-negative cocci. The presence of fungi was also noted.
A mucosal scraping was made by rubbing the ileal mucosa with a cotton-tipped swab and cultured aerobically as well as anaerobically. The material collected on the swab was inoculated onto the upper third of a Columbia agar plate (CM 331; Oxoid Pty Ltd, Basingstoke, UK) enriched with 5% citrated horse blood (BCA) and MacConkey agar without crystal violet (MAC) (CM 7B; Oxoid Pty Ltd, Basingstoke, UK). The inoculum was streaked out by means of cooled, heat-sterilised platinum loops in order to dilute bacterial growth. The agar plates were incubated aerobically at 37 °C and examined daily for the presence of bacterial colonies. All β-haemolytic or smooth lactose fermenting and non-lactose fermenting colonies were purified by streaking them onto BCA and MAC and incubating them for 24 h at 37 °C. All Gram-negative, oxidase-negative, catalase-positive, fermentative, nitrate-reducing bacteria were identified using the API10S test (10100; BioMerieux, Marcy l'Etoile, France).
The same specimen that had been used for aerobic culturing was plated onto pre-reduced BCA in the anaerobic cabinet, using the same method and incubated at 37 °C. After an incubation period of 24 h, colonies showing a zone of double β-haemolysis were streaked onto pre-reduced BCA to purify. Furthermore, the selected colonies were also inoculated onto BCA and cultured aerobically to check for aerobic growth. Any colonies that consisted of Gram-positive large rods that were oxidase negative and catalase negative, non-motile and were lecthinase positive, lactose-fermenting, but were non-proteolytic and lipase negative, were considered to be Clostridium perfringens. Fresh cultures of each C. perfringens strain isolated were emulsified in 2.2 mL NUNC cryotubes® containing 1.5 mL of brain-heart infusion broth (Oxoid Pty Ltd, Basingstoke, UK) with 0.2% cysteine and 10.0% glycerol (Merck, Whitehouse Station, USA) and stored at -86 °C (Forma Scientific, Marietta, USA). All β-haemolytic colonies consisting of Gram-negative fusiforms were identified using the Mastring antimicrobial sensitivity test (Difco Laboratories, London, UK). Pale colonies that showed no haemolysis and were not represented on the aerobic plates were identified using API 32A (Biomerieux, Marcy l'Etoile, France).
Histopathology
The intestines of 36 pigs that had died of haemorrhagic enteropathy were examined histologically. Three 1 cm sections were taken from the intestines of each pig (duodenum, jejunum and ileum) and fixed in 10% buffered formalin. Transverse blocks were cut from the fixed tissues, embedded in paraffin wax and haematoxylin and eosin (H&E) sections prepared, using routine methods. The thin sections were examined using a compound light microscope and the lesions were noted and evaluated. The lesions and presence of organisms were evaluated subjectively according to the following criteria:
• necrosis: 0% - 100% (full thickness)
• congestion: none (0), mild (1), moderate (2) or severe (3)
• haemorrhage: none (0), mild (1), moderate (2) or severe (3)
• number of organisms: none (0), few (1), moderate (2) or many (3).
Results
Deaths resulting from this syndrome tend to be in the hot summer months, producing a post-weaning mortality rate of 4% (2% above the acceptable norm).
These farms are generally well managed, with modern buildings that have more than 1000 sows. All are multi-site farms that make use of the all-in all-out management system. Two of the units that were part of the study are specific pathogen free (SPF) units that are free from contagious respiratory diseases caused by Actinobacillus pleuropneumoniae and Mycoplasma hyopneumoniae, as well as swine dysentery caused by Brachyspira hyodysenteriae. The remaining three are normal-health farms where weaners are vaccinated against M. hyopneumoniae.
Thirty-six pigs were sampled in the summer months from January 2007 until March 2007. Bacteriology was not undertaken on seven samples because of advanced autolysis.
