Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the South African Veterinary Association
versão On-line ISSN 2224-9435
versão impressa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.82 no.2 Pretoria Jan. 2011
ARTICLE ARTIKEL
The sensitivity of direct faecal examination, direct faecal flotation, modified centrifugal faecal flotation and centrifugal sedimentation/flotation in the diagnosis of canine spirocercosis
J ChristieI,*; E V SchwanII; L L BodensteinIII; J E M SommervilleIII; L L van der MerweI
IDepartment of Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort, 0110 South Africa
IIDepartment of Veterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort, 0110 South Africa
IIIDepartment of Statistics, Faculty of Natural and Agricultural Sciences, University of Pretoria, Pretoria, 0002 South Africa
ABSTRACT
Several faecal examination techniques have shown variable sensitivity in demonstrating Spirocerca lupi (S. lupi) eggs. The objective of this study was to determine which faecal examination technique, including a novel modified centrifugal flotation technique, was most sensitive to diagnose spirocercosis. Ten coproscopic examinations were performed on faeces collected from 33 dogs confirmed endoscopically to have spirocercosis. The tests included a direct faecal examination, a faecal sedimentation/flotation test, 4 direct faecal flotations and 4 modified faecal centrifugal flotations. These latter 2 flotation tests utilised 4 different faecal flotation solutions: NaNO3 (SG 1.22), MgSO4 (SG 1.29), ZnSO4 (SG 1.30) and sugar (SG 1.27). The sensitivity of the tests ranged between 42 % and 67 %, with the NaNO3 solution showing the highest sensitivity in both the direct and modified-centrifugal flotations. The modified NaNO3 centrifugal method ranked 1st with the highest mean egg count (45.24 ± 83), and was superior (i.e. higher egg count) and significantly different (P < 0.05) compared with the routine saturated sugar, ZnSO4 and MgSO4 flotation methods. The routine NaNO3 flotation method was also superior and significantly different (P < 0.05) compared with the routine ZnSO4 and MgSO4 flotation methods. Fifteen per cent (n =5)of dogs had neoplastic oesophageal nodules and a further 18 % (n = 6) had both neoplastic and non-neoplastic nodules. S. lupi eggs were demonstrated in 40 % of dogs with neoplastic nodules only and 72.9 % of the dogs with non-neoplastic nodules. The mean egg count in the non-neoplastic group (61) was statistically greater (P = 0.02) than that of the neoplastic group (1). The results show that faecal examination using a NaNO3 solution is the most sensitive in the diagnosis of spirocercosis. The modified centrifugal flotation faecal method using this solution has the highest egg count. The study also found that dogs with neoplastic nodules shed significantly fewer eggs than dogs with non-neoplastic nodules.
Keywords: dog, egg, faecal examination, Spirocerca lupi, spirocercosis.
INTRODUCTION
Spirocerca lupi (S. lupi) is a nematode of the superfamily Spiruroidea and has an indirect life cycle which may include a paratenic host. The predominant definitive host is the dog, which passes larvated eggs with the faeces and to a lesser degree with vomitus2,5. Following ingestion of an infected intermediate host (coprophagous beetles) or paratenic host (birds, lizards, frogs, snakes, mice, rabbits and rats) L3 larvae are liberated within the stomach1,2,31. Soon after ingestion the L3 penetrate the stomach mucosa and migrate, within artery walls, towards the aorta2,4,14,22. This initial part of the migration process takes approximately 3 weeks2,11. Further development of the L3 to immature adults occurs in the wall of the thoracic aorta. These immature adults then migrate in the mediastinum from the wall of the aorta towards the oesophagus, an event usually occurring 102-124 days post infection2,11. In the oesophagus the adults provoke the development of a fibrous nodule in which they undergo further maturation. These nodules may become neoplastic2,24. The adult spirurid nematode is a relatively large worm, pink-red in colour with males and females reaching 3-4 cm and 6-7 cm, respectively11. The prepatent period in the dog is 4-6 months2,13.
Oesophageal nodules may have a small opening into the oesophageal lumen through which larvated eggs are passed12. Large numbers of larvated eggs are passed with the faeces of infected dogs2.If the nodule has no opening the infection is not patent31. Owing to the complex migration route, worms are sometimes found in atypical locations within the dog. These aberrant migration sites include the lungs, trachea, pleura, diaphragm, spinal cord and skin2,4,7,13,16,23,27.
