Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.82 n.1 Pretoria Jan. 2011
ARTICLE ARTIKEL
Evaluation of the effects of long-term storage of bovine ear notch samples on the ability of 2 diagnostic assays to identify calves persistently infected with bovine viral diarrhoea virus
F KhanI,*; J H VorsterI; M van VuurenII; P MaphamIII
IVetdiagnostix - Veterinary Pathology Services, PO Box 13624, Cascades, 3202 South Africa
IIDepartment of Veterinary Tropical Diseases, University of Pretoria, Private Bag X04, Onderstepoort, 0110 South Africa
IIIVeterinary House Hospital, 339 Prince Alfred Street, Pietermaritzburg, 3201 South Africa
ABSTRACT
Research aimed at optimising diagnostic laboratory procedures is central to the development of effective bovine viral diarrhoea virus (BVDV) control programmes. BVDV is a singlestranded RNA virus that crosses the placenta to infect foetuses, resulting in reproductive losses due to foetal death or persistently infected calves that die early in life. Persistently infected animals are widely accepted to be the primary reservoir of BVDV and the largest source of infection. This poses important challenges to overall animal/herd health and can cause major losses to the cattle industry. Long-term storage of bovine ear notch samples from calves persistently infected with BVDV may adversely affect the ability of diagnostic assays to detect the virus efficiently. In order to test this hypothesis, ear notch samples from 7 animals were divided into 2 groups. One set was subjected to prompt formalin fixation and the other set stored either as fresh samples without preservatives at -2 ºC, or soaked overnight in phosphate buffered saline followed by freezing of the supernatant fluid at -2 ºC. Frozen ear notches and ear notch supernatant yielded positive results with an antigen-capture, enzyme linked immunosorbent assay (AC-ELISA) for the duration of the study (6 months) and optical density (OD) values remained significantly within range. There was no significant difference between storing fresh ear notch samples or PBS at -2 ºC. However, positive immunohistochemistry (IHC) staining on formalin fixed ear notches started to fade between Day 17 and Day 29 when stored at room temperature. It was concluded that fresh ear notches could safely be stored at -2 ºC for a period of 6 months prior to testing for BVD viral antigens.
Keywords: AC-ELISA, BVDV, ear notch, immunohistochemistry, long-term storage, PBS.
INTRODUCTION
Bovine viral diarrhoea virus (BVDV) is an important viral disease and of major concern for cattle farmers and bovine practitioners as it causes livestock production losses worldwide. Persistently infected (PI) animals are widely accepted to be the primary reservoir of BVDV1, 8and the largest source of BVDV infection6, 11. Optimal testing procedures and thorough research has therefore become paramount to assessing and dealing with the economic losses associated with animals persistently infected with BVDV. The disease cannot be controlled with vaccinations alone and therefore continuous laboratory testing followed by removal of PI animals is necessary to keep herds free of BVDV infections8, 11.
BVDV is a single-stranded RNA virus that crosses the placenta efficiently to infect foetuses, resulting in reproductive losses due to foetal death or calves that die early in life. Foetuses infected in utero between Days 40 and 125 of gestation6,8,13become immunotolerant to the virus and after birth are believed to shed the virus for life4. These PI calves carry excessive viral loads, excreting high levels in all body secretions and excretions for a long period of time. Animals in contact with them become acutely infected and these infections are coupled with immunosuppression that allows opportunistic pathogens to cause further disease and reduce overall profits4. There are 2 ways in which PI calves are produced: 1) susceptible pregnant cows infected with the virus, from any source, at1-3 months of gestation, and 2) BVDV PI cows that become pregnant. PI animals that survive calfhood are at risk of developing mucosal disease in later life, which is a severe and usually fatal condition. In addition, PI calves that become replacement heifers in the herd may experience significant morphological changes that occur in the ovaries which can result in impaired reproductive performance13.