At necropsy, the skin was usually pale, without signs of trauma. There was often a bloody discharge from the nostrils and a markedly distended abdomen (Figure 1). The most remarkable finding on incision of the abdomen was the severely distended intestinal tract that was usually dark-maroon in colour (Figure 2), but in some cases the intestines only had a slight reddish tinge. A careful examination was made of the entire length of affected intestine to identify any signs of displacement that would explain the discolouration of part of the intestine, but none of the cases had any signs of intestinal displacement. Other than the liver, which tended to be pale and pushed cranially, the remaining organs appeared normal. Indicative of a peracute disease, the stomach was always filled with food.


Other than the first 10 cm of the duodenum, which appeared normal, the luminal surface of the rest of the intestines was highly congested and the wall appeared thinner than normal. The intestinal content was reddish and foul-smelling and had a watery consistency (Figure 2). The cardio-respiratory system was normal. The carcasses of these animals showed an accelerated rate of decomposition.
Microscopic evaluation of the ileal wall ingesta
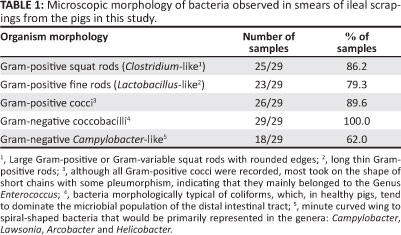
Bacterial groups observed from Gram-stained smears of the intestinal mucosa are summarised in Table 1. The results indicate that there were mixed bacterial populations on most swabs.
Aerobic cultures
It must be noted that even with the use of selective culturing methods no Salmonellae were cultured. Of interest was that β-haemolytic Escherichia coli was cultured from seven of the 29 samples (24.1%). In six of these C. perfringens was also cultured.
Anaerobic cultures
Anaerobic cultures were produced to isolate all obligate anaerobes that cause disease in pigs and included C. perfringens, Clostridium difficile and Fusobacterium necrophorum. Of these bacteria, only C. perfringens was cultured in a heavy, almost pure culture, where it was cultured from 12 (40%) of the samples (see Table 2).

Comparison of the culture of C. perfringens to the presence of clostridia-like bacteria observed on ileal smears is shown in Table 2.
Histopathology
The intestines of 36 pigs that had died of haemorrhagic enteropathy were examined histologically. Advanced autolysis of the sampled tissues precluded histological evaluation in seven of these cases.
In all of the remaining cases (29), the histopathological findings were essentially identical, varying only in degree. The three basic lesions that were consistently found in most cases, to varying degrees, were: necrosis of the intestinal mucosa and congestion and haemorrhages in the intestinal wall. Rod-shaped bacterial bacilli were usually, but not always, present on and in the intestinal mucosa.
Mucosal necrosis was present in all cases. It was invariably linear, that is, parallel to the mucosal surface, and was sharply delimited from the underlying non-necrotic mucosa. The comparative severity of mucosal necrosis in the duodenum, jejunum and ileum was calculated by dividing the sum of the percentages of mucosal necrosis in each part of the intestine by the total number of cases (excluding autolysed samples) - for the duodenum this was 19.4%, for the jejunum 69.4% and for the ileum 85.6%. It would thus appear that mucosal necrosis became more severe from the proximal to the distal end of the small intestine.
Congestion was present in every case, and was noted as distended capillaries, filled with red blood cells, in the lamina propria of the intestinal mucosa, as well as distended large blood vessels, filled with red blood cells, in the submucosa, muscular layers and serosa. A congestion index was determined by summing the congestion scores for each sampling site and then dividing this number by the number of cases - for the duodenum this index was 0.90, for the jejunum 2.85 and for the ileum 2.71. The degree of congestion was therefore mildest in the duodenum and more severe, but similar, in the jejunum and ileum.
Haemorrhage in the wall of the intestine was noted in 20 of the 29 non-autolysed cases but was absent in the samples of 11 of the pigs with 'haemorrhagic enteropathy'. Haemorrhage, if present, was noted to be almost diffuse in the lamina propria of the mucosa, usually in association with the necrotic epithelium. The haemorrhage index was calculated in the same way as the congestion index - for the duodenum this index was zero, for the jejunum 0.94 and for the ileum 1.50. Thus, haemorrhage was absent from the duodenum, intermediate in the jejunum and most severe in the ileum.
