Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the South African Veterinary Association
versión On-line ISSN 2224-9435
versión impresa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.82 no.1 Pretoria ene. 2011
ARTICLE ARTIKEL
Comparative evaluation of halothane anaesthesia in medetomidine-butorphanol and midazolam-butorphanol premedicated water buffaloes (Bubalus bubalis )
V MalikI,*; P KinjavdekarII; AmarpalII; H P AithalII; A M PawdeII; SurbhiII
IDepartment of Surgery, College of Veterinary Sciences and Animal Husbandry, DeenDayal Upadhayaya Veterinary and Animal Science University, Mathura, Uttar Pradesh, 281001, India
IIDivision of Surgery, Indian Veterinary Research Institute, Izatnagar, Uttar Pradesh, 243122, India
ABSTRACT
Six clinically healthy male water buffaloes (Bubalus bubalis ) 2-3 years of age and weighing 290-325 kg were used for 2 different treatments (H1 and H2). The animals of group H1 were premedicated with medetomidine (2.5 g/kg, i.v.) and butorphanol (0.05 mg/kg, i.v.), while in group H2 midazolam (0.25 mg/kg) and butorphanol (0.05 mg/kg) were used intravenously. Induction of anaesthesia was achieved by 5 % thiopental sodium in H1 (3.85 ± 0.63 mg/kg) and H2 (6.96 ± 0.45 mg/kg) groups. The anaesthesia was maintained with halothane in 100 % oxygen through a large animal anaesthetic machine. Better analgesia and sedation with a significantly lower dose of thiopental for induction and significantly higher values of sternal recumbency time and standing time were recorded in group H1 than in group H2 , whereas no significant (P > 0.05) difference for the halothane concentration was observed between groups H1 and H2. Significant decrease in heart rate was observed in group H1 whereas it significantly increased in group H2. In both groups, RR decreased during the preanaesthetic period, which increased significantly (P < 0.01) after halothane administration. In both groups a significant (P < 0.01) fall in RT was recorded from 20 min to the end of observation period. A significant (P < 0.05) fall in MAP was observed in group H1 from 15 min until the end, while in group H2 MAP increased nonsignificantly (P > 0.05) after premedication and a significant (P < 0.05) occurredafter thiopental administration. In both groups a significant (P < 0.01) increase in CVP and a significant (P < 0.01) decrease in SpO2 were observed after premedication which persisted up to 120 min. ECG changes included significant (P < 0.01) decrease and increase in QRS amplitudes in groups H1 and H2 respectively, a significant (P < 0.05) increase in PR interval was recorded at 15 min in group H1, a significant (P < 0.05) decrease in PR interval in group H2 , a significant (P < 0.05) decrease in T wave amplitude in group H1, and a significant (P < 0.01) increase in duration of T wave in group H1 . It is concluded that both combinations can be used safely in buffaloes for surgery of 2 h duration but better sedation, analgesia and muscular relaxation and more dose sparing effect on anaesthetics and shorter recovery times were observed in group H1.
Keywords: buffalo, butorphanol, halothane, medetomidine, midazolam, thiopental.
INTRODUCTION
In large ruminants inhalation anaesthesia maximizes effectiveness and safety, provided appropriate adaptations are made for fasting and positioning of animals. Anaesthesia for major surgery is usually maintained with volatile agents but sedation and induction of anaesthesia with injectable drugs are very important in large, aggressive animals. Medetomidine, the most potent alpha-2 agonist, has been investigated in some species of animals2,44,51. This drug has, however, not been widely used in buffaloes, particularly in combination with butorphanol. Butorphanol, a synthetic opioid analgesic, is a partial agonist and agonist at κ opioid receptors. Midazolam has been reported to produce adequate sedation when used as premedicant with thiopental sodium in human beings and several species of animals35,63,66. Synergistic interactions have been reported between alpha-2 agonists and opioids and benzodiazepines and opioids in earlier studies25,35. However, the suitability of combining butorphanol with medetomidine or midazolam is yet to be established in buffaloes.
Thiopentone sodium is the most widely used intravenous induction agent in ruminants and has been used in cattle and buffalo calves12,49. There are few reports on the use of inhalant anaesthesia in large ruminants like cattle and sheep30,47,61. However, perusal of literature reveals little information on the use of halothane in adult buffaloes. Considering the importance of this species and the scarcity of information, this study was designed to compare the suitability of halothane anaesthesia with 2 different preanaesthetic protocols.