Individual animal testing and accurate diagnosis is critical in identifying and eliminating PI calves to prevent BVDV spread in a herd. There are numerous methods that have been developed to identify PI cattle such as virus isolation, reverse transcription-polymerase chain reaction (RT-PCR), immunohistochemistry (IHC) and several antigen-and antibody ELISAs5,6. Virus isolation and RT-PCR are costly when used on individuals in a population, time-consuming and can be technically demanding whereas IHC and antigen capture-ELISA (AC-ELISA) are cost effective methods. Previous studies have verified 100 % sensitivity for both the AC-ELISA and IHC when using ear notches as the target tissues to detect the presence of BVDV3,6, provided that samples are properly collected and stored8. It was found that there was no difference in the ability of IHC and AC-ELISA to detect positive samples in individual samples tested6. Thus, current diagnostic approaches to identify PI calves predominantly make use of ear notch testing with AC-ELISA and IHC. These assays depend on specific monoclonal antibodies which target the Ernsglycoprotein of BVDV4.
Recently, researchers have begun exploring the effects of various storage and sampling methods that could ultimately influence results obtained from diagnostic assays. Little effect was found on the sensitivity of the AC-ELISA when storing samples at room temperature for prolonged periods14. All samples tested positive regardless of time stored at room temperature. However, reduced antigen detection with AC ELISA following exposure to high temperatures and drying has been reported16, 17. Similarly, reduced IHC antigen detection was documented when samples were exposed to room temperature followed by formalin fixation9. Exposure to room air for even 1 day resulted in desiccated samples that made histological processing difficult and produced false negative IHC results. Prompt formalin fixation and histological processing is recommended9. The findings advocate storage in formalin for up to 1 month but caution against false negative IHC reactions resulting from fixation longer than 36 days. A study that investigated detecting BVDV in formalin-fixed paraffinembedded tissue sections by RT-PCR showed decreases in antigen signal strength when samples were fixed for more than 24-48 h or when stored at room temperature followed by fixation after 74 h1. These studies are few and lack uniformity in results.
Numerous studies have been described for the use of PBS (phosphate buffered saline) when detecting BVDV in ear notch samples2,3,4,6,8,14,16,17 but no published research has indicated its effect on the accuracy of the AC-ELISA to detect the antigen. Previously, ear notch extracts were prepared by soaking them in PBS for 60 min16. The effects of storing ear notch extracts were evaluated but no significant differences between storage at -20 ºC, 4 ºC, and 25 ºC for 7 days in the ability of the AC-ELISA to detect antigen was found. BVDV antigen in dilutions of extracts of fresh skin biopsies was previously used for an AC-ELISA2. Samples were either frozen at -80 ºC or not frozen in either PBS or tissue diluent buffer. Mean corrected OD values differed significantly for each dilution and were greatest for tissues diluted 1:1. The effect of using different tissues showed that the mean corrected OD from frozen samples (at 1:1 dilution) was significantly higher than from non-frozen tissues. When RT-PCR ear notch pools and individual AC-ELISA tests were compared using the same PBS supernatant solutions for both tests, both methods correlated 100 % in detecting suspect PI animals.
This study set out to determine the effect of storing ear notch samples in formalin at room temperature on the ability of IHC to detect the virus over a period of 6 months. It also aimed to a) determine the effect of prolonged storage of fresh ear notches and b) to examine the effects of prolonged storage of PBS ear notch supernatant at -2 ºC on the ability of AC-ELISA to detect BVD viral antigens.
MATERIALS AND METHODS
An initial screening process, 5 August - 8 October 2008, was conducted in the quest to identify PI animals after a single initial positive test. Three animals from a feedlot, previously identified with IHC as PI animals by a different laboratory, were donated to this study on 18 September 2008. This marked the beginning of the study. On 15 October 2008, 4 pairs of BVDV-positive animal ears, previously tested positive with AC-ELISA, from another feedlot were added to the study. These remained positive with both IHC and ELISA tested on the 1st day of the study, confirming their PI status. In both cases, 1 ear from each animal was promptly placed in 10 % neutral-buffered formalin, and the other fresh ear set aside in cooler boxes for AC-ELISA procedures at the laboratory the next day.
Since animals were sampled on 2 different dates, the study was divided into Group 1 animals: 3, 7 and 8; and Group 2 animals: 10, 11, 12 and 13. Both groups of ears were subjected to the same test protocol and differed in test dates only. Tests were run twice a month with ~15-day intervals between tests. Groups 1 and 2 were eventually run on the same days starting from Day 70 and Day 43 respectively.
