Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the South African Veterinary Association
versão On-line ISSN 2224-9435
versão impressa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.83 no.1 Pretoria Jan. 2012
ORIGINAL RESEARCH
Epizootic ulcerative syndrome: Exotic fish disease threatens Africa's aquatic ecosystems
Karl D.A. HuchzermeyerI, II; Benjamin C.W. van der WaalIII
ISterkspruit Veterinary Clinic, Lydenburg, South Africa
IIDepartment of Ichthyology and Fisheries Science, Rhodes University, South Africa
IIIUniversity of Venda, Thohoyandou, South Africa
ABSTRACT
In late 2006 an unusual ulcerative condition in wild fish was reported for the first time in Africa from the Chobe and upper Zambezi Rivers in Botswana and Namibia. Concern increased with subsistence fishermen reporting large numbers of ulcerated fish in their catches. In April 2007 the condition was confirmed as an outbreak of epizootic ulcerative syndrome (EUS). The causative agent, Aphanomyces invadans, is a pathogenic water mould of fish that shows little host specificity. Ulcers follow infection of tissues by oomycete zoospores, resulting in a granulomatous inflammation associated with invading oomycete hyphae. Granulomatous tracts surrounding oomycete hyphae within the necrotic tissues characterise the diagnostic histological picture. The upper Zambezi floodplain at the confluence with the Chobe River spans the four countries of Botswana, Namibia, Zambia and Zimbabwe, making disease control a challenge. The floodplain ecosystem supports a high fish diversity of around 80 species, and is an important breeding and nursery ground. The annual cycle of flooding brings about changes in water quality that are thought to favour the infectivity of A. invadans, with diseased fish appearing soon after the plains become flooded. Since 2006 the disease has spread rapidly upstream along the upper Zambezi and its tributaries. By 2010 the disease was reported from the Okavango Delta in Botswana and in 2011 from the Western Cape Province of South Africa. EUS has the potential to disrupt floodplain ecosystems elsewhere in Africa where high fish diversity forms the basis of subsistence fisheries and local economies, and is a direct threat to freshwater fish culture.
Introduction
An epizootic ulcerative condition in fish, known as mycotic granulomatosis, was first described from Japan in 1971 (Egusa & Masuda 1971). Involvement of an invasive oomycete, subsequently named Aphanomyces invadans, has been well documented (Baldock et al. 2005). The disease has since been reported from an increasing number of countries, where it has become widespread in both cultured and wild fish populations (Blazer et al. 2002). The pattern of spread to distinctly separate geographic locations within a relatively short period is consistent with the progressive dissemination of a single infectious agent (Baldock et al. 2005; Lilley et al. 1997).
In Australia the infection has been recognised since 1972 and is known as red spot disease. It is now regarded as endemic in many of Australia's rivers and estuaries (Baldock et al. 2005). Soon after, the disease was reported from the Philippines (Callinan et al. 1995), other East Asian countries (Lilley et al. 1997) and India (Das & Das 1993). Ulcerative mycosis, an ulcerative syndrome reported in estuarine fish on the East Coast of the United States of America (USA) since the early 1980s has been ascribed to the same cause (Blazer et al. 2002). The epidemic outbreak of ulcers in fish in the Chobe and Zambezi rivers in Botswana and Namibia in 2006 represented the first report of the disease on the African continent (Andrew et al. 2008; Anon 2009).
Aphanomyces invadans is an invasive oomycete or water mould, and is regarded as a primary fish pathogen (Baldock et al. 2005). A number of other saprophytic oomycetes are known to be opportunistic pathogens and may be present as secondary invaders on surface lesions caused by A. invadans (Lilley & Roberts 1997; Sosa et al. 2007a, 2007b). A related oomycete, Aphanomyces astaci, the cause of crayfish plague, is known as a serious pathogen of the European freshwater crayfish (Astacus astacus). Crayfish plague was spread to European waters with introduction of the more resistant American signal crayfish, and resulted in disappearance of the European crayfish from much of its natural geographic range.