Organisms were present in all cases and were mostly found on the surface of the mucosa, in clumps of varying size. Such groups of bacilli were often embedded within sloughed or attached bits of necrotic mucosa. The morphology of the bacilli was typical of bacteria of the genus Clostridium, namely large brick-like organisms, which stained purple with H&E stain. A micro-organism index was determined using the average score for each sampling site - for the duodenum this index was 1.40, for the jejunum 1.62 and for the ileum 1.77. There was a tendency for the numbers to increase from the proximal to distal small intestine, but the differences were not remarkable.
Necrosis, congestion and haemorrhage appeared to become more severe from the proximal to the distal small intestine, but this did not appear to correlate with the number of clostridial organisms, as the latter were more or less evenly distributed along the length of the small intestine.
There were no lesions, either macroscopic or microscopic, that were typical for L. intracellularis infection.
Discussion
Previously, in South Africa, HBS accounted for only 2% - 5% of mortalities in grower pigs, but in the past five years it has become much more prevalent.
At the farms in this study, pigs 9-22 weeks of age were affected, with the majority being between 15 and 20 weeks of age. Usually, mortalities were spread over different age groups and it almost always appeared to be the fastest growing pig in the pen that was affected. Mortalities tended to occur in the early hours of the morning. Typically, these pigs showed no prodromal signs on the previous day. If clinical signs were observed, death usually occurred within 45 min. Initially the pig would squeal, as if in severe pain, with abdominal distension being visible within 15 min when the pig showed signs of acute and severe respiratory distress, with open mouth breathing; after a few minutes it became cyanotic. Just prior to death, the affected pig became very weak and its movements were jerky and uncoordinated (personal observations by the primary author).
These clinical signs are very similar to those seen in other species such as feedlot cattle (as mentioned by Hartwig n.d.) and dairy cows that were found to have suffered from a clostridial infection secondary to environmental and behavioural factors (Manteca et al. 2002; Van Metre, Callan & Dennison 2005). It also contrasts with proliferative haemorrhagic enteropathy (PHE), which usually affects more than one pig in a pen and where the first clinical sign is usually profuse bloody diarrhoea, instead of a dead pig (Love & Love 1977). Pigs suffering from PHE are also very anaemic, which was not the case in this study.
The clinical and pathological picture best fits C. perfringens infection, another cause of acute gastro-intestinal disease. This bacterium is a common cause of mortalities in neonatal pigs but has not yet been recognised as an important cause of disease in grower pigs (Niilo 1980).
With PHE, organisms are found in the cytoplasm of infected epithelial cells. Furthermore, there are acute inflammatory changes such as interstitial oedema and accumulation of neutrophils, lymphocytes, mononuclear cells and macrophages in the lamina propria immediately surrounding the affected crypts (Jensen, Christensen & Boye 2006). The lesions are usually restricted to the mucosa of the ileum (Love & Love 1977). In the pigs in this study, the lesions involved the whole of the small intestine, being most severe in the ileum and least severe in the duodenum. Although the lesions varied in extent and severity, in all pigs there was necrosis of the intestinal mucosa and congestion. Most pigs also showed haemorrhages in the intestinal wall. Rod-shaped bacterial bacilli were present on or in the intestinal mucosa in all affected pigs. This type of histopathology is highly suggestive of Clostridium species as the cause of death (Schwartz 2002).
Because E. coli is considered to be a cause of attaching and effacing lesions, resulting in haemorrhagic enteritis (Straw et al. 2002), this study investigated the importance of the bacterium in the HBS disease complex. Smooth E. coli were only isolated from 25% of the cases, often together with a heavy growth of C. perfringens. It is not certain whether this was because the pigs were carriers of E. coli or whether the bacterium played a role in the syndrome. The former is more likely, as growers are only rarely clinically affected with primary diarrhoea as a result of this agent. Although histological lesions in E. coli cases may include vascular congestion in the lamina propria, with some haemorrhages into the lumen and some villous atrophy (Fairbrother & Gyles 2006), attaching and effacing lesions were absent in this study.