MATERIALS AND METHODS
Experimental animals
Six clinically healthy male buffaloes 2-3 years of age and weighing 290-325 kg were used. The clinical status of the animals was assessed by recording heart rate, respiratory rate and rectal temperature and by conducting haematological examinations. The animals were fasted for 48 hours and water was withheld for 24 hours prior to the start of the experiment. The animals were secured in right lateral recumbency and the left ventrolateral aspect of the neck and dorsal side of the left ear were aseptically prepared for administration of drugs. A pre-heparinised polythene catheter was introduced into the jugular vein through a 12 gauge hypodermic needle and passed up to the level of the right atrium. This catheter was attached to a central venous pressure (CVP) saline manometer through a 3-way stopcock for recording of CVP. The 3rd end of the stopcock was attached to a glass syringe filled with heparinised saline for flushing the system. The position of the catheter in the anterior vena cava or right atrium was confirmed by pressure changes in the saline manometer due to respiration. The cuff of the non-invasive blood pressure (NIBP) monitor (Surgivet, Smith Medical PM, Waukesha, WI, 53186) was applied around the base of the tail for monitoring systolic, diastolic and mean arterial blood pressures. Subcutaneous needle electrodes were placed at the posterior border of the scapula and at the 5th costochondral junction (base apex lead) for recording of electrocardiograms at 1 mV and 25 mm/s paper speed (BPL, New Delhi). The baseline data for arterial oxygen saturation were obtained by applying the sensor of a pulse oxymeter (Nonin Medical Inc., Minneapolis, USA) to the tongue after application of a mouth gag in the sedated animals. The animals were stabilised for 30 min before recording the baseline data.
Experimental design
All the animals received 2 treatments randomly at 10-day intervals. In group H1 medetomidine (2.5 mg/kg) (Domitor; Orion Corporation, Formos Group, Turku, Finland) and butorphanol (0.05 mg/kg) (Butrum; Aristo Pharma, Mumbai) and in group H2 midazolam (0.25 mg/kg) (Mezolam; Neon Laboratories, Mumbai) and butorphanol (0.05 mg/kg) were administered intravenously. Induction of anaesthesia was achieved with 5 % thiopental sodium (Thiosol sodium; Neon Laboratories, Mumbai) in both groups. Anaesthesia was maintained with halothane (Halothane I.P. 85; Raman and Weil Pvt Ltd, 15, Mumbai) in 100 % oxygen through a large-animal anaesthetic machine (Surgivet; Smith Medical PM, Waukesha, USA). The time of administration of preanaesthetics was taken as time zero.
Technique of drug administration
After 15 min of premedication, the animals were restrained in right lateral recumbency and anaesthesia was induced with intravenous thiopental sodium. An additional dose of thiopental sodium, if required, was administered until the pinprick reflex over the ribs and coronary band ceased to occur. A mouth gag was used to open the jaws and an endotracheal tube was passed and connected to the anaesthetic machine. Halothane in 100 % oxygen was administered via a semi-closed rebreathing system for maintenance of anaesthesia for 120 min. The vaporiser setting was adjusted according to depth of anaesthesia after monitoring the animal's response to a pinprick on the tail.
Clinical observations
Sedation . Sedation was evaluated by recording behavioural changes and was graded on a 1-4 scoring scale as: 1 (no sedation) = standing alert, head high, eyes open; 2 (mild sedation) = standing but appearing tired, drooping of head and eyelids; 3 (moderate sedation) = able to sit without support, drooping of head and eyelids; 4 (excellent sedation) = unable to sit without support, drooping of head and eyelids.
Analgesia . Analgesia was assessed by recording the animal's response at 15-min intervals by pricking with a 22 G hypodermic needle on the rib periosteum and at the coronary band. The analgesia was graded on a 1-4 scoring scale as: 1 (no analgesia) = strong reaction to pinpricks; 2 (mild analgesia) = weak response to pin pricks; 3 (moderate analgesia) = occasional response to pinpricks; 4 (excellent analgesia) = no response to pinpricks.
Muscular relaxation. Muscle relaxation was recorded in the muscles of the abdomen, legs and jaws. The ease with which the jaws of recumbent animals could be opened, their hind limbs could be bent without resistance and the flaccid abdomen could be pressed was recorded as the extent of muscle relaxation. It was graded on a 1-4 scoring scale as: 1 (no relaxation) = normal abdominal muscles, tightly closed jaws and stiff limbs; 2 (mild relaxation) = moderate resistance to pressing of abdomen, opening of jaws and bending of limbs; 3 (moderate relaxation) = mild resistance to pressing of abdomen, opening of jaws and bending of limbs; 4 (excellent relaxation) = flaccid abdomen, no resistance to opening of jaws and bending of limbs.
Reflexes. The degree of loss of various reflexes, namely palpebral, corneal, pedal (interdigital skin fold pinching with a artery forceps for 1 second) and pinprick (deep pricking the last thoracic rib periosteum with a 22 G hypodermic needle) were recorded at different intervals in the animals of different groups and were graded on a (-) to (++++) scoring scale as: (-) = completely lost; (+): mild response; (++): moderate response; (+++): good response; (++++): excellent response.