Spread of A. invadans is via zoospores in the water. Secondary zoospores enter the skin following a breach of the epidermal barrier by physical or environmental causes (Baldock et al. 2005; Lilly & Roberts 1997). The zoospores germinate and hyphae invade the skin and musculature, causing a focal necrotising granulomatous dermatitis and myositis. Hyphae may invade more deeply, causing granulomatous inflammation in the internal organs (Baldock et al. 2005). The tissue necrosis in early lesions is associated with an intense inflammatory reaction that is observed as a characteristic focal reddening and swelling of the skin, hence the name 'red spot disease' used in some countries (Anon 2008). More advanced lesions are characterised by large and deep ulcers surrounded by a raised rim of inflamed tissue. The descriptive name epizootic ulcerative syndrome (EUS) has become the internationally accepted term for infection with A. invadans.
Case history 1: Chobe and upper Zambezi River outbreak
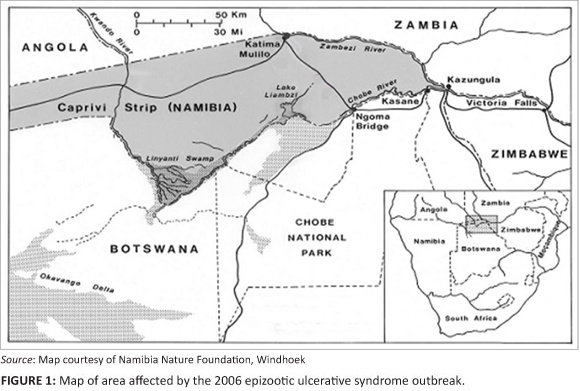
During 2006, whilst working in the Caprivi Region, Namibia, for the Integrated Management of the Zambezi/Chobe River System Fishery Resource Project, Dr N. Nyambe was informed by local manduna (headmen) of large numbers of fish with skin ulcers being caught by fishermen in the region. Fisheries authorities were alerted to the disease and then placed a ban on commercial fishing pending further investigation. One of the authors joined Dr Nyambe on the project in February 2007 and immediately started collecting information and material whilst conducting field surveys.
Surveys revealed the condition to be present in fish in the Chobe River in the vicinity of the Chobe Game Reserve in Botswana, the Caprivi Region of Namibia and on the Zambian side of the Zambezi River above Victoria Falls (Figure 1). The condition was subsequently confirmed as EUS and the causative agent A. invadans was identified by histopathology (Andrew et al. 2008; Anon 2009), culture and polymerase chain reaction (PCR) (Anon 2009). Further samples were sent to the University of Zambia for diagnostic confirmation.
Data were collected in the Caprivi Region of Namibia (Figure 1) during regular biological surveys using experimental gill nets, ad hoc scoop net sampling, inspection of fishermen's catches, annual angling competitions and casual angling. From 2007 to 2008 lesions typical of EUS were identified in 24 species of fish in the Zambezi and Chobe Rivers (Table 1).
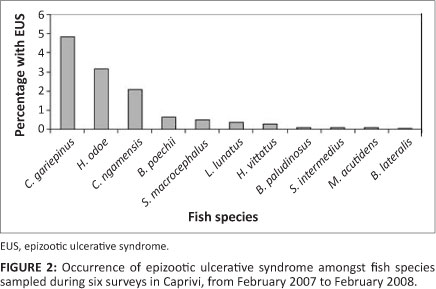
Lesions appeared in fish soon after the floodplains surrounding the Chobe and Zambezi confluence became inundated by seasonal floodwaters. Many of the juvenile and smaller floodplain species were affected and could be observed where the floodplains drained back into the main channels of the rivers. The floodplains form rich fishing grounds for local subsistence fishing, and fishermen were reporting ulcers in both small and large fish. Surveys indicated that Clarias gariepinus and Clarias ngamensis showed the highest prevalence of lesions (Figure 2). Epizootic ulcerative syndrome was confirmed from specimens of this genus that had strayed into a population of farmed Tilapia rendalli on Likunganelo fish farm near Lisikili, 25 km from Katima Mulilo in the Caprivi Region in July 2007 after flooding of the ponds.
Data from angling competitions indicated a high prevalence of lesions in larger cichlids (Table 2), whereas the formal experimental gill net surveys appeared to provide an under-representation of these fish and were unlikely to yield reliable results for large fish species.