Clinical signs associated with spirocercosis are variable but are usually as a result of the parasites' effect on the oesophagus, mediastinum or aorta. In early infections the parasite may cause no clinical signs (subclinical spirocercosis) and infection is diagnosed incidentally on faecal examination or thoracic radiography8,15. Peracute death may occur due to rupture of the aorta or other major blood vessel secondary to aneurysm formation caused by larval development and migration2,11. Classical spirocercosis clinical signs result from the spirurid nodule obstructing the oesophagus and compressing the intrathoracic structures and include vomiting, regurgitation, coughing, dysphagia, sialorrhoea, pyrexia and melaena18,20,32. Weakness and weight loss become apparent with chronicity and neoplastic transformation. Neoplastic spirocercosis results from the neoplastic transformation of the parasitic nodule. The clinical signs are similar to the classical form but hypertrophic osteopathy, anaemia, leukocytosis and thrombocytosis may also be found9. Atypical clinical signs including mediastinitis, pleuritis, pyothorax, haemopericardium and paraparesis can be associated with aberrant migrations2,8,28.
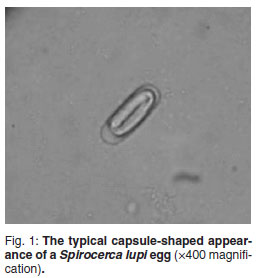
The diagnosis of spirocercosis is usually made by oesophageal endoscopy, considered the diagnostic test of choice20,25. Early to mature nodules are typically smooth, round and sessile and protrude into the oesophageal lumen. Neoplastic nodules usually show a roughened, ulcerated, necrotic surface and should be biopsied9,32. Radiography is also a sensitive diagnostic test, with the dorso-ventral and right lateral thoracic views superior for diagnosing a caudal mediastinal mass. Radiological features regarded as pathognomonic for spirocercosis include caudal oesophageal opacity, an undulating descending aorta and spondylitis of the 6th to 12th thoracic vertebrae8,11. Faecal examination (copromicroscopy) is a diagnostic test that has demonstrated a reported range of sensitivities. S. lupi eggs are thick-shelled, elongated (capsule-shaped) with parallel sides (Fig. 1) and are small compared with other nematode eggs. The typical spirurid egg measures 20-37 µ by 11-18 µ and contains a larva (L1) when laid5,6,29. Faecal examination techniques previously described include direct faecal smears, faecal flotation, faecal sedimentation and recently the FLOTAC methods3,10,15,21,25. The findings of some of these studies are contradictory and there is no clarity on how best to utilise faecal ova identification in the diagnosis of spirocercosis. The aim of the present study was to investigate this clinical problem by comparing 4 different faecal examination methods, namely direct faecal examination, direct faecal flotation, a modified centrifugal faecal flotation and centrifugal sedimentation/flotation in detecting S. lupi eggs from patients with confirmed spirocercosis. The study also compared 4 commonly used flotation solutions in the direct and modified centrifugal flotation methods to detect S. lupi eggs. The sensitivities of the different faecal examination tests performed on each faecal sample from dogs were determined.
MATERIALS AND METHODS
Faecal samples were collected from dogs presented to the Onderstepoort Veterinary Academic Hospital that met the following criteria: (1) all dogs were confirmed to have spirocercosis by oesophageal endoscopy and radiography and (2) none of the dogs had received any medication containing a macrocyclic lactone compound in the preceding 6 months. Faeces were obtained directly from the rectum or by collection of a stool sample (if defaecation was observed). All faecal samples were stored at 4 ºC and analysed within 24 hours. If a faecal sample was found to be negative for S. lupi eggs, a 2nd faecal sample was collected 24-48 h later and analysed.
The dogs were allocated to 1 of 3 groups: (1) The non-neoplastic group, where the oesophageal nodule(s) showed typical smooth endoscopic characteristics and regressed after treatment with doramectin as assessed through follow-up endoscopy at 6 weeks; (2) the neoplastic group, where the oesophageal nodule(s) appeared roughened, cauliflower-like and ulcerated and where neoplastic transformation was confirmed by histopathology of the endoscopically or surgically obtained biopsy; (3) the mixed group, consisting of dogs with multiple nodules that included both non-neoplastic and neoplastic nodules as determined by the abovementioned criteria.