Position of eyeball. Position of the eyeball in the animals of different groups was recorded at different intervals and was graded as C (central) or D (downward rotation).
Extent of salivation. The extent of salivation was recorded at different intervals and was graded on a (-) to (++++) scoring scale as follows: (-): Absent; (+): mild; (++): moderate; (+++): extensive; (++++): profuse.
Dose of thiopental and concentration of halothane. The dose of thiopental sodium (mg/kg) for induction and concentration (%) of halothane (range) for maintenance of anaesthesia was calculated after the completion of each trial.
Weak time. The time that elapsed from the time of injection of preanaesthetic agents to the time when the animals showed ptosis of the head was recorded as weak time.
Recovery time. The time from discontinuation of the administration of halothane and the 1st spontaneous movement of any body part was recorded as recovery time.
Sternal recumbency time. The time from discontinuation of the administration of halothane to the spontaneous regaining of sternal recumbency was recorded as sternal recumbency time.
Standing time. The time from discontinuation of the administration of halothane to spontaneous regaining of standing position was recorded as standing time.
Heart rate (beats/min ) (HR ), respiratory rate (breaths/min ) (RR ) and rectal temperature (ºC ) (RT ). HR, RR and RT were recorded at 0, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110 and 120 min of anaesthesia. Mean arterial pressure, central venous pressure and electrocardiogram. Mean arterial pressure (MAP) (mm Hg), central venous pressure (CVP) (cm H2O ), haemoglobin oxygen saturation (SpO2 %) and electrocardiogram (ECG) (base apex lead) at 1 mV and 25 mm/s paper speed were recorded at 0, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110 and 120 min of anaesthesia.
Statistical analysis
Analysis of variance (ANOVA) and Duncan's multiple-range test (DMRT) were used to compare the means at different intervals among different groups. Paired 't' test was used to compare the means at different intervals with their respective base values in each group. For nonparametric observations the Kruskal-Wallis 1-way test was used to compare the means between groups53,60.
RESULTS
Sedation
In group H1 the median ± SD (range) of sedation score was 4 ± 0.516 (3-4). Of 6 animals, 4 recorded a score of 4 and 2 animals recorded a score of 3. Group H2 animals recorded a sedation score of 2 ± 0.632 (1-3) (median ± SD) (range). In this group 4 animals recorded a score of 2, 1 animal recorded 3 and 1 animal scored 1.
In group H1 the signs of sedation were evident after 2-3 min of medetomidine and butorphanol premedication. Excellent sedation was recorded in this group, with characteristic signs of sedation. Two animals in group H1 attained sternal recumbency, whereas 2 animals knelt for a short period (1-2 min) and could stand up intermittently. However, other animals remained in the standing position but appeared sedated. None of the animals in this group showed signs of excitement during sedation. In 1 animal the eyelids were closed with moderate depression of palpebral and corneal reflexes and the eyeball turned ventromedially after 5 min of premedication, but it regained its central position after 10 min of drug administration.
In group H2 animals, moderate sedation was recorded. Two animals in this group attained sternal recumbency immediately after premedication with midazolam and butorphanol, but these animals stood up after 5-8 min. Other animals remained in the standing position with varying degrees of ataxia and incoordination. All the animals of this group exhibited signs of excitement like vigorous licking of muzzle, shaking of the head and tail, attempts to stand up, if recumbent within 5 min of drug administration. Two animals of this group showed signs of severe excitement and were difficult to restrain before induction. Comparison between the groups revealed that medetomidine and butorphanol produced better sedation than midazolam and butorphanol.
Analgesia
In group H1 the analgesia was graded as excellent with a score of 4. The median ± SD (range) of analgesia score recorded in group H1 was 4 ± 0.0 (4-4). However, in group H2 the analgesia was graded as good. The median ± SD (range) of analgesia score in group H2 was 2.5 ± 0.81 (2-4). In group H2, 3 animals recorded a score of 2, while 2 animals recorded 3 and only 1 animal recorded a score of 4.
Muscular relaxation
Median ± SD (range) of muscle relaxation score was 4 ± 0.40 (3-4) in group H1 and 4 ± 0.51 (3-4) in group H2, respectively. In group H1, 5 animals recorded excellent muscle relaxation (score 4) and in 1 animal good muscle relaxation (score 3) was recorded. In group H2 4 animals recorded excellent muscle relaxation (score 4) and 2 animals recorded good muscular relaxation (score 3). Signs of early muscle relaxation were observed after 8-10 min of premedication and remained so throughout the observation period. Comparison between groups did not reveal significant differences in muscle relaxation scores.