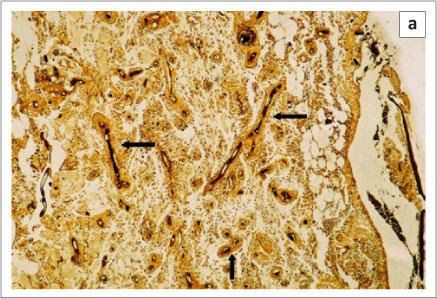
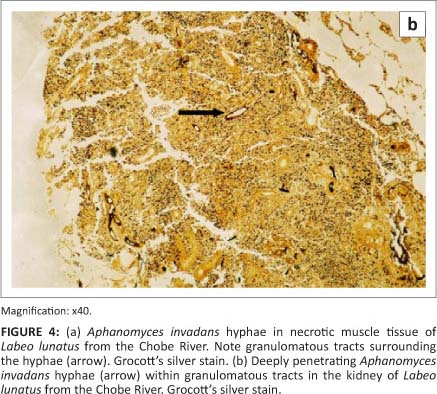
The pathology in fish collected from the Chobe and Zambezi Rivers has been described by Andrew et al. (2008). Early lesions appeared as raised hyperaemic foci in the skin of several millimetres in diameter (Figure 3). Advanced lesions consisted of large, deep skin ulcers exposing the underlying musculature. The tissues around the ulcers were characterised histologically by a granulomatous inflammatory response surrounding aseptate branched oomycete hyphae. Histological lesions in the musculature typically consisted of necrotic muscle remnants with an infiltration of large numbers of macrophages and other mixed inflammatory cells (Figure 4a). Within these areas, macrophages and fibroblasts formed dense granulomatous sheaths around hyphae. In severe cases hyphae were found to penetrate internal organs, and could be demonstrated in the kidney (Figure 4b) and, to a lesser extent, in the liver and mesentery of affected fish.

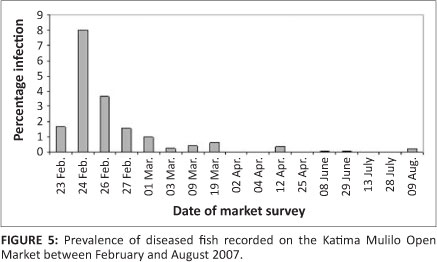
Since the 2006 outbreak in the Chobe and Zambezi Rivers, EUS-infected fish have been observed seasonally at the end of subsequent summers, but with a lower prevalence. Fish with typical EUS lesions could be found during surveys of fish brought to the Katima Mulilo Open Market during the initial period of the outbreak, between February and August 2007. However, subsequent to August 2007 the presence of infected fish in the market has been rare (Figure 5). Since fishermen were still reporting catching fish with lesions, it is assumed that fishermen or traders removed infected fish before offering them for sale.
Reports from Zambia have indicated a rapid spread upstream in the Zambezi River and some of its tributaries, in some cases associated with large-scale fish mortality (Anon 2009; Choonga et al. 2009; Songe et al. 2012). So far EUS has not been reported from the Zambezi River downstream of Victoria Falls. However, EUS has more recently been reported from the Kafue River in Zambia (Mudenda 2010). The lower
Zambezi River has two large impoundments, Lake Kariba and Lake Cahora Bassa, both of which support significant fisheries, whilst the former has one of the largest aquaculture facilities in sub-Saharan Africa.