The bacteriological and histopathological findings suggest that one of the Clostridium species might be involved in the pathogenesis of HBS. Clostridium perfringens was isolated from 40% of submitted intestinal mucosal scrapings. Histopathology revealed clostridia-type organisms on the surface of the mucosa in 76% of the affected pigs. These organisms were morphologically typical of the genus Clostridium, namely large brick-like organisms which stained purple with H&E. The discrepancy between the histological observation of clostridia-like bacteria and culture of C. perfringens was disappointing but not unexpected. This anaerobic bacterium can be overgrown by commensal bacteria. Furthermore, prolonged sampling times after death and suboptimal preservation methods may have negatively impacted the viability of the bacteria. The likelihood of the more fastidious Clostridium species being involved could also not be excluded. It is therefore suggested that sample preservation and culturing target this genus in the future and that efforts are made to identify C. perfringens and C. difficile toxins in the intestinal contents of affected pigs.
This study shows that the most likely cause of HBS for at least 40% of pigs in this project is C. perfringens. For some unknown reason, this aetiology has never been reported in pigs as a definite cause of HBS. Modern genetics have resulted in a commercial pig with improved average daily gain, feed conversion ratio and leaner meat but, perhaps because of this, they are more predisposed to metabolically induced diseases such as HBS.
This was true for the farms in the study, as the growth rates of pigs on these farms were above the national average. Pigs are probably pushed to their physiological limits from an early stage, which would explain why four pigs weighing less than 50 kg were also affected. Furthermore, these pigs cannot tolerate mistakes in management or feeding (Brumm et al. 1994), especially when rations are high in carbohydrates, which, under certain circumstances, can lead to the proliferation of C. perfringens (Pejsak 2007). Although the pig farms where the study was performed are well managed farms, one cannot ignore the impact of the environment and non-feed-related events. The extremely high ambient temperatures experienced in summer on all of these farms can lead to a decreased appetite during the day, with a compensatory increase in feeding at night. This could lead to over engorgement, with the delivery of increased amounts of intestinal carbohydrate and protein. This, in turn, could lead to enteric microflora proliferation, intestinal stasis or mechanical torsion. This is supported by the fact that most of the deaths are discovered early in the morning, with mortalities probably occurring around 04:00. The above hypothesis might explain why HBS is more prevalent in summer. Further research will be necessary to determine whether virulent strains of C. perfringens displace normal intestinal C. perfringens, as was shown in the case of necrotic enteritis in poultry (Barbary et al. 2008). If that is the case, factors enhancing the growth of virulent strains will have to be identified in order to manage the disease. Because the main ingredient of the diets fed on all the farms is corn-soya (maize and soyabean oil cake meal), one can also speculate on the importance of hypersensitivity to soya in this syndrome. This could result in a decrease in trypsin activity, preventing the inactivation of toxin β1 produced by C. perfringens types B and C (Schotte, Truyen & Neubauer 2004). Under South African conditions, in-feed medication with chlortetracycline does lower the incidence of the disease, although it does not stop it completely (personal observation by the primary author). This antimicrobial is known to be effective against clostridia as well as some of the other enteric bacteria.
Typing of the toxins is being undertaken at present, as this is deemed an important component of the investigation to confirm the organisms responsible for HBS.
Conclusion
Clostridium perfringens most likely plays a major role in HBS in the northern parts of South Africa during the hot summer months in rapidly growing pigs. Further research is necessary to investigate management factors that could act as a trigger. Because the farms that suffer from this disease recognise it as an important cause of grower mortalities, efforts should be made to fully understand any predisposing factors.
Although this study has indicated that C. perfringens is the most likely cause for 40% of the pigs in this study, efforts should be made to identify the possible toxins, both known and unknown, that may be playing a role in the pathogenisis of this disease.
Acknowledgements
Competing interests
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this paper.
Authors' contributions
A.L. (CS Vet Consultancy) was the project leader and did most of the post-mortems. B.T.S (University of Pretoria) was the primary author's mentor and provided guidance as to how to approach this study. J.A.P. (University of Pretoria) was responsible for the microbiology, whilst M.C.W. (James Cook University) was responsible for the histopathology.
References
Barbary, A.J., Trinh, H.T., Glock, R.D. & Songer, J.G., 2008, 'Necrotic enteritis-producing strains of Clostridium perfringens displace non-necrotic enteritis stains from the gut of chicks', Veterinary Microbiology 126, 377-382. http://dx.doi.org/10.1016/'.vetmic.2007.07.019, PMid:17850994 [ Links ]
Brumm, M.C., Richert, B.T., Marchant Forde, J.N. & Marchant Forde, R., 2004, 'Out-of-feed events in grow-finish pigs: Causes & consequences', Proceedings of the George A. Young Swine Health & Management 45th Conference, Sioux City, Nebraska, 05 August 1994, pp. 6-15. [ Links ]
Fairbrother, J.F. & Gyles, C.L., 2006, 'Escherichia coli Infections', in B.E. Straw, J.J. Zimmerman, S. D'Allaire & D.J. Taylor (eds.), Diseases of swine, 9th edn., pp. 639662, Blackwell Publishing, Oxford. [ Links ]
Hartwig, N.R., n.d., Controlling clostridial diseases in cattle, viewed 18 January 2009, from http://www.iabeef.org [ Links ]
Jensen, T.K., Christensen, B.B. & Boye, M., 2006, 'Lawsonia intracellularis infection in the large intestines of pigs', Acta Pathologica, Microbiologica et Immunologica Scandinavica 114, 225-264. http://dx.doi.org/10.1111/j.1600-0463.2006.apm_53.x, PMid:16689824 [ Links ]
Labuscagne, A., 2008, 'Elisa profiles of L. intracellularis on pig farms in Southern Africa', Proceedings of the 20th International Pig Veterinary Society Congress, Durban, South Africa, 22-26 June 2008, p. 145. [ Links ]
Love, R.J. & Love, D.N., 1977, 'Pathology of proliferative haemorrhagic enteropathy in pigs', Veterinary Record 100, 65-68. http://dx.doi.org/10.1136/vr.100.4.65, PMid:299966 [ Links ]
Manteca, C., Daube, G., Jauniaux, T., Linden, A., Pirson, V., Detilleux, J. et al., 2002, 'A role for the Clostridium perfringens β2 toxin in bovine enterotoxaemia?', Veterinary Microbiology 86, 191-202. http://dx.doi.org/10.1016/S0378-1135(02)00008-1 [ Links ]
Niilo, L., 1980, 'Clostridium perfringens in animal disease, a review of current knowledge', Canadian Veterinary Journal 21, 141-148. PMid:6253040 [ Links ]
Pejsak, Z., 2007, 'Krwotoczny zespót jelitowy u swin [Haemorrhagic bowel syndrome in pigs]', Zycie Weterynaryjne 82, 398-400. [ Links ]
Quinn, P.J., Carter, M.E., Markey, B. & Carter, G.R., 1994, Clinical veterinary microbiology, Mosby/Elsevier Limited, Edinburgh. [ Links ]
Schotte, U., Truyen, U. & Neubauer, H., 2004, 'Significance of β2-toxigenic Clostridium perfringens infections in animals and their predisposing factors - a review', Journal of Veterinary Medicine 51, 423-426. http://dx.doi.org/10.1111/j.1439-0450.2004.00802.x, PMid:15606864 [ Links ]
Schwartz, K.J., 2002, 'Hemorrhagic bowel syndrome (HBS): A diagnostic laboratory perspective', Proceedings of the 33rd Annual Meeting of the American Association of Swine Veterinarians, Kansas City, Kansas, 02-05 March 2002, pp. 405-408. [ Links ]
Straw, B., Dewey, C., Kober, J. & Henry, S.C., 2002, 'Factors associated with death due to hemorrhagic bowel syndrome in two large commercial swine farms', Journal of Swine Health and Production 10, 75-79. [ Links ]
Van Metre, D.C., Callan, R.J. & Dennison, A.C., 2005, 'Hemorrhagic bowel syndrome: What we do and don't know', Proceedings of the North American Veterinary Conference, Orlando, Florida, 08-12 January 2005, pp. 47-48. [ Links ]
 Correspondence to:
Correspondence to:
Annemarie Labuscagne
PO Box 95315
Waterkloof 0145, South Africa
annie@csvet.co.za
Received: 03 Apr. 2012
Accepted: 29 Oct. 2012
Published: 13 Dec. 2012