Experimental design
Immunohistochemistry . Ears remained in formalin for ~24 h before arriving at the laboratory. On arrival, rectangular ear notches ~1.0 × 1.0 cm were obtained from the ventral margin of the pinna for each animal, using sterile surgical blades. Ear notches were embedded in paraffin wax (Tissue-Tek®VIPTM ) in 6-chambered cassettes, sectioned at 5 µ and mounted on poly-L-lysine-coated slides. Slides were sent to the Faculty of Veterinary Science, University of Pretoria, for staining. Briefly, tissue sections were de-waxed and then blocked with 3 % hydrogen peroxide for 15 min, followed by incubation with a BVDV monoclonal antibody at a working dilution of 1:500. Monoclonal antibody was produced by and obtained from .E.J. Dubovi, Animal Health Diagnostic Centre, Cornell University. A Nova Red counter stain was used to show BVDV antigen in the sections. Positive and negative control slides were also included with each stain. Control slides were prepared from known positive and negative samples collected at the University. Samples were repeatedly processed for IHC approximately twice a month for 6 months.
AC-ELISA. Upon arrival at the laboratory, each ear was cut into 2 parts for both ELISA studies. These were further divided into 12 pieces for each animal, measuring ~2.0 × 2.0 mm (HerdChek BVDV Ag/Serum Plus, IDEXX Laboratories B.V.) for 12 test runs over 6 months and stored either fresh or as PBS ear notch supernatant at -2 ºC.
Preparation of reagents and test protocol was followed as described by IDEXX HerdChek* BVDV Ag Test Kit/Serum Plus insert. On arrival, a fresh ear notch from each animal was submerged in 150 µℓ of IDEXX Ear Notch Soaking Buffer in individual dilution tubes and allowed to soak overnight (12-18 h) at 4 ºC. The remaining 11 fresh ear notches were stored at -2 ºC. All test reagents were brought to room temperature the next day and ear notches were removed from the buffer solution and discarded. Briefly, detection antibodies were added to all wells of a microtitre plate coated with Ernsmonoclonal antibodies. Positive and negative kit controls were added in duplicate to appropriate wells, followed by 50 µℓ of the ear-notch supernatant buffer to the remaining wells. The plate was left in the incubator at 37 ºC for 2 h before washing and addition of conjugate and substrate. The optical density (OD) values were measured at 450 nm and recorded as the starting OD for each animal. Frozen ear notches were thereafter tested approximately twice a month for 6 months using the same procedure described above.
For the 2nd part of the ELISA study, on arrival, 12 ear notches from each animal, measuring 2.0 × 2.0 mm, were submerged in individual aliquots of 100 µℓ 0.1 M PBS (pH 7.4) 3. Ear notches were left to soak overnight (12-18 h) at 4 ºC. They were removed the next day and the PBS supernatant aliquots were stored at -2 ºC for the duration of the study. On Day 3, a frozen aliquot for each animal was removed from the freezer, thawed and tested with the ELISA, as described above. This procedure was repeated approximately twice a month for 6 months.
Data analysis
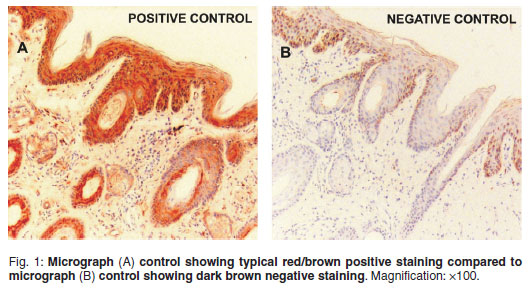
Immunohistochemistry. A specialist veterinary pathologist examined stained slides. Positive reactions were identified by evident Nova Red staining in the epidermis and hair follicles of the ear. Slides were graded based on the intensity of antigen staining using a 3-tier grading system18of 2 (strong, dense), 1 (diminished, scattered) and 0 (none). Positive and negative controls were examined for consistent staining for the duration of the study (Fig. 1).
AC-ELISA. Results were calculated as described by IDEXX HerdChek* BVDV Ag Test Kit/Serum Plus insert. For the assay to be valid, the difference between the positive control mean and the negative control mean must be greater than or equal to 0.150 OD. Also, the negative control mean must be less than or equal to 0.250 OD. The presence or absence of the viral antigen is determined by the corrected OD value (S-N) for each sample.
Calculation for test sample:
S-N = Sample A450- Negative control mean A450
A 1-tailed paired t -test was performed to determine whether there was a significant difference between OD values recorded on the 1st and last day for both test treatments. P < 0.05 was considered to be statistically significant. All calculations were computed using Microsoft ExcelTM software.
RESULTS
Immunohistochemistry
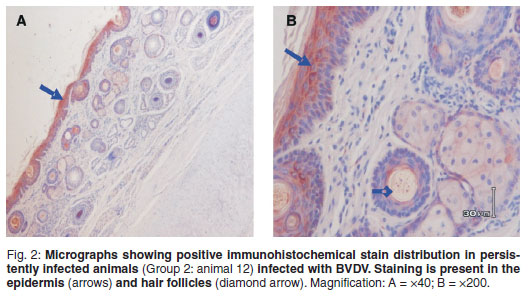
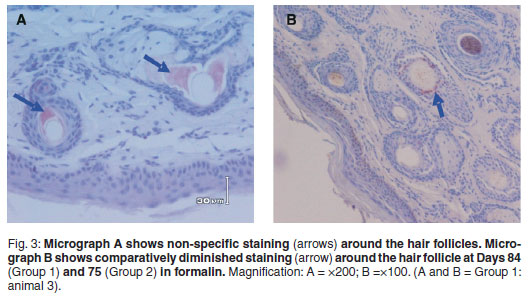
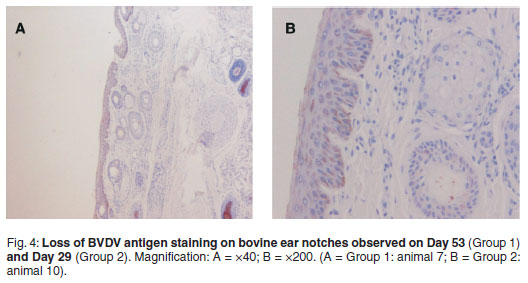
Positive BVD antigen staining was graded as follows: 2 = strong, dense, 1 = diminished, scattered, 0=no staining (Table 1). Shaded areas highlight days on which both groups were processed for IHC. Strong and dense staining patterns within the cytoplasm of keratinocytes (epidermal), sebaceous epithelial cells, follicular epithelial cells, mononuclear cells in the dermis and smooth muscle cells were observed between Days 1 and 35 (Group 1) and Days 1 and 15 (Group 2) (Fig. 2). A clear loss of antigen staining was evident on Days 53 and 29 respectively (Fig. 4). Markedly diminished and scattered antigen staining was observed for both groups from Days 84 and 75, respectively (Fig. 3). Staining was predominant in the follicular epithelial cells and virtually no staining was present in the epidermis (Fig. 3A). Other cells that revealed positive staining included dermal mononuclear cells, smooth muscle cells and cartilage cells (data not shown).
AC-ELISA
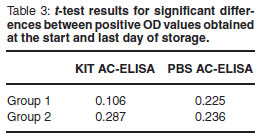
A 1-tailed paired t -test revealed no significant differences between OD values for all positive BVD ear notches tested on the 1st day samples arrived and the last day of storage (Table 2 and 3). Samples remained positive throughout the storage period regardless of buffer treatments applied.
DISCUSSION
Immunohistochemistry
Immunohistochemistry is a useful diagnostic tool for antigen detection and has been applied to detect various pathogens. While formalin provides excellent preservation of tissue architecture, formalin fixation is known to be a limiting factor for IHC. High concentrations of proteins and other solutes within tissue lead to the formation of a dense irregular network of cross-links and over-fixation can render some epitopes inaccessible to immunoreagents18, decreasing the sensitivity of an IHC test.
This study has shown that long-term storage of bovine ear notches in formalin is associated with reduced reactivity of BVD viral antigens with immunohistochemical staining procedures. The pattern of IHC staining for all animals on the 1st day of storage in formalin was strong and dense in epidermal and hair follicle epithelial cells, (Fig. 2). In Group 1, the distribution and intensity of staining decreased by the 3rd test and was completely lost by the 4th test (Fig. 4). In Group 2, positive staining ear notches were completely lost by the 3rd test. Although an exact day could not be established, it is evident from both groups that BVDV antigen staining starts to fade between Days 17 and 29 when stored in formalin (Table 1). The effect of prolonged formalin fixation on IHC detection of 61 different antigens has been evaluated18. The results showed that long-term formalin fixation had little effect on antigen detection 'for most commonly used antibodies' while other antibodies showed 'moderate to marked decreases in their detection abilities' following long-term fixation. It was found that moderate variations in fixation times up to 7 weeks should not significantly affect IHC results. Therefore it was concluded that it is likely that the effects of long-term formalin fixation would depend on the antibody, the antigen of interest and the antigen detection method.
The diminished and scattered appearance of antigen staining from Day 84 (Group 1) and Day 75 (Group 2) (Table 1; Fig. 3B) could be attributed to one or a combination of the following factors. Tissue and slide processing procedures whereby non-immunological binding of proteins to substrate reaction products could lead to false positive results. They may also be the result of endogenous biotin activity, a known source of nonspecific staining in IHC procedures based on the biotin-avidin system10. Biotin-avidin interactions form the basis for the identification of antigens in IHC. Also, autolysis will continue until all tissues are penetrated by formalin. Therefore, variations in immunoreactivity are attributed to the differing degrees of autolysis that usually occur before fixation18. Necrotic or degenerated cells can also exhibit non-specific staining. Non-specific staining is typically of a diffuse appearance (Fig. 3A). The reason for this discrepancy is uncertain considering that negative controls were certified and consistent throughout (Fig. 1). Ear notches were taken from different areas of a whole ear on each day of IHC processing and it is possible that variations in antigen concentration and distribution and variation in tissues evaluated could have resulted in different protein-protein cross-links and differential effects of prolonged fixation18. Similarly, weak follicular staining and no epidermal IHC reactions have been described after 176 days in formalin9.
Formalin fixation times are rarely standardised18and this variability lends itself to vast inconsistencies when interpreting IHC results, especially when results are unexpected. The subjective nature of interpreting IHC results is also a cause of inconsistency and open to interpreters' biases. The observations in this study reiterate the importance of proper optimisation and standardisation of antigen retrieval protocols for antibody and tissues used in IHC techniques18to obtain repeatable results every time.
From this study and a previous report9on prolonged formalin fixation on IHC detection of BVDV, the most favourable procedure to follow would be to promptly fix samples for no less than 24 h to minimise the risk of desiccated samples, and to minimise the risk of over-fixation, store in formalin for no more than 17 days to minimise risk of false negative antigen staining. Storing formalin fixed paraffin embedded tissues is practised routinely and while these may remain stable over time in terms of antigenicity, only controlled studies on the effect of nucleic acid detection on archival paraffin blocks have been published. Alternatively, cryostat preservation of skin tissue followed by IHC staining for BVD maintains histological appearances while avoiding the drawbacks of formalin fixation4. However this method still requires extensive validation before use as a routine diagnostic test.
AC-ELISA
Skin biopsies have been shown to be useful for diagnosis of BVDV and their use is widely accepted by researchers worldwide. Long-term storage of bovine ear notches kept at -20 ºC proved to be an efficient storage method for subsequent BVDV antigen detection. All samples remained positive over the 6-month storage period (Table 2(A) ) with no significant differences between start OD values and OD values obtained on the last day of storage (Table 3). A similar finding of strong positive results and low variation in testing multiple samples from the same ear of a PI animal was reported7. However, analysing OD values obtained on different days can lead to inconsistencies due to its erratic nature affected by uncontrolled parameters in the laboratory. Corrected OD values -S-N values confirmed positive results (Table 2(A and B) ).
The use of PBS as ear notch supernatant/buffer diluent as an alternative to the manufacturer 's soaking buffer proved to be relatively reliable. Similar to frozen ear notches, all PBS ear notch supernatants remained positive over the 6-month storage period (Table 2(B) ) with no significant differences between OD values from the first and last day of storage (Table 3). It is common practice to maintain ear notches or PBS ear notch supernatant4,16at -20 ºC4,8during transport to a laboratory to prevent desiccation17. The findings in this study contrast with those of a previous report of significantly higher mean OD values for ear notches frozen in PBS or tissue diluent buffer2.In this study, start OD values for 4 (animals 3, 7, 8 and 11) out of 7 unfrozen ear notches (Table 2(A) ) were higher than ODs obtained after ~15 days storage at -20 ºC. Furthermore, OD values for frozen PBS in Group 1 (Table 2(B) ) were lower than the ODs obtained from unfrozen kit buffer ear notch supernatants (Table 2(A) ). Similarly, the lowest sample to positive (S/P) ratio in foetal ear punch samples were observed when stored at -20 ºC and the highest S/P values for samples when stored at 37 ºC15. It is not clear from the previous report and others4,14whether samples are frozen with or without ear notches. The protocol followed here instructs overnight (12-18 h) soaking and conducting the test with only the supernatant. The effect of excessive soaking has not been documented and could be an element to be seriously considered in future studies. Also, samples were frozen at -80 ºC(ref. 2) compared with -20 ºC in this study. The possibility of the release of Ernsviral proteins during desiccation hence higher S/P values has been expressed previously15. The destructive effects of freezing (-80 ºC) PBS with the ear notch may also contribute to release of viral proteins after cell lysis.