Palpebral reflex
In group H1 the palpebral reflex was moderately depressed up to 15 min after premedication and remained absent during the rest of the observation period, whereas in group H2 the palpebral reflex was good to moderately depressed after midazolam and butorphanol administration, but was later completely depressed and remained absent during the rest of the observation period. Comparison between the 2 groups did not show significant differences in the depression of the palpebral reflex.
Corneal reflex
In both groups the corneal reflex showed good response to moderate depression after premedication. However, it disappeared after administration of thiopental sodium in both groups and remained absent up to the end of anaesthesia.
Pedal reflex
After premedication, a good response to moderate depression of pedal reflex was recorded and it remained so up to 15 min of anaesthesia in both groups. After induction of anaesthesia, the pedal reflex disappeared completely and this persisted until the end of maintenance anaesthesia in animals of group H1. However, in group H2 the pedal reflex was lost after induction and remained so, but 2 animals in this group showed a mild response up to the end of the observation period. Comparison between groups revealed that the degree of depression of the pedal reflex was higher in group H1 than in group H2.
Position of eyeball
The eyeball remained central in position during the sedation period in group H2, whereas in group H1 the eyeball rotated downward 5 min after premedication. During the maintenance period it rotated downward in most of the animals of both groups.
Salivation
Mild to moderate salivation was observed after 5-10 min of premedication in both groups. However, in group H1 the extent of salivation was moderate but group H2 animals produced only mild salivation during the sedation period. Moderate to profuse salivation was noticed throughout the observation period in both groups. When the concentration of halothane was increased to maintain the desired depth of anaesthesia, the extent of salivation increased immediately. An increase in halothane concentration also increased lacrimation.
Doses of drugs
Thiopental sodium
Mean doses of thiopental sodium for induction of anaesthesia in groups H1 and H2 were 3.85 ± 0.63 mg/kg and 6.96 ± 0.45 mg/kg, respectively. Group H1 required significantly (P < 0.05) lower doses than group H2.
Halothane
Halothane concentration for maintenance of anaesthesia in group H1 ranged from 2.5 to 3.5 %, whereas in group H2 it ranged from 2.75 to 4.0 %. Comparison between the groups revealed no significant (P > 0.05) differences in the halothane concentration used for maintenance.
Weak time
The median ± SD of weak times recorded in groups H1 and H2 were 3.00 ± 1.60 min and 4 ± 1.89 min, respectively, which did not differ significantly between groups (Fig. 1 ). However, in group H1 sedation developed smoothly and the onset of limb incoordination was gradual but the animals took less time to show ptosis of the head. In contrast, in group H2 the onset of limb incoordination was rapid but the animals took longer to show ptosis of the head.
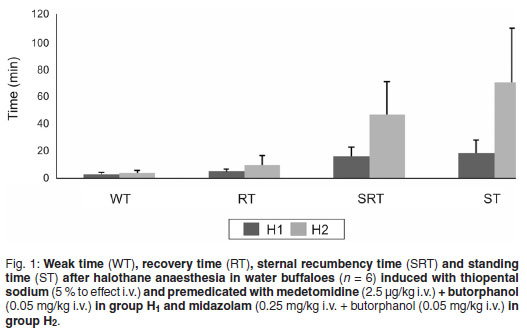
Recovery time
The median ± SD of recovery time recorded in groups H1 and H2 were 5 ± 1.86 min and 9.5 ± 7.00 min, respectively. Group H1 recovered earlier than group H2, The recovery times did, however, not differ significantly (P > 0.05) (Fig. 1).
Sternal recumbency time
The median ± SD of sternal recumbency time recorded in groups H1 and H2 were 16 ± 6.83 min and 46.5 ± 23.86 min, respectively. Group H2 took significantly (P < 0.05) longer to resume sternal recumbency than group H1 (Fig. 1).
Standing time
The median ± SD of standing time recorded in groups H1 and H2 were 18.50 ± 9.43 min and 70 ± 39.82 min, respectively. Standing time in group H2 was significantly (P < 0.05) longer than in group H1 (Fig. 1 ).
Heart rate (HR)
Medetomidine and butorphanol combination produced significant (P < 0.01) bradycardia immediately after premedication in group H1, which persisted up to 40 min. After 50 min HR improved slightly but remained significantly (P < 0.05) decreased until the end of the observation period. In group H2 HR increased after midazolam and butorphanol administration and remained significantly (P < 0.05) increased from 10-40 min and returned to the base value at 120 min. HR remained significantly (P < 0.05) higher in group H2 than in group H1 (Fig. 2).
Respiratory rate (RR)
In group H1 a non-significant (P > 0.05) decrease in RR was recorded up to 15 min of premedication but an increasing trend in RR was recorded after halothane administration, which was significant (P < 0.01) throughout the maintenance period. In group H2 RR decreased nonsignificantly (P > 0.05) after premedication and remained so up to the post-induction period (20 min). It increased after the administration of halothane and remained significantly (P < 0.01) increased throughout the maintenance period (Fig. 3).
Rectal temperature (RT)
In groups H1 and H2 a significant (P < 0.01) decrease in RT was recorded at 20 min which persisted up to the end of the observation period. Significantly higher RT values were recorded in group H1 up to 15 min, but no significant differences between the groups was recorded in RT after induction until the end of anaesthesia (Fig. 4).
Mean arterial pressure (MAP)
In group H1 a significant (P < 0.05) drop in MAP was recorded after 15 min of premedication and the decrease continued for the entire observation period. However, from 30 to 50 min a highly significant (P < 0.01) decrease in MAP was recorded. In group H2 a nonsignificant (P < 0.05) increase in MAP was recorded after 5 min of premedication, but after induction a significant (P < 0.05) decrease in MAP was recorded, which persisted for the entire observation period (Fig. 5).
Central venous pressure (CVP)
In group H1 CVP increased significantly (P < 0.01) 5 min after premedication and this persisted up to 120 min. However, a maximum increase in CVP was recorded at 10 min. CVP increased significantly (P < 0.01) after 5 min of premedication in group H2, which decreased but remained nonsignificantly (P > 0.05) higher throughout the observation period. CVP remained significantly (P < 0.05) higher in group H1 thab in group H2 throughout the observation period (Fig. 6).
Haemoglobin oxygen saturation (SPO2)
In group H1 SpO2 decreased significantly (P < 0.01) at 5 min, and this persisted for 120 min. However, the lowest SpO2 value was observed after induction of anaesthesia. Group H2 exhibited a significant (P < 0.01) decrease in SpO2 at 10 min, which remained decreased up to the end of the observation period. No significant differences in SpO2 between groups were recorded throughout the observation period (Fig. 7).
Electrocardiography
Electrocardiographic parameters recorded in both groups are presented in Table 1. A normal sinus rhythm was recorded before premedication in all the animals of both groups. However, sinus bradycardia after premedication was a consistent finding in group H1.On the other hand sinus tachycardia was consistently observed after premedication in group H2. After induction of anaesthesia, however, sinus bradycardia disappeared in group H1 and did not reappear during the rest of the observation period. In group H1 the QRS amplitude recorded a highly significant (P < 0.01) decrease after premedication which remained decreased up to 90 min, whereas in group H2 the QRS amplitude showed a significant (P < 0.01) increase from 15 min until the end of the observation period.
A significant (P < 0.05) increase in PR interval was recorded at 15 min in group H1. A further highly significant (P < 0.01) increase was recorded from 45 min to 120 min. By contrast, in group H2 a significant (P < 0.05) decrease in PR interval was recorded at 30 min, which persisted until the end of the observation period. In group H1 the T wave amplitude decreased significantly (P < 0.05) at 30 min from the baseline and remained decreased until the end of the observation period except at 1 or 2 intervals. In both groups the duration of T wave exhibited a highly significant (P < 0.01) increase at 15 min and 30 min and thereafter up to 120 min.
DISCUSSION
The doses of medetomidine (2.5 g/kg) and butorphanol (0.05 mg/kg) were selected on the basis of pilot trials conducted before the start of the experiment. It was found that smaller doses of medetomidine (2.5 g/kg) and butorphanol (0.05 mg/kg) enhanced the sedation and analgesia and reduced the adverse effects. Similarly, decreased doses of medetomidine ranging from 2 to 10 g/kg with butorphanol have been reported to enhance sedation and analgesia, while potentially reducing the duration of the adverse cardiovascular effects associated with its use45. Synergistic sedative and analgesic activity between alpha-2 agonists and opioid agonist-antagonists has been reported in horses and ruminants10, 35. Midazolam and butorphanol produced moderate sedation and adequate analgesia but induced varying degrees of excitement in all the animals. Although midazolam does not have any analgesic effect, addition of butorphanol might have resulted in an adequate level of analgesia. Thiopental and halothane have no or minimal intrinsic analgesic effect31. Excellent analgesia throughout the observation period in both groups might be due to the long-lasting analgesic effect of medetomidine and butorphanol and CNS depressant effects of thiopental and halothane. Alpha-2 agonists have been reported to produce profound muscle relaxation alone and in combination with opioid agonist-antagonists24. Midazolam possesses muscle-relaxant properties typical of benzodiazepines. The muscle relaxation and motor incoordination induced by midazolam have been reported in different species32,58. Moderate depression of all the reflexes was recorded in both groups. Moderate to complete abolition of palpebral and corneal reflexes after medetomidine (10 g/kg) and pentazocine (3mg/kg) in goats, mild to moderate palpebral reflex and full corneal reflex after midazolam administration (0.2 mg/kg, i.v.) in bovine and complete abolition of palpebral and corneal reflexes and ventro-medial rotation of eyeball after thiopental administration and during maintenance with halothane in bovine have been reported4,5,21,49.
Increased salivation during the maintenance period could be due to the effect of alpha-2 agonists on salivary glands, decreased swallowing reflex or partially opened jaws for placement of the endotracheal tube 26,50. Regurgitation, a frequent complication during general anaesthesia in ruminants, could be effectively prevented in the present study by keeping the animals off feed prior to the start of the experiment and by placing the caudal cervical and anterior thoracic region higher than the rest of the body46.
Reduction in the induction dose of thiopental was recorded in both groups as the usual dose of thiopental in large ruminants is around 8-10 mg/kg. Synergism between medetomidine and butorphanol and thiopental might have played an important role in reducing the induction dose of thiopental in the present study. Synergistic interaction between midazolam and thiopental has also been reported where preanaesthetic medication with midazolam reduced the dose of thiopental by about 25 % and 50 % in cattle and 40 % in buffaloes5,7,21. The concentration of halothane required for maintenance in group H1 was slightly lower than in group H2, which suggested a greater halothane sparing effect of medetomidine in comparison to midazolam. Similarly, medetomidine (30 g/kg) has been reported to cause a 47.2 % decrease in MAC of isoflurane compared to a 23 % reduction in MAC of isoflurane after midazolam administration in dogs9,64.
The animals of group H2 required significantly longer time to stand than those of group H1. It might be due to the greater induction doses used in group H2. Consciousness has been reported to return within 15-20 min after discontinuation of halothane in diazepam and chloral hydrate induced calves, which were able to stand within 90 min55. However, it has been reported that buffaloes took 190 minutes to stand after thiopental-induced halothane anaesthesia6.In another study, return of consciousness, within 15-20 min following halothane anaesthesia, irrespective of induction agents used, has also been reported in different animals11,31.
Significant bradycardia has been recorded in medetomidine and butorphanol premedicated buffaloes. Inhibition of sympathetic tone from the CNS, vagal activity in response to medetomidine induced vasoconstriction and direct increase in the release of acetylcholine from sympathetic nerves in the heart have been reported as the possible mechanisms by which medetomidine induced bradycardia32. Similar effects have been reported in earlier studies after medetomidine administration in different species28,67. Midazolam has been reported to cause transient hypotension in humans and, as the baroreflex is preserved, the increase in HR may be a reflex response to decreased blood pressure in humans29. A similar finding has been recorded in the present study, where midazolam caused tachycardia. An appreciable tachycardia with no change in MAP but decreased CVP in calves sedated with diazepam has also been reported37. Mild increase in HR after thiopentone administration in both groups in the present study supported the findings in buffaloes administered thiopentone sodium and glyceryl guiacolate1. It has been observed that during halothane anaesthesia tachycardia was a consistent finding and during controlled ventilation HR increased approximately 50 % over resting values68. Similar findings of tachycardia have also been reported in cattle anaesthetised with halothane13. By contrast, bradycardia has been shown to develop after halothane anaesthesia in cattle calves and no change in HR was reported when it was used at clinical doses in these animals52,65.
Respiratory depression was a consistent finding in both groups. Respiratory depression associated with alpha-2 adrenergic agonists might be secondary to the CNS depression produced by alpha-2 adrenoceptor stimulation or due to direct depression of the respiratory centres by preanaesthetics27,54. A similar decrease in RR has also been reported after medetomidine administration in sheep38,39. In contrast, only a small effect on RR has been reported after intravenous medetomidine administration in goats41. In both groups, shallow and rapid respiration was recorded throughout the observation period. Shallow and rapid respiration has been reported to be a characteristic feature of halothane anaesthesia36. Similar observations have been recorded in camels during halothane anaesthesia68.
Significant hypothermia was recorded in both groups throughout the observation period. Alpha-2 agonists have been reported to induce prolonged depression of thermoregulation43and similar findings have also been reported after medetomidine administration in goats59. These agents have also been found to depress hypothalamic noradrenergic alpha-2 adrenergic receptors to cause hypothermia34. Reduced basal metabolic rate and muscle activity on one hand and depression of thermoregulation on the other might have resulted in hypothermia43.
Hypotension was a consistent finding in both groups. A biphasic response, i.e. transient initial hypertension followed by prolonged hypotension, has been considered a classic response after intravenous administration of alpha-2 adrenergic agonists48. However, an initial hypertensive response was not recorded in the present study and instead only a hypotensive phase was recorded. It has been reported that preservation of haemodynamic functions occurred with midazolam, which involves an intact sympathetic reflex as demonstrated by release of endogenous catecholamine14, and this might be the reason for increase in MAP after midazolam and butorphanol administration. However, in contrast with the findings of the present study, intravenous administration of midazolam and butorphanol has been reported to induce significant decrease in MAP in isoflurane anaesthetised cats16. In both groups the MAP remained lower than the baseline during the maintenance period. However, the depression was significant only in group H1. The hypotensive effect of halothane has been shown in cattle, horses, sheep, buffalo and camels12,42,52,57,62. Halothane, when used in clinical doses, produced a decrease in arterial blood pressure, which resulted from the depression of myocardial contractility and cardiac output65.
The significant and prolonged increase in CVP as recorded in the present study was probably a reflection of medetomidine-induced bradycardia and possibly vasoconstriction resulting in the pooling of blood in the venous circulation27. Significant increase in CVP has been demonstrated after medetomidine and butorphanol administration in goats41. A slight decrease in CVP was recorded immediately after thiopentone administration in the present study. Similar findings have been reported after thiopentone administration in buffaloes and crossbred calves5,56. In group H1, however, the CVP showed a decline after the start of the maintenance period but it remained significantly higher than the base value. Continued maintenance of CVP at higher levels in both groups might possibly have been due to the depressive influence of medetomidine and butorphanol on the heart that gradually subsided with the elimination of the drugs20,22. Compensatory mechanisms might have been affected in the present study, as the CVP did not return completely to the baseline at the end of the observation. In group H2, the CVP increased significantly after premedication but the increase was less significant than in group H1 and this trend was recorded for the entire observation period. A lesser rise in CVP in group H2 might be attributed to increased HR and decreased systemic vascular resistance.
Decrease in SpO2 was possibly due to a certain degree of respiratory depression in both groups. Low SpO2 values with medetomidine and ketamine anaesthesia have been reported in rabbits15,40. Slightly higher values of SpO2 recorded during the maintenance period in the present study might be due to the administration of 100 % oxygen with halothane.
In the present study, no abnormality in the ECG except for slight variations in the amplitude of P wave, T wave and QRS complex was recorded under halothane anaesthesia in buffaloes as has also been reported in other studies6. Similar findings have also been reported after medetomidine administration in goats8,19. Ventricular premature depolarisation has been reported in dogs and cats under halothane anaesthesia18. The elevation of ST segment, T wave changes, wandering pacemakers and ectopic pacing in sheep during thiopentone and halothane anaesthesia have been reported42. None of the above abnormalities except for some T wave changes like notched or biphasic T waves were observed in the present study during halothane anaesthesia. Similar findings have been reported under thiopentone and halothane anaesthesia in calves55.
It is concluded that both anaesthetic drug combinations can be used safely in buffaloes for surgery of 2-hour duration However, medetomidine (2.5 g/kg) and butorphanol (0.05 mg/kg) provide better sedation, analgesia and muscle relaxation with transient but slightly more cardiac depression than midazolam (0.25 mg/kg) and butorphanol (0.05 mg/kg) when used as preanaesthetics to thiopental and halothane anaesthesia in buffaloes. Medetomidine and butorphanol combination provides more dose sparing effect on anaesthetics used for induction and maintenance with shorter recovery times than that of the midazolam-butorphanol combination.