Case history 2: Subsequent southern African outbreaks
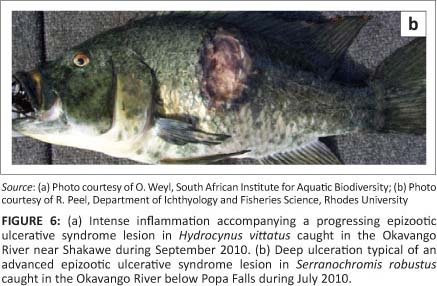
In 2010 the Botswana Government reported an outbreak of EUS in fish from the Okavango Delta to the World Organisation for Animal Health (OIE) (Anon 2011). Three species of fish, sharptooth catfish (C. gariepinus), African pike (Hepsetus odoe) and silver catfish (Schilbe intermedius) were affected. The disease was confirmed by histopathological examination in H. odoe and in S. intermedius (Anon 2011). During 2010 typical EUS lesions were also found on large tiger fish (Hydrocynus vittatus) (Figure 6a) (O. Weyl [South African Institute for Aquatic Biodiversity, Grahamstown, South Africa], pers. comm., 2011) and in nembwe (Serranochromis robustus) (Figure 6b) caught in the Okavango River (R. Peel [Department of Ichthyology and Fisheries Science, Rhodes University, Grahamstown, South Africa], pers. comm., 2011). The Okavango Delta in Botswana represents a unique inland basin where the Kavango River that arises in the highlands of Angola discharges its water into the Kalahari Desert. The Okavango River is intermittently connected to the Chobe and Zambezi catchment. Such a link was established in 2008 following a number of dry years. The Kwando River, where the link via the Selinda spillway enters the Linyati/Chobe system, was included in monitoring for EUS from 2007 to 2009, but during this time no infected fish were recorded. How the disease spread to the Okavango Delta remains speculative. Figure 6a and 6b show EUS lesions in fish caught in the Okavango River in 2010.
In 2011 two outbreaks of EUS were reported from South Africa (Anon 2011). The disease was first noticed in juvenile largemouth bass (Micropterus salmoides), bluegill sunfish (Lepomis macrochirus) and an unidentified cichlid species in an artificial impoundment (Arrieskraal Dam) (Van Helden & Grewar 2011) on the Palmiet River in the Western Cape Province. Rainbow trout being farmed in the same impoundment initially appeared unaffected, although they later developed lesions. The disease was confirmed by histopathology (A. Mouton [Amanzi Biosecurity, Hermanus, South Africa], pers. comm., October 2011) and by PCR (K. Christison [Department of Agriculture,
Forestry and Fisheries, Cape Town, South Africa], pers. comm., August 2011). A further outbreak in October 2011 caused serious mortality amongst free-living sharptooth catfish (C. gariepinus) in a farm dam on the Eerste River near Stellenbosch in the Western Cape (Anon 2011; Van Helden & Grewar 2011). The Western Cape Province is home to 11 endangered endemic species of freshwater fish (Skelton 1993; Skelton et al. 1995), with small populations in a few rivers, increasing their vulnerability. It is not known how the disease was introduced into South Africa.
Discussion
Africa has a unique aquatic biodiversity, that in many areas forms the basis of important subsistence fisheries. The Zambezi River supports at least 134 species of freshwater fish (Skelton 1993). Serious regional biosecurity shortcomings were identified in Botswana and Namibia at the time of the 2006 EUS outbreak (Andrew et al. 2008; Anon 2009) and a number of urgent recommendations were made (Anon 2009). Subsequently, however, in 2010 the disease was reported from the pristine wilderness area of the Okavango Delta in Botswana (Anon 2011) and in February 2011 from South Africa (Anon 2011).
From the list of susceptible fish species (Anon 2010) it is clear that A. invadans shows little host specificity. Broad species susceptibility has similarly been reported from Australia (Anon 2008), the USA (Sosa et al. 2007b) and elsewhere (Anon 2010, 2011). The disease spreads by spores released into the water. These may disseminate through water, contaminated equipment and transport of live and dead fish.
A number of predisposing factors leading to infection have been identified (Baldock et al. 2005; Choonga et al. 2009). Disease often manifests when water temperatures drop (Baldock et al. 2005), possibly as a consequence of a retarded immune response in the fish. Heavy rainfall and soils which are either naturally acidic or disturbed by agriculture or residential development may lead to a lowering of water pH (Baldock et al. 2005; Choonga et al. 2009). This has been shown to increase susceptibility to infection with A. invadans (Baldock et al. 2005) and may partly explain the relatively high seasonal prevalence of infection in fish in floodplains.
During 2007 severe floods in the upper Zambezi followed several years of low rainfall. Changes in water quality resulting from flooding of previously dry land are suspected to have played a role in increasing susceptibility of fish to infection by A. invadans. The natural acidification of groundwater following periods of drought and subsequent contamination of surface waters of floodplains was identified as the main predisposing factor for EUS outbreaks in Zambia (Choonga et al. 2009). Low water pH may have played a similar role in the Okavango outbreaks, where seasonal flooding is an integral driver of the delta ecosystem. Other causes of skin trauma, providing a portal of entry for infective zoospores, cannot be discounted in an aquatic environment where predation is common.
It remains unclear how EUS was introduced to the southern African region and how it has spread between catchments. It has been suggested that A. invadans may spread locally by flood events, but that regional and international spread may occur by movement of fish for aquaculture or ornamental purposes (Lilley et al. 1997). A number of tropical ornamental fish are known to be susceptible to EUS (Anon 2010), as is the goldfish (Carassius auratus) (Miyazaki & Egusa 1972). Goldfish as well as species of tropical fish are farmed in the warmer regions of Southern Africa, often in open water systems close to natural water sources containing wild fish. The Okavango Delta and Chobe and upper Zambezi Rivers are popular sport fishing venues. Both sport and subsistence fishing may be associated with movement of potentially infected fish and equipment.
A few fish species have been found to be less susceptible to EUS. Both the common carp (Cyprinus carpid) and Nile tilapia (Oreochromis niloticus) are believed to be resistant (Anon 2010). The role of O. niloticus in the spread of EUS is not known. One outbreak of EUS was noted in a tributary of the Kabompo River, part of the upper Zambezi system in western Zambia, just downstream from a farm holding O. niloticus (R. Bills [South African Institute of Aquatic Biodiversity, Grahamstown, South Africa], pers. comm., 2011). Other indigenous tilapia species are, however, susceptible (Anon 2010). Both Nile tilapia and carp are actively farmed in many parts of the world, including the southern regions of Namibia. Although neither species is actively farmed along the upper Zambezi and Chobe Rivers, they have been introduced to the Kafue River in Zambia and elsewhere in Southern Africa, and the Nile tilapia forms the basis of a major aquaculture fishery in Lake Kariba.
Since October 2007 a Food and Agriculture Organization Regional Technical Cooperation Programme (TCP/ RAF/311[E]), 'Emergency Assistance to Combat EUS in the Chobe-Zambezi River', was approved for implementation covering seven participating Southern African countries (Angola, Botswana, Malawi, Mozambique, Namibia, Zambia and Zimbabwe) (Anon 2009). The programme stresses the importance of enhancing surveillance and diagnostic capacity, as well as formulation of a regional emergency response strategy to increase education and awareness, promoting responsible trade in aquatic animals in both affected and unaffected areas. The OIE has since organised several regional training seminars to heighten awareness of aquatic animal diseases amongst OIE focal points and delegates in Africa.
The veterinary school at the University of Zambia in Lusaka in 2008 developed a pathology laboratory where suspect fish material can be evaluated for EUS. In Cape Town the Aquaculture Research Laboratory of the Department of Agriculture, Forestry and Fisheries has developed the capacity to detect the presence of A. invandans in infected tissue by PCR. Rhodes University in Grahamstown, South Africa, has embarked on providing basic training in aquatic animal diseases for South African State veterinarians and border control officials. Such initiatives need to be expanded to broaden awareness of risks posed by diseases such as EUS.
Conclusion
South Africa has many well-managed conservation areas. Many lie in the drainage pathways of major river catchments. With increasing urbanisation and industrialisation, many of the major catchments in South Africa have been degraded through anthropogenic activity and pollution (Ashton 2007). Aquatic diseases such as EUS have the potential to spread along such river courses, and conservation authorities must be aware of the risk of such diseases entering national parks. The subsequent outbreaks in the Okavango Delta and in the Western Cape Province highlight the urgent need to implement effective surveillance. In countries further north in Africa, fish from local fisheries form a major source of protein for the population, with fishing an important income generator. As natural stocks become increasingly over-harvested, the expansion of EUS into Africa threatens potential development of fish farms
Acknowledgements
The Ministry of Fisheries and Marine Resources, Republic of Namibia, as well as the Namibian Nature Foundation and World Wide Fund for Nature, are acknowledged for making it possible for Dr Van der Waal to survey fish in the region during 2007 and 2008. The staff of the Ministry and of the Integrated Management of the Zambezi/Chobe River System Fishery Resource Project are thanked for assistance during the extensive fish-collecting surveys. The work of Dr N. Nyambe, in particular, is acknowledged. The Director, Mr S.M. Nengu, and staff of the Department of Wildlife and National Parks, Botswana, and Dr T.G. Andrew of the Department of Ichthyology and Fisheries Science at Rhodes University, South Africa, are acknowledged for providing the opportunity to access infected fish. Dr B.C. Mbeha of the Department of Animal Health and Production, Botswana, is thanked for providing information on the initial outbreak in the Chobe River. Drs R. Bills and O. Weyl of the South African Institute for Aquatic Biodiversity and Mr R. Peel of the University of Namibia are thanked for providing photos of diseased fish. Drs A. Mouton and K. Christison are thanked for providing information on the EUS outbreak in South Africa.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this paper.
Authors' contributions
B.C.W.v.d.W. (University of Venda) provided earlier reported results, and K.D.A.H. (Sterkspruit Veterinary Clinic) provided the description of the pathology of the disease and wrote the manuscript with contributions by B.C.W.v.d.W. (University of Venda).
References
Andrew, T.G., Huchzermeyer, K.D.A., Mbeha, B.C. & Nengu, S.M., 2008, 'Epizootic ulcerative syndrome affecting fish in the Zambezi River system in southern Africa', Veterinary Record 163, 629-632. http://dx.doi.org/10.1136/vr.163.21.629, PMid:19029110 [ Links ]
Anon, 2008, 'Diseases of finfish: Fungal diseases - epizootic ulcerative syndrome/ redspot disease', in Australian Government Department of Agriculture, Fisheries and Forestry, Aquatic animal diseases significant to Australia: Identification field guide, pp. 1-3, Australian Government Department Agriculture Forestry and Fisheries, Canberra, viewed in 2011, from http://www.daff.gov.au/_data/assets/pdf_file/0007/976210/epizootic-ulcerative-syndrome.pdf [ Links ]
Anon, 2009, Report of the international emergency disease investigation task force on a serious finfish disease in Southern Africa, 18-26 May 2007, Food and Agriculture Organization of the United Nations, Rome, viewed in 2011, from http://www.fao.org/docrep/012/i0778e00.htm [ Links ]
Anon, 2010, Manual of diagnostic tests for aquatic animals, World Organisation for Animal Health, Paris, viewed in 2011, from http://www.oie.int/international-standard-setting/aquatic-manual/access-online/ [ Links ]
Anon, 2011, World animal health information database interface: Exceptional epidemiological events, World Organisation for Animal Health, Paris, viewed in 2011, from http://web.oie.int/wahis/public.php?page=country_reports [ Links ]
Ashton, P.J., 2007, 'Riverine biodiversity and conservation in South Africa: Current situation and future prospects', Aquatic Conservation: Marine and Freshwater Ecosystems 17, 441-445. http://dx.doi.org/10.1002/aqc.886 [ Links ]
Baldock, F.C., Blazer, V., Callinan, R.B., Hatai, K., Karunasagar, I., Mohan, C.V. et al., 2005, 'Outcomes of a short expert consultation on epizootic ulcerative syndrome (EUS): Re-examination of causal factors, case definition and nomenclature', in P. Walker, R. Lester & M.G. Bondad-Reantaso (eds.), Diseases in Asian aquaculture V, pp. 555-585, Fish Health Section, Asian Fisheries Society, Manila. [ Links ]
Blazer, V.S., Lilley, J.H., Schill, W.B., Kiryu, Y., Densmore, C.L., Panyawachira, V. et al., 2002, 'Aphanomyces invadans in Atlantic menhaden along the East Coast of the United States', Journal of Aquatic Animal Health 14, 1-10. http://dx.doi.org/10.1577/1548-8667(2002)014<0001:AIIAMA>2.0.CO;2 [ Links ]
Callinan, R.B., Paclibare, J.O., Bondad-Reantaso, M.G., Chin, J.C. & Gogolewski, R.P., 1995, 'Aphanomyces species associated with epizootic ulcerative syndrome (EUS) in the Philippines and red spot disease (RSD) in Australia: Preliminary comparative studies', Diseases of Aquatic Organisms 21, 233-238. http://dx.doi.org/10.3354/dao021233 [ Links ]
Choonga, K., Hang'ombe, B., Samui, K.L., Syachaba, M., Phiri, H., Maguswi, C. et al., 2009, 'Environmental and climatic factors associated with epizootic ulcerative syndrome (EUS) in fish from the Zambezi floodplains, Zambia', Bulletin of Environmental Contamination and Toxicology 83, 474-478. http://dx.doi.org/10.1007/s00128-009-9799-0, PMid:19565173 [ Links ]
Das, M.K. & Das, R.K., 1993, 'A review of the fish disease epizootic ulcerative syndrome in India', Environmental Sciences 11, 134-135. [ Links ]
Egusa, S. & Masuda, N., 1971, 'A new fungal disease of Plecoglossus altivelis', Fish Pathology 6, 41-46. http://dx.doi.org/10.3147/jsfp.6.41 [ Links ]
Lilley, J.H., Hart, D., Richards, R.H., Roberts, R.J., Cerenius, L. & Sóderàll, K., 1997, 'Pan-Asian spread of single fungal clone results in large scale fish kills', Veterinary Record 140, 653-654. http://dx.doi.org/10.1136/vr.140.25.653, PMid:9226850 [ Links ]
Lilley, J.H. & Roberts, R.J., 1997, 'Pathogenicity and culture studies comparing the Aphanomyces involved in epizootic ulcerative syndrome (EUS) with other similar fungi', Journal of Fish Diseases 20, 135-144. http://dx.doi.org/10.1046/j.1365-2761.1997.d01-116.x [ Links ]
Miyazaki, T. & Egusa, S., 1972, 'Studies on mycotic granulomatosis in freshwater fish. I: Mycotic granulomatosis in goldfish', Fish Pathology 7, 15-25. http://dx.doi.org/10.3147/jsfp.7.15 [ Links ]
Mudenda, B.H., 2010, 'Epizootic ulcerative syndrome (EUS)', in P. Bastiaensen, M. le Groumellec, G. Mylrea, N. Mapitse & B. Mtei (eds.), OIE Report. Regional training seminar for OIE focal points for aquatic animal diseases in Africa - Swakopmund, pp. 69-70, World Organisation for Animal Health (OIE), Paris. [ Links ]
Skelton, P.H., 1993, A complete guide to the freshwater fishes of southern Africa, Southern Book Publishers, Halfway House. [ Links ]
Skelton, P.H., Cambray, J.A., Lombard, A. & Benn, G.A., 1995, 'Patterns of distribution and conservation status of freshwater fishes in South Africa', South African Journal of Zoology 30, 71-81. [ Links ]
Songe, M.M., Hang'ombe, M.B., Phiri, H., Mwase, M., Choonga, K., Van der Waal, B. et al., 2012, 'Field observations of fish species susceptible to epizootic ulcerative syndrome in the Zambezi River basin in Shesheke District of Zambia', Tropical Animal Health Production 44, 179-183. http://dx.doi.org/10.1007/s11250-011-9906-1, PMid:21647772 [ Links ]
Sosa, E.R., Landsberg, J.H., Kiryu, Y., Stephenson, C.M., Cody, T.T., Dukeman, A.K. et al., 2007a, 'Pathogenicity studies with the fungi Aphanomyces invadans, Achlya bisexualis, and Phialemonium dimorphosporum: Induction of skin ulcers in striped mullet', Journal of Aquatic Animal Health 19, 41-48. http://dx.doi.org/10.1577/H06-013.1, PMid:18236631 [ Links ]
Sosa, E.R., Landsberg, J.H., Stephenson, C.M. & Forstchen, A.B., 2007b, 'Aphanomyces invadans and ulcerative mycosis in estuarine and freshwater fish in Florida', Journal of Aquatic Animal Health 19, 14-26. http://dx.doi.org/10.1577/H06-012.1, PMid:18236628 [ Links ]
Van Helden, L. & Grewar, J., 2011, 'Epizootic ulcerative syndrome - Eerste River system', Epidemiology Report, Veterinary Services, Western Cape Government 3(11), 1-2. [ Links ]
 Correspondence to:
Correspondence to:
Karl Huchzermeyer
PO Box 951
Lydenburg 1120
South Africa
aquavet@telkomsa.net
Received: 27 Jan. 2012
Accepted: 19 May 2012
Published: 25 Sept. 2012