The ultimate goal of fixation is to preserve tissues and antigenicity. While formalin fixation allows that, long-term storage in formalin can limit further investigations of the sample at hand. Misinterpretation of a PI animal on a single IHC stained ear notch sample is prone to occur, even after 2 weeks of fixation in formalin. It is therefore recommended that ear notches be frozen at -20 ºC for future BVDV investigations. Since kit reagents are limited and sufficient for just the plates provided, it is suitable to use a 0.1 M phosphate buffered saline (pH 7.4) solution to obtain ear notch supernatant. This supernatant can be frozen at -20 ºC and later used for retrospective studies. The option of freezing ear notch supernatant may prove more reliable than freezing ear notch tissue, which may affect the ability of the ELISA to detect the BVDV virus over time. Four diagnostic tests for detecting BVD in buffy coat samples after long-term storage have been compared12. Findings indicated that RT-PCR is more suitable for detecting BVDV in frozen buffy coats stored over a year. This lower immunodetection sensitivity of the other tests was attributed to the presence of toxic elements after autolysis following freezing and thawing.
The development of the ear notch AC-ELISA has paved the way for delivering quicker, easier to interpret and more reliable results with only 1 technician required, compared with IHC which is more time-consuming and requires a team. Unfortunately, this study was only performed on 7 animals and further divided into 2 time frames, therefore results could not be interpreted collectively. In addition, the study was only conducted over 6 months and a longer storage period would be worthy of consideration for future examination. Furthermore, although antigen was successfully detected in all samples in frozen PBS throughout the 6 months, a longer storage period may also allow for detecting any differences between the buffers. This principle comes from a previous finding where OD values differed significantly by each dilution and were greatest at 1:12. Since the IDEXX tissue diluent buffer concentration and constituents are unknown and only speculated to be PBS, it is possible that the constituent components of the 2 buffers vary considerably. Consistent results from multiple studies are still required to reach firm conclusions.
ACKNOWLEDGEMENTS
This study was conducted at the Vet-diagnostix-Veterinary Pathology Services laboratory under the supervision of Drs J H Vorster, R Mapham and R D Last and by Prof. M van Vuuren of the University of Pretoria. Bovine samples were made available through correspondence with Dr S Morris and kindly donated by Sparta Beef Farm and Karan Estate. The authors wish to extend their gratitude to everybody who contributed towards the completion of this study.
REFERENCES
1. Bhudevi B, Weinstock D 2003 Detection of bovine viral diarrhoea virus in formalin-fixed paraffin-embedded tissue sections by real time RT-PCR (Taqman). Journal of Virological Methods 109: 25-30 [ Links ]
2. Brodersen B W, Huchzermeier R, Galeota J, Smith, D R, Steffen D J 2002 Dilution of extracts of fresh skin biopsies for an antigen capture ELISA (ACE) for detection of BVDV. Detecting and controlling BVDV infections [Institute for International Cooperation in Animal Biologics Training and Meetings]. 4-5 April 2002. Ames, Iowa [ Links ]
3. Cornish T E, L. van Olphen A, Cavender J L, Edwards J M, Jaegar P T, Vieyra L L, Woodard L F, Miller D R, O'toole D 2005 Comparison of ear notch immunohistochemistry, ear notch antigen-capture ELISA, and buffy coat virus isolation for detection of calves persistently infected with bovine viral diarrhoea virus. Journal of Veterinary Diagnostic Investigation 17: 110-117 [ Links ]
4. Gripshover E M, Givens M D, Ridpath J F, Brock K V, Whitelly E M, Sartin E A 2007 Variation in Ernsviral glycoprotein associated with failure of immunohistochemistry and commercial antigen capture ELISA to detect a field strain of bovine viral diarrhoea virus. Veterinary Microbiology 125: 11-21 [ Links ]
5. Hilbe M, Stalder H, Peterhans E, Heasig M, Nussbaumer M, Egli C, Schelp C, Zlinszky K, Ehrensperger F 2007 Comparison of five diagnostic methods for detecting bovine virus infection in calves. Journal of Veterinary Diagnostic Investigation 19: 28-34 [ Links ]
6. Kennedy J A, Mortimer R G, Powers, B 2006 Reverse transcription-polymerase chain reaction on pooled samples to detect bovine viral diarrhoea virus by using fresh ear-notch sample supernatants. Journal of Veterinary Diagnostic Investigation 18: 89-93 [ Links ]
7. Kuhne S, Schroeder C, Holmquist G, Wolf G, Horner S, Brem G, Ballagi 2005 Detection of bovine viral diarrhoea virus infected cattle - testing tissue samples derived from ear tagging using an Ernscapture ELISA. International Journal of Veterinary Medicine Series B 52: 272-277 [ Links ]
8. Larson R L, Brodersen B W, Grotelueschen D M, Hunsaker B D, Burdett W, Brock K V, Fulton R W, Goehl D R, Sprowls R W, Kennedy J A, Loneragan G H Dargatz D A 2005 Considerations for Bovine Viral Diarrhoea (BVD) testing. Bovine Practice 39: 96-100 [ Links ]
9. Miller M A, Ramos-Vara J A, Kleiboeker S B, Larson R L 2005 Effects of delayed or prolonged fixation on immunohistochemical detection of bovine viral diarrhoea virus type 1 in skin of two persistently infected calves. Journal of Veterinary Diagnostic Investigation 17: 461-463 [ Links ]
10. Mount S L, Cooper K 2001 Beware of Biotin: a source of false-positive immunohistochemistry. Current Diagnostic Pathology 7: 161-167 [ Links ]
11. Njaa B L., Clark E G, Janzen E, Ellis J A, Haines D M 2000 Diagnosis of persistent bovine viral diarrhoea virus infection by immunohistochemical staining of formalin-fixed skin biopsy specimens. Journal of Veterinary Diagnostic Investigation 12: 393-399 [ Links ]
12. Ozkul A, Yeşilbag K, Burgu I 2001 Comparison of four diagnostic techniques for detecting bovine viral diarrhoea virus (BVDV) in buffy coat samples after long-term storage. Turkish Journal of Veterinary Animal Science 26: 1043-1048 [ Links ]
13. Potgieter L N D 2004 Bovine viral diarrhoea and mucosal disease. In Coetzer J A W, Tustin R C (eds), Infectious diseases of livestock Vol. 2 (2nd edn). Oxford University Press South Africa, Cape Town: 946-969 [ Links ]
14. Reed M C, O'Connor A M, Yoon K, Cooper V L 2008 Assessing the effect of sample handling on the performance of a commercial bovine viral diarrhoea virus antigencapture enzyme-linked immunoabsorbent assay. Journal of Veterinary Diagnostic Investigation 20: 124-126 [ Links ]
15. Ridpath J F, Chiang Y W, Waldbillig J, Neill J D 2009 Stability of bovine viral diarrhea virus antigen in ear punch samples collected from bovine fetuses. Journal of Veterinary Diagnostic Investigation 21: 346-349 [ Links ]
16. Ridpath J F, Hessman B E, Neil J D, Fulton R W, Step D L 2006 Parameters of ear notch samples for BVDV testing: stability, size requirements and viral load. In Proceedings of the American Association of Bovine Practitioners 39th Annual Convention, Saint Paul, 21-23 September 2006, Minnesota. Online at: http://www.aabp.org/meeting/program2006.pdf [ Links ]
17. Ushijima A, Cleveland S 2005 Proper handling of ear notch samples for the detection of BVDV by antigen capture ELISA. Lab-Lines 10: 1-3 [ Links ]
18. Webster J D, Miller M A, Dusold D, Ramos-Vara J 2009 Effects of prolonged formalinfixation on diagnostic immunohistochemistry in domestic animals. Journal of Histochemistry and Cytochemistry DOI: 10.1369/jhc. 2009.953877 [ Links ]
Received: July 2010.
Accepted: January 2011.
* Author for correspondence. E-mail: khanfirdaus@gmail.com