REFERENCES
1. Agrawal K B P, Prashad B, Sobti V K 1983 Clinical observations during anaesthesia after administration of glyceryl guaicolate with and without chloral hydrate/thiopental sodium in buffalo calves. Indian Journal of Veterinary Surgery 4: 64-69 [ Links ]
2. Aithal H P, Amarpal, Tyagi S P, Singh G R, Mogha I V 1998 Sedative, analgesic and cardiorespiratory effects of diazepam-medetomidine anaesthesia in atropinized dogs. Indian Journal of Animal Sciences 68: 1012-1014 [ Links ]
3. Amarpal, Aithal H P, Pratap K, Singh G R 1998 Neuroleptanalgesia with medetomidine and pentazocine in goats. Indian Veterinary Journal 75: 150-154 [ Links ]
4. Amarpal, Aithal H P, Pratap K, Singh G R 1998 Neuroleptanalgesia with medetomidine and pentazocine in goats. Indian Veterinary Journal 75: 150-154 [ Links ]
5. Bisnoi P 2001 Studies on midazolam and its combination with chloral hydrate and thiopentone sodium anaesthesia in calves. PhD thesis, Punjab Agriculture University, Ludhiana, India [ Links ]
6. Bose A S, Kohli R N 1983 Studies on halothane anaesthesia in buffaloes with special reference to thoracic surgery. Indian Journal of Veterinary Surgery 4: 50-57 [ Links ]
7. Cheema J S 2002 Studies on midazolamthiopentone anaesthesia in bovine diaphragmatic herniorrhaphy. MVSc thesis, Punjab Agriculture University, Ludhiana, India [ Links ]
8. Chitale D, Pratap K, Amarpal, Gupta O P, Aithal H P, Singh G R 1998 Observations on some clinical aspects of alpha-2 agonists with diazepam as preanaesthetic to ketamine anaesthesia in goats. Indian Veterinary Journal 76: 112-114 [ Links ]
9. Ewing K K, Mohamed H O, Scarlett J M 1993 Reduction of isoflurane anaesthetic requirement by medetomidine and its restoration by atipamezole in dogs. American Journal of Veterinary Research 54: 294-299 [ Links ]
10. Faulkner D B, Eurell T, Tranquilli W J, Ohl M W, Zinn G 1992 Performance and health of weaning bulls after butorphanol-xylazine administration at castration. Journal of Animal Sciences 70: 2970-2974 [ Links ]
11. Fisher E W, Jennings S 1958 The use of fluothane in horses and cattle. Veterinary Record 70: 567-573 [ Links ]
12. Gahlawat J S, Singh A P, Peshin P K, Singh J 1986 Evaluation of halothane anaesthesia with and without thiopental sodium induction in spontaneously ventilating buffalo calves. Archiv für Experimentelle Veterinärmedizin 40: 861-869 [ Links ]
13. Gates J B, Botta J A, Teer P A 1971 Blood and pH determination in cattle anaesthetized with halothane. Journal of the American Veterinary Medical Association 158: 1678-1682 [ Links ]
14. Glisson S N, Belusco R J Kubak M A, Heiber M F 1982 Midazolam on stimulatory response to hypotension-pre-induction and during anaesthesia (Abstract). Anaesthesiology 59: 328 [ Links ]
15. Grint N J, Murison P J 2008 A comparison of ketamine-midazolam and ketaminemedetomidine combinations for induction of anaesthesia in rabbits. Veterinary Anaesthesia and Analgesia 35: 113-121 [ Links ]
16. Gross M E, Smith J A, Tranquilli W J 1993 Cardiorespiratory effects of combined midazolam and butorphanol in isoflurane anaesthetized cats. Veterinary Surgery 22: 159-162 [ Links ]
17. Hall K D, Norris F H 1958 Respiratory and cardiovascular effects of fluothane in dogs. Anaesthesiology 19: 339-352 [ Links ]
18. Hubbell J A E, Muir W W, Bednarski R M, Bednarski L S 1984 Change in inhalation anaesthetic agents for management of ventricular premature depolarizations in anaesthetized cats and dogs. Journal of the American Veterinary Medical Association 185: 643-646 [ Links ]
19. Hugar B, Gupta O P, Singh G R 1998 A note on the effects of medetomidine with and without ketamine in goats. Indian Veterinary Medical Journal 139-140 [ Links ]
20. Kallio A, Saraste M, Scheinin M 1990 Acute hemodynamic effects of medetomidine and clonidine in healthy volunteers: on noninvasive electrocardiographic study. Journal of Cardiovascular Pharmacology 16: 28-33 [ Links ]
21. Kaur A, Singh S S 2004 Clinical effects of midazolam-ketamine and midazolam thiopentone anaesthesia in bovines. Indian Journal of Veterinary Surgery 25: 80-82 [ Links ]
22. Kinjavdekar P, Pawde A M, Amarpal, Gupta O P, Aithal H P, Pratap K 2005 Medetomidine and ketamine anaesthesia in buffalo calves. Buffalo Journal 19: 349-356 [ Links ]
23. Klein L, Sherman J 1977 Effects of preanaesthetic medication, anaesthesia, and the position of recumbency on central venous pressure in horses. Journal of the American Veterinary Medical Association 170: 216-219 [ Links ]
24. Ko J C H, Bailey J E, Pablo L S, Heaton-Jones T G 1996 Comparison of sedative and cardiorespiratory effects of medetomidine and medetomidine-butorphanol combination in dogs. American Journal of Veterinary Research 57(4): 535-540 [ Links ]
25. Kojima K, Nishimura R, Mutoh T, Hong S H, Mochizuki M, Sasaki N 2002 Effects of medetomidine-midazolam, acepromazine-butorphanol, and midazolam-butorphanol on induction dose of thiopental and propofol and on cardiopulmonary changes in dogs. American Journal of Veterinary Research 63: 1671-1679 [ Links ]
26. