Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the South African Veterinary Association
versión On-line ISSN 2224-9435
versión impresa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.82 no.3 Pretoria ene. 2011
ARTICLE ARTIKEL
Serial haematology results in transfused and non-transfused dogs naturally infected with Babesia rossi
E ScheepersI,*; A L LeisewitzI; P N ThompsonII; M M ChristopherIII
IDepartment of Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort, Pretoria, 0110 South Africa
IIDepartment of Production Animal Studies, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort, Pretoria, 0110 South Africa
IIIDepartment of Pathology, Microbiology & Immunology, School of Veterinary Medicine, University of California, One Shields Avenue, Davis, CA, USA
ABSTRACT
This prospective longitudinal study investigated the progression of haematological changes in 32 transfused and 54 non-transfused dogs naturally infected with Babesia rossi over the 1st 6 days following diagnosis and treatment. The effect of patient age on the results of complete blood counts was determined. Haematology data were analysed at presentation and at 24 hours, 3 days and 6 days after presentation. Dogs were treated with diminazene aceturate at diagnosis and a blood transfusion was given if deemed clinically required. Mildly to moderately regenerative normocytic normochromic anaemia was observed in all dogs throughout the study period. Transfused dogs more often had an inflammatory leukogram at presentation and at 24 hours, than dogs that were not transfused. In dogs with a left shift, a concurrent normal or decreased segmented neutrophil count was found more commonly than neutrophilia. Severe thrombocytopenia that resolved within a week was common. Blood transfusion alleviated the anaemia, but had no significant effect on white blood cell or platelet responses. Blood cell responses were not significantly influenced by age. In conclusion, the red blood cell and white blood cell responses were less than expected in dogs with babesiosis, given the degree of anaemia and inflammation present. The magnitude of thrombocytopenia and rapid return of the platelet count to normal suggested a possible immune-mediated mechanism for the thrombocytopenia.
Keywords: anaemia, Babesia rossi, canine babesiosis, haematology, left shift, thrombocytopenia.
INTRODUCTION
Canine babesiosis is caused by the haemoprotozoan parasites Babesia canis and B. gibsoni12. The 3 subspecies initially described for B. canis, namely B. c. canis, B. c. rossi and B. c. vogeli34, are now considered true species7,36. B. rossi is the most virulent of the canine babesial parasites5,13,34. Recently a new large Babesia species probably capable of infecting immunocompromised domestic dogs only has been described4,20.
Babesia spp. are thought to cause anaemia by both antibody-mediated erythrocyte destruction and direct parasite damage to erythrocytes. This results in both extravascular and intravascular haemolysis21. The decrease in haematocrit found in experimental babesiosis before parasites were detectable in peripheral blood was hypothesised to be caused by haemodilution, splenomegaly and sequestration21,27,28. Acute canine babesiosis, characterised by life-threatening haemolytic anaemia, is the most common presentation in South Africa14. Treatment of uncomplicated cases consists of antibabesial drugs and, in severely anaemic animals, a blood transfusion15. Severe babesiosis shares clinical and pathological similarities with other pro-inflammatory diseases, such as bacterial sepsis, endotoxaemia and human malaria9,24, and requires more intensive treatment.
Haematological information in dogs with a natural infection of babesiosis remains fragmented and scarce. The most frequently reported haematological findings are anaemia14,16,24 and thrombocytopenia16,17,24. Limited haematological studies in naturally occurring disease showed that dogs in France23, Spain25, Poland2,11,37, Croatia19 and the Philippines6 had anaemia and thrombocytopenia but leukocyte abnormalities were not fully described. Canine babesiosis in these studies was most likely caused by B. canis and/or B. vogeli, as only these 2 species have been described in these regions13,34. Two haematological studies on canine babesiosis in Sub-Saharan Africa showed that dogs with the disease had severe anaemia, neutrophilia and monocytosis1,24. This concurs with the fact that B. rossi, the most prevalent African species12,13,34, causes more severe haematological changes than the less virulent B. canis found in Europe5,13,34. B. vogeli has also been described in Africa22, but generally causes subclinical disease13. A study presumably describing haematological changes in B. rossi, showed that dogs with mild anaemia had a very mild leukocyte response and were older compared with dogs with severe anaemia24. These authors also described thrombocytopenia. Platelets were not examined in the other African study1.
Comprehensive haematological studies involving dogs with naturally occurring babesiosis are therefore lacking, yet correction of anaemia and resolution of systemic inflammation are pivotal to recovery. The course of haematological changes over time in babesiosis caused by a natural infection with B. rossi has not been described previously. Presently, many clinicians in general veterinary practice make decisions according to treatment and progression of disease based on assumptions that have not been tested. Evaluation of the regenerative red blood cell response, white blood cell response and platelet response will be valuable for veterinarians evaluating patients before, during, and after treatment. This study provides a series of haematological results that can be used in the evaluation of different therapies for Babesia-induced anaemia and the associated inflammatory response, and will allow comparisons to be made between anaemia induced by haemoprotozoal infection and anaemia caused by other aetiologies. Because the haematological kinetics of the canine haematopoietic response to infection are generally poorly documented, the results from this study may also be applicable to other types of infections or disease processes in dogs. Finally, the results of this study may be used to better compare haematological changes in canine babesiosis and human malaria and could strengthen the basis for using canine babesiosis as a study model for some aspects of malaria in humans9,24.
The objective of this study was therefore to describe the haematological abnormalities in 2 treatment groups of dogs naturally infected with babesiosis over the 1st 6 days following diagnosis and treatment and thereby assess the kinetics of blood cell production, demand or utilisation, and destruction. Because of the potential effect of age on haematological variables, an additional objective was to determine how patient age affected the results. Although not a primary goal of the study, the effect of a blood transfusion on the evolving haematological changes was also necessarily assessed because of the presence of 2 treatment groups (with and without transfusion). Further, because the clinical nature of this study meant that both whole blood and packed cell transfusions were utilised, these different treatments were also assessed.
MATERIALS AND METHODS
Study population
Ninety-six client-owned dogs with canine babesiosis presented to the Onderstepoort Veterinary Academic Hospital (OVAH) were considered for inclusion in this prospective, descriptive longitudinal study. The diagnosis of B. rossi was based initially on microscopic examination of a thin capillary blood smear and confirmed by polymerase chain reaction (PCR) analysis, as previously described22. Dogs of any breed, age and either sex weighing more than 3 kg were included. Dogs were excluded (1) if Ehrlichia canis morulae were seen on a thin capillary blood smear; (2) the result of an in-saline agglutination test3 was positive; (3) results of PCR analysis were positive for E. canis or B. vogeli22 infection; (4) if the dogs were vaccinated or treated with cortisone in the previous month, diagnosed with unrelated metabolic illness or babesiosis in the previous month or received cortisone therapy during the course of the study. Dogs were also excluded if they had severe complicated babesiosis (i.e. acute renal failure, cerebral babesiosis, coagulopathy, icterus and hepatopathy, immune-mediated haemolytic anaemia, haemoconcentration ('red biliary') and acute respiratory distress syndrome. Dogs were also excluded if a blood transfusion was deemed necessary but not given because of financial constraints.
All dogs were managed according to the OVAH standard treatment protocol for babesiosis, which includes anti-babesial treatment with diminazene aceturate at a dosage of 3.5 mg/kg, and a blood transfusion if required. Supportive treatment (i.e. fluid, electrolyte and glucose supplementation, anti-emetic therapy and enteral feeding) was given as deemed necessary. Either whole blood or packed red cells were given. The volume of blood transfused in all dogs was calculated according to the following formula: body weight (kg) × 90 × {(packed cell volume (PCV) desired (25 %) - PCV patient)/ (PCV donor)}. Thus the volume of red blood cells transfused, whether packed cells or whole blood, was patient specific, taking cognisance of both patient and transfusion PCVs. The attending clinician made the decision to give a blood transfusion, based on the clinical signs and PCV of the dog at presentation. As a general guideline, dogs with PCV of <15 % were transfused, but in many cases the clinical status of the dog (e.g. collapsed vs non-collapsed) played a role in decision-making. Dogs were treated at presentation, and then either discharged or hospitalised for further supportive treatment and monitoring. The Animal Use and Care Committee of the University of Pretoria approved the study.
Experimental procedures
An air-dried thin blood smear was made from capillary blood obtained from the anterior edge or hairless ventral surface of the ear, stained with Cam's Quick-Stain (Kyro-quick Solution®, Kyron Laboratories (Pty) Ltd, Benrose, South Africa) and examined microscopically for Babesia sp. organisms at presentation. In-saline agglutination3 was determined using heparinised whole blood. Heparinised whole blood was transferred to a microhaematocrit tube and centrifuged for 5 minutes using a microhaematocrit centrifuge. The PCV was determined using a Hawksley haematocrit reader (Laboratory and Scientific Equipment Co., Johannesburg, South Africa). A 0.5 mℓ (dogs 3-5 kg body weight) or 3.0 mℓ (dogs >5 kg body weight) whole blood sample was collected from the jugular vein of the dog in a tube containing EDTA (Vacutainer or BD Microtainer, Becton Dickinson Co., Franklin Lakes, NJ, USA) and submitted to the Clinical Pathology Laboratory, Faculty of Veterinary Science, Onderstepoort, at presentation, 24 hours (Day 1), 3 days and 6 days after presentation, or until death. All dogs in the study population were sampled at these intervals, irrespective of whether they were hospitalised or treated as outpatients, as owners returned their dogs for sampling as necessary. A complete blood count (CBC) was performed, using a CELL-DYN® 3700 analyser (Abbot Laboratories, Santa Clara, CA, USA). An experienced veterinary haematology technologist performed manual leukocyte differential counts by counting 100 cells on a Cam's Quick-stained peripheral blood smear. The remainder of the EDTA sample collected at presentation was submitted for PCR analysis.
The haematological variables, with reference intervals for the Onderstepoort Clinical Pathology Laboratory29, were haematocrit (HCT), absolute reticulocyte count (ARC), mean cell volume (MCV), mean cell haemoglobin concentration (MCHC), total white cell count (WCC), segmented neutrophil count, band neutrophil count (metamyelocytes were also included in this category), lymphocyte count, monocyte count, eosinophil count and platelet (PLT) count. PLT counts of samples that had marked platelet aggregation were not included in data analysis. Anaemia was classified as very severe if HCT <0.13 ℓ/ℓ , severe if HCT = 0.13-0.19 ℓ/ℓ , moderate if HCT = 0.20-0.29 ℓ/ℓ and mild if HCT = 0.30-0.36 ℓ/ℓ33. Reticulocyte responses were classified as marked if ARC >500 × 109/ℓ , moderate if ARC = 150-300 × 109/ℓ and mild if ARC = 80-150 × 109/ℓ
Statistical analysis
Data were captured into an Excel spreadsheet (Microsoft Excel 2003, Microsoft Corp, Redmond, WA, USA). The results for each haematological variable were screened for normality using the Shapiro-Wilk test, log-transformed and used for statistical analysis. The medians and range values of the haematological variables for each of the treatment groups (non-transfused and transfused) at each time point were determined, as the vast majority of data were not normally distributed. Data distribution was represented in box plots and median was used as a measure of central location. Treatment groups were compared with respect to sex and outcome using Fisher's exact test, and age and body weight using the Wilcoxon ranksum test. Multiple regression models were fitted in order to investigate the effect of age and treatment group on each of the haematological variables. Multiple regression models were also fitted in order to investigate the effect of a whole blood transfusion vs a transfusion with packed cells in the group that received a blood transfusion. A significance level (α) of 0.05 was used throughout. Data analysis was performed using NCSS 2004 (NCSS, Kaysville, Utah, USA).
RESULTS
Study population
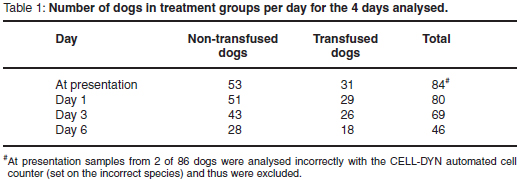
Of 96 dogs with babesiosis initially considered for this study, 86 dogs (54 dogs in the non-transfused group, 32 dogs in the transfused group) met the inclusion criteria. Three dogs (1 dog from the non-transfused group and 2 dogs from the transfused group) were excluded during the study because they received glucocorticoid therapy, but their CBCs prior to receiving glucocorticoids (i.e. CBCs at presentation and 24 hours later for 2 dogs and a CBC at presentation for 1 dog) were included in the data analysis. These 3 dogs had negative saline agglutination results at presentation, but as glucocorticoid treatment was deemed necessary during the course of the study they were subsequently excluded.
Baseline data were tabulated (Table 1). Results on breed (represented by more than 1 dog), sex, age, body weight, admission to hospital and outcome were summarised (Table 2). The transfused group was significantly younger than the non-transfused group (P = 0.003). There was no significant difference between the 2 groups with respect to sex (P = 0.47), outcome (P = 0.62) or weight (P = 0.05). While 82 dogs survived, 4 dogs died during the course of the study, without the exact Babesia-related cause being known, as no post mortem examinations were done.
Red blood cell responses
The anaemia at presentation was moderate in the non-transfused group (median HCT 0.28 ℓ/ℓ , range 0.12-0.56) and very severe in the transfused group (median HCT 0.09 ℓ/ℓ , range 0.06-0.23). The anaemia was normocytic (based on median MCV; data not shown) and normochromic (based on median MCHC; data not shown) throughout the study period for all dogs. The median HCT (0.36 ℓ/ℓ in the non-transfused group and 0.31 ℓ/ℓ in the transfused group) was still below the reference interval for both treatment groups on Day 6 (Fig. 1).

There was no regenerative response for the non-transfused group at presentation (median ARC 33.67 × 109/ℓ , range 10.45-500.35) or Day 1 (median ARC 38.67 ×109/ℓ , range 5.75-708.76), a moderate regenerative response on Day 3 (median ARC 227.31 × 109/ℓ , range 6.64-1377.96), and a mild regenerative response on Day 6 (median ARC 124.25 × 109/ℓ , range 24.94-766.34) (Fig. 2). The regenerative response for the transfused group was moderate at presentation (median ARC 155×109/ℓ , range 15.74-455.07), Day 1 (median ARC 187.37 × 109/ℓ , range 8.80-964.88) and Day 3 (median ARC 323.78 × 109/ℓ , range 44.77-1079.7), and mild on Day 6 (median ARC 91.88 × 109/ℓ , range 20.58-537.19) (Fig. 2).

White blood cell responses
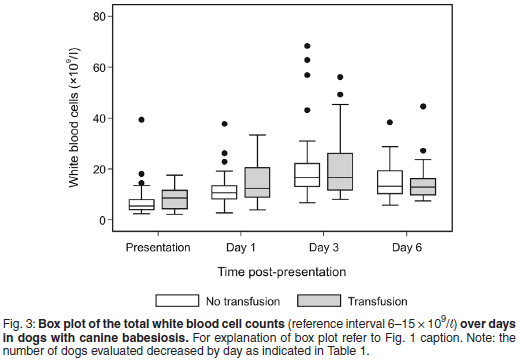
The median total WCC was within the reference interval for both treatment groups throughout the study period, except at presentation, when very mild leukopenia was found for the transfused group (median total WCC 5.4 × 109/ℓ , range 2.4-39.4); and on Day 3, when mild leukocytosis was found for the non-transfused group (median total WCC 16.6 ×109/ℓ , range 6.7-68.3) and for the transfused group (median total WCC 16.6 × 109/ℓ , range 8.1-56.1) (Fig. 3).
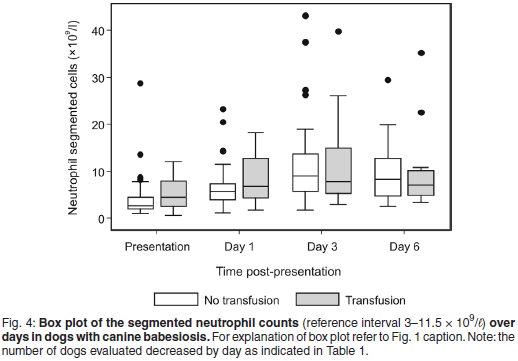
The median segmented neutrophil count was within the reference interval for both treatment groups throughout the study period (Fig. 4). The median band neutrophil count was within reference interval at presentation and Day 6, and increased on Day 1 (median band neutrophil count 0.54 × 109/ℓ , range 0-4.51) and Day 3 (median band neutrophil count 1.23 × 109/ℓ , range 0-23.22) for the non-transfused group (Fig. 5). The median band neutrophil count was increased throughout the study period for the transfused group, with a peak response on Day 3 (median band neutrophil count 1.76 × 109/ℓ , range 0-15.25). The more anaemic group of dogs had a significantly higher percentage of dogs with a left shift at presentation (P = 0.002 ) and on Day 1 (P = 0.001). Four of 86 (5 %) dogs had a degenerative left shift at presentation only. The prevalence of neutrophilia, neutropenia, and left shift with neutrophilia was tabulated (Table 3).


The median lymphocyte count was within the reference interval throughout the study period for both treatment groups (Fig. 6).

The median monocyte count was within the reference interval at presentation, Day 1 and Day 6 for the non-transfused group, with mild monocytosis on Day 3 (median monocyte count 1.46 × 109/ℓ , range 0.13-6.20) (Fig. 7). The median monocyte count was within the reference interval at presentation and Day 6 for the transfused group, with mild monocytosis on Day 1 (median monocyte count 1.46 × 109/ℓ , range 0.12-3.27) and Day 3 (median monocyte count 1.73 × 109/ℓ , range 0.38-4.98)( Fig. 7).

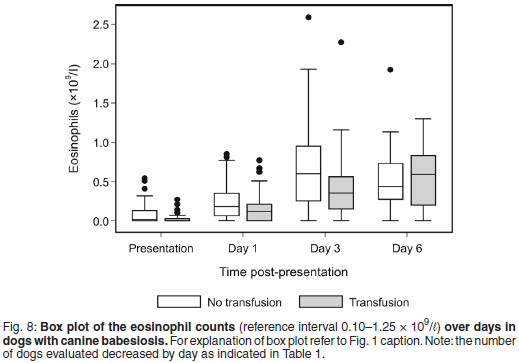
The median eosinophil count was within reference interval for all dogs throughout the study period, except at presentation where mild eosinopenia was found for both treatment groups (median eosinophil count 0.02 × 109/ℓ , range 0.00-0.54 for the non-transfused group and 0.00 × 109/ℓ , range 0.00-0.27 for the transfused group) (Fig. 8).
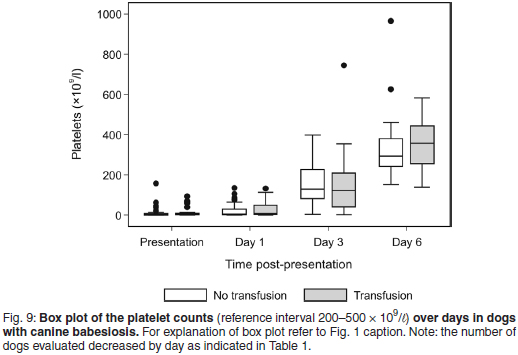
Platelet responses
Both treatment groups were severely thrombocytopenic at presentation and Day 1, and moderately thrombocytopenic on Day 3. The median PLT count at presentation was 3.6 × 109/ℓ , range 0-157, for the non-transfused group; and 4.1 × 109/ℓ , range 0-92.8, for the transfused group (Fig. 9). The median PLT count was within the normal reference interval on Day 6 for both treatment groups.
Results of multiple regression analysis
Based on multiple regression models, the treatment group (transfused or non-transfused) had a significant effect on HCT and ARCs, with the more anaemic dogs having lower median HCT counts (P < 0.022) and higher median ARCs (P < 0.031). Treatment group also had a significant effect on band neutrophil counts and left shifts at presentation and Day 1, with dogs with more severe anaemia having higher mean band neutrophil counts (P < 0.001), and a higher prevalence of left shifts (P = 0.002). Treatment group had no significant effect on any other leukocyte or platelet responses.
Based on multiple regression models, age had no significant effect on red blood cell responses. Older dogs had significantly higher median segmented neutrophil counts than younger dogs at presentation (P = 0.042), significantly higher median PLT counts than younger dogs at presentation (P = 0.037) and significantly lower median lymphocyte counts than younger dogs throughout the study period (P < 0.001).
Based on multiple regression models, whether a packed cell or whole blood transfusion was given had no significant effect on any of the blood cell parameters on any of the 4 days (P > 0.09).
DISCUSSION
The results of this study describe clearly, for the 1st time, changes over time in a wide range of haematological parameters in a large population of dogs naturally infected with B. rossi and subsequently treated for the infection. Dogs in this study had a mild to moderate regenerative response in the face of severe anaemia. In addition, a left shift was observed in most dogs, without neutrophilia. These red blood cell and white blood cell responses were less robust than expected based on the severity of haemolysis and inflammation as previously described and could indicate impaired haematopoiesis in these cell lines or other factors. Rapid resolution of severe thrombocytopenia suggested a strong megakaryocytic response.
In dogs with severe acute haemolytic anaemia and a functional bone marrow, a strong erythroid regenerative response is expected within a few days, with peak reticulocytosis at 4-7 days and a return to normal HCT within 2-3 weeks10. The mild to moderate (rather than marked) regenerative response in dogs in this study could indicate a suppressive effect of the organism on the bone marrow, or other factors. It has been shown in falciparum malaria in humans that the anaemia is multifactorial, with erythrocyte destruction and ineffective erythropoiesis playing equal parts in the aetiology of malarial anaemia8. Ineffective erythropoiesis is caused by host cytokines such as TNF-α, IL-10, MIF (macrophage migration inhibitory factor) and IL-12, inflammatory mediators, and parasite products such as lysate and haemozin8. Although administration of a blood transfusion can blunt the regenerative response by alleviating hypoxia, dogs receiving transfusions had persistently moderate regenerative responses until Day 6, when ARCs decreased in all dogs, regardless of transfusion status. A previous study describing reticulocytosis in South African dogs with babesiosis presumably caused by B. rossi, showed that only dogs with severe anaemia (PCV < 15 %) at presentation had mild reticulocytosis (mean reticulocyte percentage 3.43 %10)24. We are unaware of other studies that examined the red blood cell response over time in a natural infection with B. rossi. Further studies on the nature and underlying pathophysiological mechanisms of the bone marrow response to canine babesiosis are warranted.
Although this study did not monitor the dogs until complete recovery, the HCT increased gradually and median HCT values on Day 6 were nearing the lower limit of the reference interval (0.37 ℓ/ℓ ), consistent with the resolution of anaemia. HCT in the non-transfused group was lower at Day 1 than at presentation, consistent with the expected loss of parasitised red blood cells after chemotherapeutic treatment. In addition to the blunting effect of a transfusion, the slower increase in HCT in transfused dogs (compared with non-transfused dogs) could be the result of rapid destruction of the transfused red cells. Monocytosis in dogs in the transfused group could be consistent with a higher level of erythrocyte destruction, much of which occurs extravascularly by macrophages in the spleen. Anaemia in most dogs was normocytic and normochromic, indicating the importance of an ARC to document and monitor regeneration in dogs with babesiosis.
Babesiosis has previously been described as an inflammatory disease comparable to human falciparum malaria8,9,24,35. Studies on South African dogs with babesiosis documented a systemic inflammatory response35 accompanied by an acute phase response, with increased serum C-reactive protein concentration18.A previous study reported a left shift with normal neutrophil counts in dogs with babesiosis (including cases with complicated babesiosis) having moderate to very severe anaemia at presentation24 (as classified in this study). This study similarly found a left shift without neutrophilia at presentation. In addition, nearly 50 % of the dogs with babesiosis in this study had neutropenia, with or without a left shift, at presentation, while only about5%of dogs had neutrophilia. Indeed, although neutrophil counts increased in dogs in this study from presentation to Day 3, only 38 % of dogs with a left shift on Day 3 had concurrent neutrophilia. These findings could indicate excessive neutrophil destruction or utilisation, an early stage of bone marrow response, impaired bone marrow response, or previous depletion of the storage pool of neutrophils from the bone marrow.
The neutrophil changes in this study of dogs with less complicated babesiosis may differ from those with more complicated babesiosis. In a previous study of dogs with complicated babesiosis in South Africa, only 10 % had leukopenia and 60 % had leukocytosis35. The magnitude of leukocytosis may also in part be due to age; consistent with the findings in a previous study of babesiosis, dogs in the transfused group (more severe anaemia) in our study were younger, and also had higher neutrophil and lymphocyte counts16. Importantly, neutropenia or lack of a neutrophil response in dogs with babesiosis could be misinterpreted as evidence of concurrent infection by E. canis, which was ruled out in our study as a potential cause. While dogs with clinical evidence of secondary immune-mediated haemolytic anaemia (IMHA) (typically with severe leukocytosis, neutrophilia and a left shift3) were excluded from this study, haemolysis caused by babesiosis is not less severe than that caused by IMHA. This suggests that other factors may contribute to the higher prevalence and severity of an inflammatory response in dogs with IMHA and to the lack of neutrophilia in dogs with babesiosis.
In addition to haemolysis, monocytosis and eosinopenia also may have been associated with endogenous corticosteroid release. In a study on the endocrine predictors of mortality in both uncomplicated and complicated canine babesiosis, it was found that 42 % of dogs had basal serum cortisol levels higher than the established reference interval30. With treatment, corticosteroid-mediated stress diminishes, as seen by the eosinophil counts returning to within the reference interval in most dogs in this study on Day 3.
The prevalence of thrombocytopenia (100 %) in dogs in this study was in agreement with that reported in a study on dogs infected with presumably B. rossi, which found thrombocytopenia to be present in 99 % of cases17. This was also reported in another study on B. rossi24.The severity of thrombocytopenia was much greater in our study than in these 2 studies. Decreases in the PLT counts due to artefacts were ruled out in our study because samples with marked platelet aggregation were excluded. Although it has been speculated that the severity of thrombocytopenia might be influenced by the severity of the anaemia17, this study found no difference between the 2 groups of dogs. The pathogenesis of the thrombocytopenia in dogs with babesiosis remains unclear; however, resolution of normal PLT numbers in the majority of dogs in this study by Day 6 suggests a megakaryocytic response in the bone marrow, supporting increased PLT destruction (i.e. immune-mediated thrombocytopenia) as a likely mechanism. Sequestration of PLTs in the spleen and hypersplenism, a marked primary PLT sequestration in the spleen, are both considered rare causes of thrombocytopenia in animals26. While animals with haemolytic anaemia have splenomegaly, it is unlikely that mild redistribution alone was responsible for the magnitude and consistency of thrombocytopenia seen in this study.
With the exception of lymphocyte count, which is usually higher in young animals32, CBC results were not statistically significantly influenced by patient age. In a study on the prevalence of hypoglycaemia in dogs with babesiosis16,itwas found that dogs younger than 6 months were 7 times more likely than dogs older than 1 year to be severely anaemic. In this study the effect of age (treated as a continuous rather than categorical variable) on HCT, was not statistically significant. The differences in statistical significance are likely due to differences between the 2 studies in the classification of age and the use of HCT rather than degree of anaemia as the outcome. In agreement with the study mentioned16, we found that the median age of the transfused group was significantly lower than that of the group that was not transfused.
There were several limitations in this study. One was the slight subjectivity in the choice to transfuse dogs, which resulted in the overlap in HCT between the 2 groups. A 2nd limitation was that dogs with complicated babesiosis were excluded, and therefore the results might not be representative of the broader population of dogs with B. rossi. Further studies on serial haematologies of complicated babesiosis are required to determine this. However, dogs with complicated disease often die soon after presentation18,35, making it difficult to monitor a haematopoietic response to peripheral cytopenias. A final limitation of this study was the inability to determine the exact day of infection but this is the case in all studies of natural infections.
CONCLUSIONS
The results of this study showed that during treatment of uncomplicated canine babesiosis, a mildly to moderately regenerative normocytic normochromic anaemia occurs that does not completely resolve by the end of 1 week. An inflammatory leukogram also occurs, characterised mainly by a left shift without concurrent neutrophilia. Both of these haematological changes are less robust than might be expected for the degree of anaemia and inflammation, suggesting the influence of factors unique to B. rossiinduced haemolysis. The severe thrombocytopenia at presentation resolved within a week after commencing treatment, suggesting a possible immune-mediated mechanism.
ACKNOWLEDGEMENTS
This study was carried out in fulfilment of the requirements for the MSc (Veterinary Science) degree, Faculty of Veterinary Science, University of Pretoria. Funding was received from the Faculty of Veterinary Science, University of Pretoria. Abbot Laboratories, South Africa, donated automated cell counter reagents. Our gratitude is expressed to Mrs Gertie Pretorius and Mrs Cheryl Booth of the Onderstepoort Clinical Pathology Laboratory, Department of Companion Animal Clinical Studies, Faculty of Veterinary Science for their dedication in performing the manual differential leukocyte counts.
REFERENCES
1. Abdullahi S U, Mohammed A A, Trimnell A R, Sannusi A, Alafiatayo R 1990 Clinical and haematological findings in 70 naturally occurring cases of canine babesiosis. Journal of Small Animal Practice 31: 145-147 [ Links ]
2. Adaszek L, Winiarczyk S, Skrzypczak M 2009 The clinical course of babesiosis in 76 dogs infected with protozoan parasites Babesia canis canis. Polish Journal of Veterinary Science 12: 81-87 [ Links ]
3. Balch A, Mackin A 2007 Canine immune-mediated hemolytic anemia: pathophysiology, clinical signs and diagnosis. Compendium on Continuing Education for the Practicing Veterinarian 29: 217-224 [ Links ]
4. Birkenheuer A J, Neel J, Ruslander D, Levy M G, Breitschwerdt E B 2004 Detection and molecular characterization of a novel large Babesia species in a dog. Veterinary Parasitology 124: 151-160 [ Links ]
5. Bourdoiseau G 2006 Canine babesiosis in France. Veterinary Parasitology 138: 118-125 [ Links ]
6. Carlos E T, Rodelas E R, Manzon J C 1989 Hematologic studies of clinical cases of canine babesiosis. Philippine Journal of Veterinary Medicine 26: 29-36 [ Links ]
7. Carret C, Walas F, Carcy B, Grande N, Precigout E, Moubri K, Schetters T P, Gorenflot A 1999 Babesia canis canis, Babesia canis vogeli, Babesia canis rossi: differentiation of the three subspecies by a restriction fragment length polymorphism analysis on amplified small subunit ribosomal RNA genes. Journal of Eukaryotic Microbiology 46: 298-303 [ Links ]
8. Casals-Pascual C, Roberts D J 2006 Severe malarial anaemia. Current Molecular Medicine 6: 155-168 [ Links ]
9. Clark I A, Jacobson L S 1998 Do babesiosis and malaria share a common disease process? Annals of Tropical Medicine and Parasitology 92: 483-488 [ Links ]
10. Cowgill E S, Neel J A, Grindem C B 2003 Clinical application of reticulocyte counts in dogs and cats. Veterinary Clinics of North America Small Animal Practice 33: 1223-1244 [ Links ]
11. Fabisiak M, Sapierzyn'ski R, Klucin'ski W 2010 Analysis of haematological abnormalities observed in dogs infected by a large Babesia. Bulletin of the Veterinary Institute in Pulawy 54: 167-170 [ Links ]
12. Friedhoff K T 1988 Transmission of Babesia. In Ristic M (eds) Babesiosis of domestic animals and man. CRC Press, Boca Raton: 23-52 [ Links ]
13. Irwin P J 2009 Canine babesiosis: from molecular taxonomy to control. Parasites and Vectors 2 (Suppl. 1): 24 [ Links ]
14. Jacobson L S 2006 The South African form of severe and complicated canine babesiosis: clinical advances 1994-2004. Veterinary Parasitology 138: 126-139 [ Links ]
15. Jacobson L S, Swan G E 1995 Supportive treatment of canine babesiosis. Journal of the South African Veterinary Association 66: 95-105 [ Links ]
16. Keller N, Jacobson L S, Nel M, De Clerq M, Thompson P N, Schoeman J P 2004 Prevalence and risk factors of hypoglycemia in virulent canine babesiosis. Journal of Veterinary Internal Medicine 18: 265-270 [ Links ]
17. Kettner F, Reyers F, Miller D 2003 Thrombocytopaenia in canine babesiosis and its clinical usefulness. Journal of the South African Veterinary Association 74: 63-68 [ Links ]
18. Koster L S, Van Schoor M, Goddard A, Thompson P N, Matjila P T, Kjelgaard-Hansen M 2009 C-reactive protein in canine babesiosis caused by Babesia rossi and its association with outcome. Journal of the South African Veterinary Association 80: 87-91 [ Links ]
19. Kucer N, MatijatkoV, Kis I, Grden D, Brkljacic M, Forsek J, Zvorc Z, Rafaj R B 2010 White blood cell count and neutrophil to lymphocyte ration in uncomplicated and complicated canine babesiosis caused by Babesia canis canis. Veterinarski Arhiv 78: 321-330 [ Links ]
20. Lehtinen L E, Birkenheuer A J, Droleskey R E, Holman P J 2008 In vitro cultivation of a newly recognized Babesia sp. in dogs in North Carolina. Veterinary Parasitology 151: 150-157 [ Links ]
21. Maegraith B, Gilles H M, Devakul K 1957 Pathological processes in Babesia canis infections. Zeitschrift für Tropenmedizin und Parasitologie 8: 485-514 [ Links ]
22. Matjila P T, Penzhorn B L, Bekker C P, Nijhof A M, Jongejan F 2004 Confirmation of occurrence of Babesia canis vogeli in domestic dogs in South Africa. Veterinary Parasitology 122: 119-125 [ Links ]
23. Pages J, Vidor E, Trouillet J, Bissuel G, Lecointre O, Moreau Y 1990 Description clinique, hematologique et serologique de 133 cas de babesiose canine. Pratique medicale et chirgicale de l'animal de compagnie 25: 89-96 [ Links ]
24. Reyers F, Leisewitz A L, Lobetti R G, Milner R J, Jacobson L S, Van Zyl M 1998 Canine babesiosis in South Africa: more than one disease. Does this serve as a model for falciparum malaria? Annals of Tropical Medicine and Parasitology 92: 503-511 [ Links ]
25. Ruiz de Gopegui R, Penalba B, Goicoa A, Espada Y, Fidalgo L E, Espino L 2006 Clinico-pathological findings and coagulation disorders in 45 cases of canine babesiosis in Spain. Veterinary Journal 174: 129-132 [ Links ]
26. Russel K E, Grindem C B 2000 Secondary thrombocytopenia. In Feldman B F, Zinkl J G, Jain N C (eds) Schalm's veterinary hematology. Lippincott Williams & Wilkins, Philadelphia: 487-495 [ Links ]
27. Schetters T P, Kleuskens J, Scholtes N, Gorenflot A 1998 Parasite localization and dissemination in the Babesia-infected host. Annals of Tropical Medicine and Parasitology 92: 513-519 [ Links ]
28. Schetters T P, Kleuskens J A, Scholtes N C, Pasman J W, Goovaerts D 1997 Vaccination of dogs against Babesia canis infection. Veterinary Parasitology 73: 35-41 [ Links ]
29. Schmidl M 1989 Laboratory testing in veterinary medicine, diagnosis and treatment Boehringer Mannheim, Mannheim [ Links ]
30. Schoeman J P, Rees P, Herrtage M E 2007 Endocrine predictors of mortality in canine babesiosis caused by Babesia canis rossi. Veterinary Parasitology 148: 75-82 [ Links ]
31. Stockham S L, Keeton K S, Szladovits B 2003 Clinical assessment of leukocytosis: distinguishing leukocytoses caused by inflammatory, glucocorticoid, physiologic, and leukemic disorders or conditions. Veterinary Clinics of North America Small Animal Practice 33: 1335-1357 [ Links ]
32. Toman M, Faldyna M, Knotigova P, Pokorova D, Sinkora J 2002 Postnatal development of leukocyte subset composition and activity in dogs. Veterinary Immunology and Immunopathology 87: 321-326 [ Links ]
33. Tvedten H W 1981 Hematology of the normal dog and cat. Veterinary Clinics of North America Small Animal Practice 11: 209-217 [ Links ]
34. Uilenberg G, Franssen F F, Perie N M, Spanjer A A 1989 Three groups of Babesia canis distinguished and a proposal for nomenclature. Veterinary Quarterly 11: 33-40 [ Links ]
35. Welzl C, Leisewitz A L, Jacobson L S, Vaughan-Scott T, Myburgh E 2001 Systemic inflammatory response syndrome and multiple-organ damage/dysfunction in complicated canine babesiosis. Journal of the South African Veterinary Association 72: 158-162 [ Links ]
36. Zahler M, Schein E, Rinder H, Gothe R 1998 Characteristic genotypes discriminate between Babesia canis isolates of differing vector specificity and pathogenicity to dogs. Parasitology Research 84: 544-548 [ Links ]
37. Zygner W, Gojska O, Rapacka G, Jaros D, Wedrychowicz H 2007 Hematological changes during the course of canine babesiosis caused by large Babesia in domestic dogs in Warsaw (Poland). Veterinary Parasitology 145: 146-151 [ Links ]
Received: November 2009.
Accepted: June 2011.
* Author for correspondence. E-mail: elrien.scheepers@up.ac.za