All faecal samples were examined using 10 different faecal examination tests. Four faecal examination techniques were used: direct faecal examination, direct faecal flotation, modified centrifugal flotation and a centrifugal sedimentation/flotation. Four different flotation fluids were used in the direct and modified centrifugal flotation techniques. These flotation fluids included: sugar solution (SG 1.27); zinc sulphate (ZnSO4) solution (SG 1.30); sodium nitrate (NaNO3) solution (SG 1.22) and magnesium sulphate (MgSO4) solution (SG 1.29). Only the NaNO3 flotation solution (SG 1.22) was commercially available (Faecalyser® - Kyron Laboratories (Pty) Ltd, South Africa). All other solutions were prepared in a laboratory using de-ionised water and the corresponding solute. Forty per cent formaldehyde solution was added to the sugar solution to preserve it, at a concentration of 40 mℓ /ℓ . The specific gravities of all the flotation solutions were monitored using a hydrometer and were also tested at 3-month intervals to ensure they were maintained. All solutions were kept at room temperature in airtight containers and away from light.
Faecal examination techniques:
(1) Direct faecal examination: 1 g of faeces was placed in a plastic test tube and 5mℓ of saline was added. The mixture was manually agitated using a wooden spatula for 30 s. 0.1 mℓ of the mixture was then aspirated using an adjustable micropipette and placed on a microscope slide, covered with a 22 mm × 22 mm coverslip and examined under a light microscope at ×100 magnification. All S. lupi eggs under the coverslip and those seen on the perimeter of the coverslip were manually counted (Fig. 2). The direct faecal examination method was used to estimate the number of eggs per gram using the formula number of eggs per 0.1 mℓ mixture multiplied by 50 (sample size was 1/50 of mixture).
(2) Direct faecal flotation: 1 g of faeces was placed into a routine faecal flotation test kit (Ovatector® - Kyron Laboratories (Pty) Ltd, South Africa). The faecal flotation solution was added and the mixture was agitated with a wooden spatula for 30 s and the strainer inserted. The faecal flotation test kit was filled to the rim with the specified solution and covered with a 22 mm × 22 mm coverslip. Twenty minutes was allowed for egg flotation after which the coverslip was removed and placed on a glass slide for light microscopic examination at ×100 magnification. All the S. lupi eggs under the coverslip and those seen on the perimeter of the coverslip were manually counted. Four direct faecal flotations were performed on each faecal sample using each of the flotation solutions, namely sugar, ZnSO4,NaNO3 and MgSO4.
(3) Modified centrifugal faecal flotation: 1 g of faeces was placed in a plastic test tube and 5mℓ of the flotation solution was added. The mixture was agitated using a wooden spatula for 30 s. The test tube cap was tightened and the tube placed into a fixed-armrotor of a centrifuge. The sample was centrifuged at 1400G for 10 min. An adjustable micropipette was used to aspirate 0.1mℓ of the supernatant, in 2 aliquots of 50 µℓ , which was then placed on a glass slide. A 22 mm × 22 mm coverslip was placed and the slide was examined under a light microscope at ×100 magnification. All the S. lupi eggs under the coverslip and those seen on the perimeter of the coverslip were manually counted. Four modified faecal flotations were performed on each sample using each of the flotation solutions, namely sugar, ZnSO4, NaNO3 and MgSO4.
(4) Centrifugal sedimentation flotation: 1 g of faeces was placed in a 50 mℓ graduated tube and mixed with 30mℓ of artificial gastric juice (2 % pepsin and 1 % concentrated hydrochloric acid). The faecal suspension was agitated for 5 min at room temperature with a magnetic stirrer prior to being strained through a fine tea strainer into a beaker. The strained suspension was centrifuged at 1400 G for 10 min after which the supernatant fluid was removed, leaving faecal sediment at the bottom of the tube. MgSO4 solution was then added to the faecal sediment which was re-suspended and centrifuged for a further 10 min. The tube was then filled to the rim with the MgSO4 solution and covered with a 22 mm × 22 mm coverslip and 10 min allowed for egg flotation. The resultant slide was examined under light microscopic examination at ×100 magnification. All the S. lupi eggs under the coverslip and those seen on the perimeter of the coverslip were manually counted. Thiswasa modification of the Markovics and Medinski19 method as our initial attempts to place coverslips over the test tubes in the swing-out rotor for the centrifuge were unsuccessful and had to be abandoned. Artificial gastric juice was also added instead of the described tap water to increase the sensitivity in this technique.
All egg counts for the different faecal tests, excluding the centrifugal sedimentation flotation test, were performed by the same author. The centrifugal sedimentation flotation test was also always performed by the same individual.
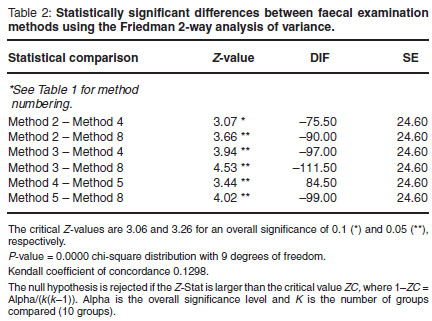
A total of 10 faecal examination tests were performed on each sample. The Friedman non-parametric repeated measures analysis of variance was used to compare the egg counts for the 10 different faecal examinations. Multiple comparisons, using a 0.05 level of significance with the Bonferroni correction, were used to determine which pairs of methods were statistically different. BMDP Statistical software (BMDP Statistical software, Inc. Los Angeles, USA) was used to compute the statistics. The non-neoplastic and neoplastic groups egg counts were statistically compared using the Mann-Whitney U-test.
RESULTS
Thirty-three patients were included in this study, which ran from April 2008 to December 2009. The sensitivity of the various tests to detect S. lupi eggs ranged between 42.4 % and 66.7 %. The routine and modified NaNO3 flotation tests showed the highest sensitivity (Table 1). No eggs were found in 12 of 33 initial faecal samples (36.6 %) and an additional sample taken 24 to 48 hours later identified only 1 additional infected patient using the direct and modified NaNO3 flotation tests only.

Table 1 summarises the findings of all the faecal examination tests performed. The Friedman test was statistically significant at the 0.05 level (P = 0.0000). The modified NaNO3 flotation test ranked 1st with the highest mean egg count (45, Table 1). The NaNO3 modified faecal flotation test was found to be superior to (higher egg count)andstatistically different (Table 2) from the sugar, ZnSO4 and MgSO4 direct flotation tests (P < 0.05). The NaNO3 direct faecal flotation test was also found to be superior to and statistically different from the ZnSO4 and MgSO4 direct flotation tests (P < 0.05). When applying a 0.1 level of significance the NaNO3 direct flotation was also found to be superior to and statistically different from the direct sugar flotation test. No further statistically significant differences were found between the techniques.
The average egg count per gram was 1100 (22 eggs per 0.1 mℓ faecal suspension) with the highest egg count per gram being 21 250 (425 eggs per 0.1 mℓ faecal suspension).
The non-neoplastic group made up 67 % of the samples (n = 22), the neoplastic group made up a further 15 % of the samples (n = 5) and the mixed group 18 % (n = 6). The sensitivity of faecal flotation (using the NaNO3 modified flotation method) to detect S. lupi eggs was 72.7 % in the non-neoplastic group and 40 % in the neoplastic group. The mixed group was not included in this assessment. The mean egg count using this method was found to be 61 ± 95.8 in the non-neoplastic group and 1 ± 1.7 in the neoplastic group. This difference was significant (P = 0.024, Mann-Whitney U-test, 1-sided test).
DISCUSSION
This study was able to show that the sensitivity of coprological examination for detecting S. lupi eggs in dogs diagnosed with spirocercosis is variable and depends on which faecal examination technique and flotation fluid is utilised and also the stage of infection. The sensitivity ranged between 42 % and 67 % in this study, which confirms that faecal flotation per se is not a sensitive tool to confirm a diagnosis of spirocercosis. Egg shedding from S. lupi infected dogs is variable and does not occur in all infected individuals. Reasons for this include a prepatent infection with an immature worm, neoplastic conversion of the nodule, the lack of a patent operculum in the nodule, infection with female or male nematodes only or the intermittent shedding pattern of the female worm. This study also showed that a 2nd faecal sample (24 to 48 h later) was only able to detect eggs in an additional 8.4 % of patients. A previous study found a higher number of cases (28 %) to be positive upon a 2nd examination but did not mention exactly how many days elapsed after the 1st sample was taken20. For ethical reasons the client-owned dogs in this study had to be treated as soon as possible after diagnosis was confirmed, which accounted for the 48 h timeframe. Diagnosis by faecal analysis is only possible when eggs are passing in the faeces and this passage can occur for an unpredictable, relatively short period11.
Previously it was shown that peak egg production appeared between 140 and 205 days post infection with a maximum of 2100 eggs per gram of faeces26. A subsequent study showed that eggs per gram of faeces counts ranged from 2000 to 11 000 in laboratory infected animals2. The aim of this study was not to demonstrate when peak egg production occurs but it was shown, using the direct faecal examination method, that egg production could be very high, with 21 250 eggs per gram of faeces recorded in 1 patient. Three additional patients were found to have egg counts above the 1971 maximum levels, with 2650 and 3150 eggs per gram of faeces, respectively. The average egg count per gram of faeces using the direct faecal examination method was 1100, therefore even though shedding is intermittent, infected dogs are a massive source of environmental contamination and this could explain the increasing incidence of the disease in regions of South Africa . The importance of rapid removal of faeces from the environment is thus emphasised as a practical control measure. Treatment causes a 99.3 % decrease in egg count within 10 days after the 1st dose of a doramectin17, thus early diagnosis and treatment will also obviously decrease environmental contamination. It was hypothesised that the typical early Spirocerca cases without neoplastic transformation would have higher egg counts than those with advanced neoplastic disease as the worms were younger and shedding more actively. This would have made faecal flotation a valuable screening tool in these often asymptomatic cases. Unfortunately the sensitivity of faecal egg counts did not increase significantly when only these early cases were included in the analysis. The sensitivity of faecal egg counts was different in the early non-neoplastic versus the neoplastic group, 72.7 % vs 40 %; these were not determined to be statistically significant and a sensitivity of 72.7 % is still inadequate as a screening test. It was also determined that the mean number of eggs shed by the dogs in the neoplastically transformed group (1 ± 1.7) was significantly lower than that of the non-neoplastic group (61.1 ± 95.8). This may indicate that these nodules contain fewer worms or that these worms shed fewer eggs. Although the difference between the mean egg counts between the nonneoplastic and neoplastic group was statistically significant there is no real clinical relevance as both groups shed eggs. A combination of neoplastic and non-neoplastic nodules was found in 18 % of cases. Faecal examination is thus not a suitable tool to assist in differentiating between non-neoplastic and neoplastic canine spirocercosis.
This study showed that NaNO3 (SG 1.22) had the highest sensitivity in both the direct and modified centrifugal flotation tests. This was unexpected as previous reports indicate that these eggs appear heavier than those of other nematodes and require flotation solutions with higher specific gravities to improve recovery of these eggs3,10. In this study the ZnSO4 (SG 1.30) and the MgSO4 (SG 1.29) flotation fluids did in fact damage the eggs as previously reported5. The eggs became rectangular in shape and the edges appeared to fold in on themselves. The sugar flotation solution crystallised rapidly, was sticky and was difficult to work with compared with the other solutions. The NaNO3 (SG 1.22) solution was easy to work with and the solution was also extremely clear, making visualisation of the S. lupi eggs far easier compared with the other fluids. This study demonstrates that a high specific gravity is not required to visualise S. lupi eggs. Markovics and Medinski19 found that sugar flotation using a laboratory run centrifugal technique was 100 % sensitive in detecting S. lupi eggs in 8 samples with low egg numbers (100 eggs per gram) compared with the direct faecal examination (50 %). This study demonstrated equal sensitivities
(57.6 %) for direct faecal examination and centrifugal sedimentation/flotation, but the centrifugal sedimentation/flotation method did have a higher mean egg count (35) compared with the direct faecal examination method (22) (Table 1). The addition of artificial gastric juice did not appear to enhance the sensitivity of the Markovics and Medinski method. A recent study found that S. lupi egg detection using a ZnSO4 (SG 1.35) flotation and the Markovics and Medinski technique were insensitive as diagnostics tests for spirocercosis, detecting eggs in only 7/31 and 4/31 S. lupi PCR positive faecal samples30. The study showed that the FLOTAC® apparatus was more sensitive, as it detected eggs in 10 of these 31 faecal samples. This study also found that the PCR was able to increase faecal spirocercosis sensitivity by 45 % and 38.2 % in 2 groups of faecal samples when compared with a combination of faecal flotation methods.
The modified flotation method in our study used centrifugation to separate the solid and fluid matter in the faecal mixture, aiding egg flotation, and it was hypothesised that it would result in a higher mean egg count and increased sensitivity of the faecal egg count. Although the modified flotation method using NaNO3 (SG 1.22) solution did result in the highest egg counts, it had an equal sensitivity to that of routine faecal flotation using the same fluid. Both of the above methods are extremely simple to perform, do not require specialised equipment and can be completed in less than 20 minutes, which makes them ideal for laboratories and veterinary hospitals. The relatively low SG of the commercially available flotation fluid is also easily maintained as it does not sediment out as easily as the more concentrated fluids. A limitation of this study was the manner in which egg counts were performed. As no grid was available for counting, all eggs under the microscope cover and on the perimeter were counted in a grid-like fashion by the same individual to ensure consistency. Statistical difference was found throughout the comparison but the power of the study could have been increased with a greater number of cases.
The results of this study show that dogs with neoplastic spirocercosis do shed eggs although much fewer than the nonneoplastic spirocercosis patients. Faecal examination using a NaNO3 (SG 1.22) solution is the most sensitive in the diagnosis of spirocercosis, with the modified centrifugal flotation method using this solution achieving the highest egg counts. This faecal examination method is simple and inexpensive to perform and is not technically challenging, making it a suitable test in the diagnosis of spirocercosis.
ACKNOWLEDGEMENTS
The authors would like to thank Dawn Durand from the Department of Tropical Diseases Helminthology Laboratory, University of Pretoria, for her help during this project, the Onderstepoort Veterinary Academic Hospital and Faculty of Veterinary Science, University of Pretoria, for their support and financial assistance towards this project, and the South African Veterinary Foundation (SAVF) for financial assistance to the S. lupi project at Onderstepoort. The authors would also like to thank Prof. R.M. Kirberger, Department of Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, for all his input during the preliminary stages of this project.
REFERENCES
1. Anataraman M, Krishna S 1966 Experimental spirocercosis in dogs with larvae from a paratenic host, Calotes versicolor, the common garden lizard in Madras. Journal of Parasitology 52: 911-912 [ Links ]
2. Bailey W S 1972 Spirocerca lupi: a continuing inquiry. Journal of Parasitology 58: 3-22 [ Links ]
3. Cabrera D J, Bailey W S 1964 A modified Stoll technique for detecting eggs of Spirocerca lupi. Journal of the American Veterinary Medical Association 145: 573-575 [ Links ]
4. Chandraskharon K P, Sastry G A, Menon M N 1958 Canine spirocercosis with special reference to the incidence and lesions. British Veterinary Journal 114: 388-395 [ Links ]
5. Chhabra R C, Singh K S 1972 On the life cycle of Spirocerca lupi: preinfective stages of the intermediate host. Journal of Helminthology 46: 125-137 [ Links ]
6. Dixon K G, McCue J F 1967 Further observations on the epidemiology of Spirocerca lupi in the southeastern United States. Journal of Parasitology 53: 1074-1075 [ Links ]
7. Du Plessis C J, Keller N, Millward I R 2007 Aberrant extradural spinal migration of Spirocerca lupi: four dogs. Journal of Small Animal Practice 48: 275-278 [ Links ]
8. Dvir E, Kirberger R M, Malleczek D 2001 Radiographic and computed tomographic changes and clinical presentation of spirocercosis in the dog. Veterinary Radiology and Ultrasound 42: 119-129 [ Links ]
9. Dvir E, Kirberger R M, Mukorera V, Van der Merwe L L, Clift S J 2008 Clinical differentiation between dogs with benign and malignant spirocercosis. Veterinary Parasitology 155: 80-88 [ Links ]
10. Evans L B 1983 Clinical diagnosis of Spirocerca lupi infestation in dogs. Journal of the South African Veterinary Association 54: 189-191 [ Links ]
11. Fox S M, Burns J, Hawkins J 1988 Spirocercosis in dogs. Compendium of Continuing Education for the Practicing Veterinarian 10: 807-822 [ Links ]
12. Georgi J R, Georgi M E 1990 Parasitology for veterinarians (5th edn). W B Saunders, Philadelphia, USA [ Links ]
13. Harrus S, Harmelin A, Markovics A, Bark H 1996 Spirocerca lupi infection in the dog: aberrant migration. Journal of the American Animal Hospital Association 33: 125-130 [ Links ]
14. Hu C H, Hoeppli R J C 1936 The migration route of Spirocerca sanguinolenta in experimentally infected dogs. Chinese Medicine Journal-Peking Supplement 1: 11-99 [ Links ]
15. Kashchula J M, Malherbe W D 1964 The incidence and diagnosis of spirocercosis in dogs in the Transvaal. Journal of the South African Veterinary Association 25: 53-59 [ Links ]
16. Kirberger R M, Zambelli A 2007 Imaging diagnosis: aortic thromboembolism associated with spirocercosis in a dog. Veterinary Radiology and Ultrasound 48: 418-420 [ Links ]
17. Lavy E, Aroch I, Bark H, Markovics A, Aizenberg I, Mazaki-Tovi M, Haga A, Harrus S 2002 Evaluation of doramectin for the treatment of experimental canine spirocercosis. Veterinary Parasitology 109: 65-73 [ Links ]
18. Lobetti R G 2000 Survey of the incidence, diagnosis, clinical manifestations and treatment of Spirocerca lupi in South Africa. Journal of the South African Veterinary Association 71: 43-45 [ Links ]
19. Markovics A, Medinski B, 1996 Improved diagnosis of low intensity Spirocerca lupi infection by the sugar flotation method. Journal of Veterinary Diagnostic Investigation 8: 400-401 [ Links ]
20. Mazaki-Tovi M, Baneth G, Aroch I, Harrus S, Kass P H, Ben-Ari T, Zur G, Aizenberg I, Bark H, Lavy E 2002 Canine spirocercosis, clinical, diagnostic, pathological and epidemiological characteristics. Veterinary Parasitology 107: 235-250 [ Links ]
21. Minnaar W N, Krecek R C, Fourie L J 2002 Helminths of dogs from a peri-urban resource-limited community in the Free State Province, South Africa. Veterinary Parasitology 107: 343-349 [ Links ]
22. Murray M 1968 Incidence and pathology of Spirocerca lupi in Kenya. Journal of Comparative Pathology 78: 401-405 [ Links ]
23. Pereira W L A, Guimarães F ABDA,MartinsAKP,PeixotoPC 1995 Haemopericardium in a dog associated with hyperparasitism by Spirocerca lupi. Boletim de Faculdade de Ciencias Agrárias do Pará 23: 45-51 [ Links ]
24. Ranen E, Lavy E, Aizenberg I, Perl S, Harrus S 2004 Spirocercosis-associated esophageal sarcomas in dogs. A retrospective study of 17 cases (1997-2003). Veterinary Parasitology 119: 209-221 [ Links ]
25. Reche-Emonot M, Beugnet F, BourdoiseauG 2001 Étude epidemiologique et clinique de la spirocercose canine a Î'lle de la Réunion a partir de 120 cas. Revue de Médecine Vétérinaire (Toulouse) 152: 469-477 [ Links ]
26. Sen K, Anataraman M 1971 Some observations on the development of Spirocerca lupi in its intermediate and definitive hosts. Journal of Helminthology 45: 123-131 [ Links ]
27. Singh B, Juval P D, Sobti V K 1999 Spirocerca lupi in a subcutaneous nodule in a dog in India. Veterinary Parasitology 13: 59-60 [ Links ]
28. Stephens S C, Gleiser C A, Jardine J H 1983 Primary pulmonary fibrosarcoma associated with Spirocerca lupi in a dog with hypertrophic pulmonary osteoarthropathy. Journal of the American Veterinary Medical Association 182: 496-498 [ Links ]
29. Soulsby E J L 1982 Helminths, arthropods and protozoa of domestic animals. Lea and Febiger, Philadelphia, USA [ Links ]
30. Traversa D, Avolio S, Modry D, Otranto D, Iorio R, Aroch I, Cringoli G, Milillo P, Albrechtová K, Milhalca A D, Lavy E, 2008 Copromicroscopic and molecular assays for the detection of cancer-causing parasitic nematode Spirocerca lupi. Veterinary Parasitology 157: 103-116 [ Links ]
31. Urquhart G M, Armour J, Duncan J L, Dunn A M, Jennings F Q 1994 Veterinary parasitology. Longman Scientific and Technical, Essex, England [ Links ]
32. van der Merwe L L, Kirberger R M, Clift S J, Williams M, Keller N, Naidoo V 2008 Spirocerca lupi infection in the dog: a review. Veterinary Journal 197: 294-309 [ Links ]
Received: November 2010.
Accepted: April 2011.
* Author for correspondence. E-mail: jevan.christie@up.ac.za














