Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.82 n.2 Pretoria Jan. 2011
CLINICAL COMMUNICATION KLINIESE MEDEDELING
Bilateral multiple cystic kidney disease and renal cortical abscess in a Boerboel
A M KitshoffI,*; V McClureII; C K LimIII; R M KirbergerIII
ISection Small Animal Surgery, Department Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort, 0110 South Africa
IISection of Small Animal Medicine, Department Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort, 0110 South Africa
IIISection of Diagnostic Imaging, Department Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort, 0110 South Africa
ABSTRACT
Cystic renal disease is rare in dogs and although infected renal cysts have been reported in humans, no report could be found in dogs. A 58 kg, 5-year-old, castrated, male Boerboel presented with weight loss, pyrexia, lethargy and vomiting, 20 months after an incident of haematuria was reported. The initial ultrasonographic diagnosis was bilateral multiple renal cysts of unknown aetiology. The cysts had significantly increased in size over the 20-month period and some contained echogenic specks which could be related to infection, normal cellular debris or haemorrhage. In both kidneys the renal contours were distorted (the left more than the right). The abnormal shape of the left kidney was largely due to multiple cysts and a large crescent-shaped septate mass on the cranial pole of the kidney. Aspirates of the septate mass were performed (left kidney) and the cytology and culture were indicative of an abscess. It is suggested that the previous incident of haematuria provided a portal of entry for bacteria into the cysts resulting in renal cortical abscess formation.
Keywords: abscess, Boerboel, cyst, haematuria, renal.
INTRODUCTION
Cystic structures in the kidney can be classified as true cysts (epithelial cell membrane separating them from the surrounding tissue) or pseudocysts (wall consisting of granulation and/or fibrous tissue as a result of inflammation)14,36.
Renal cysts can be acquired or congenital, solitary or multiple and involve either one or both kidneys14,19,30,35. Furthermore these cysts can be classified as either simple or complicated, in which case they contain cells, bacteria or fungi34. Renal cysts in dogs, unlike those in cats, are usually small, solitary and incidental14.An autosomal dominant mode of inheritance for polycystic kidney disease (PKD) has been demonstrated in Persian cats6,12, and Bull terriers36. Ultrasonographically simple renal cysts can mimic renal neoplasia and renal cortical and perinephric abscesses in humans11. Simple renal cysts usually have a distinct sonographic appearance, which includes a round to oval contour, anechoic contents, smooth walls (with enhancement of the far wall) and marked distal acoustic enhancement6,35. Cysts can have internal echoes that can be related to haemorrhage or cellular debris, or might be artefactual due to a slice thickness artefact mimicking sediment in the cyst14,30,35. In humans complications such as infection and acute rupture of these cysts have been reported3,9,13,15,16,20,23-25,27,29,39-45.To the authors' knowledge, there are no previous case reports that documents renal cyst infection in dogs with multiple-, solitary cystic kidney disease or PKD.
CASE HISTORY
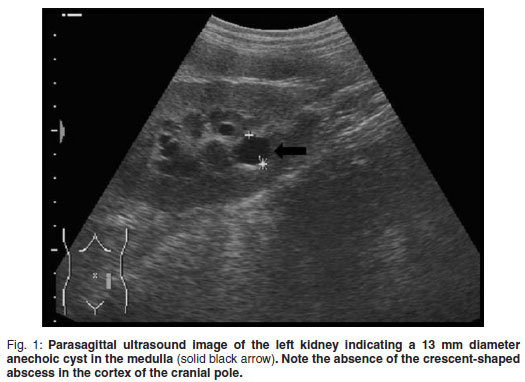
A 5-year-old, fawn, fully vaccinated, intact, male Boerboel weighing 58 kg was presented to the Onderstepoort Veterinary Academic Hospital (OVAH). The history suggested a change in the sexual behaviour of the dog with an increased tendency to 'mount' other dogs. Red discoloration of the urine for 2 weeks before presentation was noted. The only abnormalities that were seen on clinical examination were areas of echymoses on the penile mucosa. Faecal analysis was normal. Urine was collected via cystocentesis and analysed with the following findings: urine specific gravity (SG) 1.018, pH 7, proteins 1+, blood/haemoglobin 3+, erythrocytes 2+ and reticuloendothelial cells 1+. Abdominal ultrasound was performed using a Siemens Sonoline Omnia (Siemens AG, Erlangen, Germany) ultrasound machine and a mutli-frequency curvilinear array transducer operated at 5 MHz. This revealed multiple bilateral cystic structures measuring up to 13 mm in the medulla of the kidneys (Fig. 1). The prostate was normal in size with patchy hypo-to anechoic mottled areas. Bilaterally on the cranio-lateral aspect of the prostate there were more anechoic focal fluid accumulations up to 20 mm in diameter. The cytology of the ultrasoundguided fine-needle aspirates (FNA) was consistent with benign prostatic hyperplasia. Owing to financial constraints the owners declined further diagnostics and blood tests. The dog was castrated by the referring veterinarian.
The dog was presented to the OVAH 20 months after initial presentation with a history of vomiting of 2 weeks duration. The dog had lost 12 kg and had a temperature of 40.2ºC that was partially responsive to amoxicillin with clavulanic acid (Ranclav, Ranbaxy, South Africa, 625 mg) tablets at a dose of 20 mg/kg tid as prescribed by the referring veterinarian. The rest of the clinical examination was normal as was the faecal analysis. Urine was collected via cystocentesis and revealed the following: SG 1.026, pH 6, proteins 3+, bilirubin 1+, and blood/haemoglobin 4+. Cytological examination of the sediment from the spun down sample of urine revealed 4+ red blood cells. Blood samples were collected via cephalic venipuncture and sent for complete blood count and biochemistry profiles. The results revealed a moderate left shift neutrophilia, mild thrombocytopenia and a mild hyperglobulinaemia.
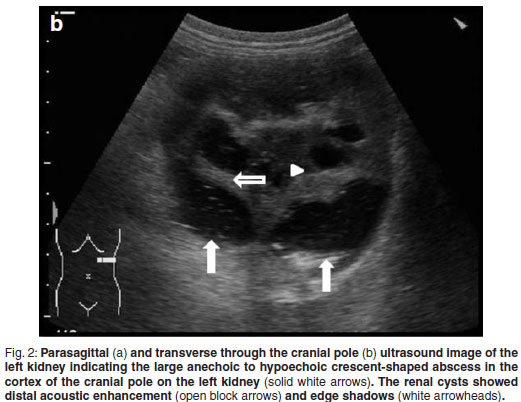
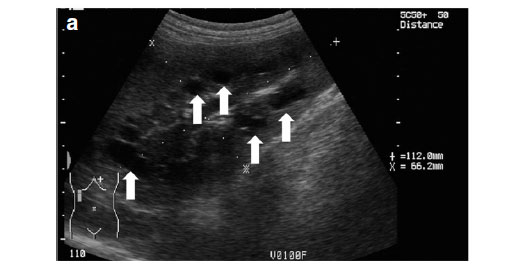
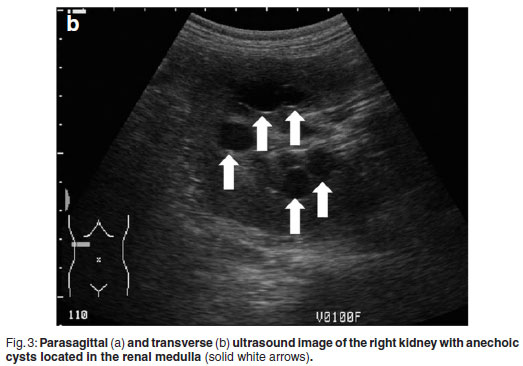
Abdominal ultrasonography was performed under sedation using medetomidine (Domitor, Pfizer, 1 mg/kg) at a dose of 10 µg/kg intravenously. The left kidney measured 10×6×7.5 cm with a slightly uneven margin (cranial pole was larger). A small amount of hypoechoic subcapsular fluid surrounded the kidney. Multiple round, well-marginated, anechoic, cystic structures were seen mainly in the medulla with some extending into the cortex. The largest of these measured 2.5 × 1.5 × 2 cm. These cysts had a clear edge shadow, distal acoustic enhancement with a thin but clear hyperechoic far wall (Fig. 2a,b). Some cysts contained multiple internal hyperechoic specks. The renal cortex was normoechoic and the corticomedullary junction was distinct. Two of the cranial pole cysts of the kidney were more hypoechoic and communicated with a large 5×3×3cm hypoechoic well-marginated, crescentshaped mass which distorted the cranial pole of the kidney. This structure had hyperechoic septa and multiple hyperechoic internal specks that swirled on ballotment. Acoustic enhancement and edge shadowing were seen. Slice thickness artefact was noted in the mass mimicking sediment. No gas was noted in the mass. The right kidney measured 11 × 6 × 5 cm. Multiple smaller (largest about 1×1×1.5 cm) anechoic structures were present mainly in the medulla (Fig. 3a,b). Some of these cysts communicated with each other and some had multiple hyperechoic internal specks. No cysts were found in any of the other abdominal organs, including the pancreas and the liver. The ultrasonographic diagnosis was atypical multiple renal cysts with a suspected large left renal cranial pole cortical abscess. Other individual complicated cysts could not be excluded. The differential diagnosis for cyst-like structures included uniform blood clots, unclotted blood, abscesses without debris, lymphomatous masses and necrosis related to tumours or cystadenocarcinomas35.
An ultrasound-guided aspirate was taken of the left cranial pole renal mass and appeared to be blood-stained pus, which was confirmed cytologically. Aerobic and anaerobic culture was positive for a rough Escherichia coli sensitive to doxycycline, trimethroprim sulphas, enrofloxacin and chloramphenicol. The patient was treated with oral antibiotics, 5 mg/kg enrofloxacin sid (Baytril 10 % oral solution, Bayer, Germany) but showed no improvement and became azotaemic. As ultrasound-guided percutaneous aspiration of the infected cyst was not feasible due to the size of the dog and the septation, surgical drainage was performed via a ventral midline celiotomy. Macroscopically the left kidney had an undulating shape with a large soft, yellow tinged fluctuant mass on the cranial pole (Fig. 4). The right kidney appeared macroscopically normal except for having an undulating contour (Fig. 5). The area surrounding the left kidney was packed with moist abdominal swabs. During manipulation of the left kidney the abscess ruptured inadvertently and the purulent material was removed by suction. The defect in the kidney was flushed, after which the abdomen was flushed with 10ℓ of lukewarm lactated Ringers solution (Ringers lactate, Fresenius kabi). The abdomen was partially closed leaving the cranialmost part of the incision open for drainage. A sterile non-adherent primary wound dressing and a large amount of cotton wool in the secondary layer for absorption of the exudate were applied. After the drainage the dog was placed on 5 mg/kg enrofloxacin sid (Baytril 10 % oral solution, Bayer, Germany) for a period of 3 weeks. Post-operative pain was treated with 0.2 mg/kg morphine sulphate (Morphine sulphate Fresenius PF, Fresenius Kabi, 10 mg/mℓ) intravenously every 4 hours for the 1st 48 hours. The bandage was changed daily for 7 days in a sterile manner under general anaesthesia, after which the abdomen was closed surgically. During this period the albumin levels were tested daily with a lowest recorded level of 21 g/ℓ (n = 27-35). The dog made a full recovery following surgery and the pyrexia and azotaemia resolved. He was discharged with instructions to complete the antibiotics. The follow-up ultrasound revealed remarkable improvement. There were few residual renal cysts up to 22 mm in diameter, but no evidence of the renal abscess.


DISCUSSION
Polycystic kidney disease has been diagnosed in the Persian cat and English bull terrier, West highland white terrier and Cairn terrier dogs4-7,12,32,33,36,3 and the cysts have been reported to originate from mainly the collecting duct and the distal part of the nephron17. The sensitivity and specificity for the detection of PKD in Persian cats at an age of 3 months using ultrasonography has been shown to be 92.6 % and 91 %, respectively8. Results were 100 % repeatable and disease progression was noted in 75 % of cats over a 1-year period45 but no data could be found for dogs. The disease also progressed in the case reported here with the cysts increasing in size from about 13 mm to 22 mm in diameter over a 2-year period. Although the disease in the current report presented much like PKD, potential cystic renal disease in related dogs was not investigated.
Renal abscesses are rare in dogs14,21,46 and their pathophysiology is poorly understood21. The true incidence of renal abscesses is uncertain but 1 study reported it to be 0.003 % of all cases seen over a period of 15 years21. Sonographically specifically be looked for. Depending on the amount of gas, it can present as areas of increased echogenicity with or without dirty acoustic shadowing25 rising to the non-dependent surface of the abscess. Predisposing conditions to renal abscess formation include diabetes mellitus21, nephrolithiasis1,31, surgery2,18, toxin exposure22 and pyelonephritis18.28,31. In humans corticomedullary abscesses form as a result of ascending urinary tract infection, hence the common isolates of E.coli, Klebsiella spp. and Proteus spp.38 Most patients with renal cortical abscesses do not have urinary tract symptoms because the localised process in the cortex does not generally communicate with the excretory passages11. One study reported only 42 % positive renal cultures in humans with renal abscesses10. The authors hypothesise that a traumatic incident might have damaged the cysts and hence contributed to the haematuria. This allowed bacteria to gain access to the cysts with resultant abscess formation.
CONCLUSION
Polycystic kidney disease is a rare condition in dogs. Although infection of renal cysts has been reported extensively in humans, no such reports exist for dogs. This case report presents a large breed dog with slowly progressive multiple cystic kidney disease with focal abscessation of unknown origin. The patient responded well to surgical drainage and antibiotic therapy.
REFERENCES
1. Agut A, Laredo F G, Belda E, Seva J, Soler M 2004 Left perinephric abscess associated with nephrolithiasis and bladder calculi in a bitch. Veterinary Record 154:562-565 [ Links ]
2. Aronson L R 2002 Retroperitoneal fibrosis in four cats following renal transplantation. Journal of the American Veterinary Medical Association 221:984-989 [ Links ]
3. Azumi M, Kato Y, Saga Y, Kakizaki H 2007 A case of infected renal cyst suspected of originating from retrograde infection. Acta Urologica Japonica 53:53-56 [ Links ]
4. Barthez P Y, Rivier P, Begon D 2003 Prevalence of polycystic kidney disease in Persian and Persian related cats in France. Journal of Feline Medicine and Surgery 5:345-347 [ Links ]
5. Beck C, Lavelle R B 2001 Feline polycystic kidney disease in Persian and other cats: a prospective study using ultrasonography. Australian Veterinary Journal 79:181-184 [ Links ]
6. Biller D S, DiBartola S P, Eaton K A, Pflueger S, Wellman M L, Radin M J 1996 Inheritance of polycystic kidney disease in Persian cats. Journal of Heridity 87:1-5 [ Links ]
7. Bonazzi M, Volta A, Gnudi G, Bottarelli E, Gazzola M, Bertoni G 2007 Prevalence of the polycystic kidney disease and renal and urinary bladder ultrasonographic abnormalities in Persian and exotic shorthair cats in Italy. Journal of Feline Medicine and Surgery 9:387-391 [ Links ]
8. Bonazzi M, Volta A, Gnudi G, Cozzi M C, Strillacci M G, Polli M, Longeri M, Manfredi S, Bertoni, G 2009 Comparison between ultrasound and genetic testing for the early diagnosis of polycystic kidney disease in Persian and exotic shorthair cats. Journal of Feline Medicine and Surgery 11:430-434 [ Links ]
9. Cho K J, Maklad N, Curran J, Ting Y M 1976 Angiographic and ultrasonic findings in infected simple cysts of the kidney. American Journal of Roentgenology 127:1015-1019 [ Links ]
10. Coelho R F, Schneider-Monteiro E D, Mesquita J L, Mazzucchi E, Marmo Lucon A, Srougi M 2007 Renal and perinephric abscesses: analysis of 65 consecutive cases. World Journal of Surgery 31:431-436 [ Links ]
11. Dembry L M, Andriole V T 1997 Renal and perirenal abscesses. Infectious Disease Clinics of North America 11:663-680 [ Links ]
12. Eaton K A, Biller D S, DiBartola S P, Radin M J, Wellman M L 1997 Autosomal dominant polycystic kidney disease in Persian and Persian-cross cats. Veterinary Pathology 34:117-126 [ Links ]
13. Feldberg M A M, Mali Th. W P 1980 An infected renal cyst. Urologic Radiology 2:47- 49 [ Links ]
14. Finn-Bodner S T 1995 The kidneys. In Cartee R E, Selcer B A (eds) Practical veterinary ultrasound. Williams and Wilkins, Philadelphia: 157-185 [ Links ]
15. Frishman E, Orron D E, Heiman Z, Kessler A, Kaver I, Graif M 1994 Infected renal cysts: sonographic diagnosis and management. Journal of Ultrasound in Medicine 13:7-10 [ Links ]
16. Gakiya M 2000 Spontaneous rupture of infected renal cyst: a case report. Hinyokika Kiyo 46: 265-267 [ Links ]
17. Garel L 2010 Renal cystic disease. Ultrasound Clinics 5: 15-59 [ Links ]
18. Gookin J L, Stone E A, Spaulding K A, Berry C R 1996 Unilateral nephrectomy in dogs with renal disease: 30 cases (1985-1994). Journal of the American Veterinary Medical Association 208:2020-2026 [ Links ]
19. Greco D S 2001 Congenital and inherited renal disease of small animals. Veterinary Clinics of North America: Small Animal Practice 31:393-399 [ Links ]
20. Haraguchi T, Tachibana Y, Kawabata G 1999 Infected renal cyst: a report of two cases. Nishinihon Journal of Urology 61:479-481 [ Links ]
21. Hess R S, Ilan I 2003 Renal abscess in a dog with transient diabetes mellitus. Journal of Small Animal Practice 44:13-16 [ Links ]
22. Hylands R 2006 Veterinary diagnostic imaging: retroperitoneal abscess and regional cellulitis secondary to a pyelonephritis within the left kidney. Canadian Veterinary Journal 47:1033-1035 [ Links ]
23. Ishibiki Y, Matsumura T 2005 Gas-forming infection in a simple renal cyst: a case report. Nishinihon Journal of Urology 67:128-131 [ Links ]
24. Ishizuka E, Kitajima N, Fujii H, Iwasaki A 1984 Clinical study on the treatment of infected renal cyst. Acta Urologica Japonica 30:609-614 [ Links ]
25. Jeffrey R B, Wing V W, Laing F C 1985 Real-time sonographic monitoring of percutaneous abscess drainage. American Journal of Roentgenology. 144:469-470 [ Links ]
26. Jemni M, Jemni H, Kraiem C, Mosbah A 1991 Infection and rupture of a serous renal cyst. Annales d'Urologie 25:45-47 [ Links ]
27. Kinder P W, Rous S N 1978 Infected renal cyst from hematogenous seeding: a case report and review of the literature. Journal of Urology 120:239-240 [ Links ]
28. Konde L J, Park R D, Wrigley R H, Lebel J L 1986 Comparison of radiography and ultrasonography in the evaluation of renal lesions in the dog. Journal of the American Veterinary Medical Association 188:1420- 1425 [ Links ]
29. Kopp W, Tolly E, Ebner F, Kullnig P 1986 Spontaneous rupture of an infected kidney cyst. Röntgen-Blätter 39:203-204 [ Links ]
30. Lang J 2006 Urinary tract. In Mannion P (ed.) Diagnostic ultrasound in small animal practice. Blackwell Science, Ames, Iowa: 109-119 [ Links ]
31. Lewis D C, Adamson D R, Jacobs K A, Lamb W A 1988 Pyelonephritis, nephrolithiasis and perinephric abscessation in a dog. Australian Veterinary Journal 65:195-196 [ Links ]
32. McAloose D, Casal M, Patterson D F, Dambach D M 1998 Polycystic kidney and liver disease in two related West highland white terrier litters. Veterinary Pathology 35:77-81 [ Links ]
33. McKenna S C, Carpenter J L 1980 Polycystic disease of the kidney and liver in the Cairn terrier. Veterinary Pathology 17:436-442 [ Links ]
34. Murshidi M M, Suwan Z A 1997 Simple renal cysts. Archivos Espanoles Urologia 50:928-931 [ Links ]
35. Nyland T G, Mattoon J S, Herrgesell E J, Wisner E R 2002 Urinary tract. In Nyland T G and Mattoon J S (eds) Small animal diagnostic ultrasound (2nd edn). Saunders, Philadelphia: 158-195 [ Links ]
36. O'Leary C A, Mackay B M, Malik R, Edmondston J E, Robinson W F, Huxtable C R 1999 Polycystic kidney disease in bull terriers: An autosomal dominant inherited disorder. Australian Veterinary Journal 77: 361-366 [ Links ]
37. O'Leary C A, Turner S 2004 Chronic renal failure in an English bull terrier with polycystic kidney disease. Journal of Small Animal Practice 45:563-567 [ Links ]
38. Patterson J E, Andriole V T 1987 Renal and perirenal abscesses. Infectious Disease Clinics of North America 1:907-926 [ Links ]
39. Rainio J, De Giorgio F, Carbone A 2006 Death from renal cyst: spontaneous or traumatic rupture? American Journal of Forensic Medicine and Pathology 27:193-195 [ Links ]
40. Suwabe T, Ubara Y, Higa Y, Nakanishi S, Sogawa Y, Nomura K, Nishimura H, Hoshinoa J, Sawa N, Katori H, Takemoto F, Nakamura M, Tomikawa S, Hara S, Takaichi K 2009 Infected hepatic and renal cysts: differential impact on outcome in autosomal dominant polycystic kidney disease. Nephron Clinical Practice 112:157-163 [ Links ]
41. Tokuchi H, Yamamoto M, Kamoto T 2004 Spontaneous rupture of infected renal cyst presenting sudden onset of right flank distension: a case report. Hinyokika Kiyo 50:323-326 [ Links ]
42. Vaidyanathan S, Hughes P L, Oo T, Soni B M 2008 Spontaneous rupture of an infected renal cyst and external drainage through a lumbar surgical scar in a male patient with cervical spinal cord injury: a case report. Journal of Medical Case Reports 2:154 [ Links ]
43. Van Zijl P S, Chai T C 2004 Gas-forming infection from Clostridium perfringens in a renal cyst of a patient with autosomal dominant polycystic kidney disease. Urology 63:1178-1179 [ Links ]
44. Wills S J, Barrett E L, Barr F J, Bradley K J, Helps C R, Cannon M J, Gruffydd-Jones T J 2009 Evaluation of the repeatability of ultrasound scanning for detection of feline polycystic kidney disease. Journal of Feline Medicine & Surgery 11:993-996 [ Links ]
45. Yokoyama S, Fukuhara S, Imazu T, Hara T, Yamaguchi S 2007 Infected renal cyst: a case report. Nishinihon Journal of Urology 69:547-549 [ Links ]
46. Zatelli A, D'Ippolito P 2004 Bilateral perirenal abscesses in a domestic neutered shorthair cat. Journal of Veterinary Internal Medicine 18:902-903 [ Links ]
Received: December 2010.
Accepted: May: 2011.
* Author for correspondence. E-mail: adriaan.kitshoff@up.ac.za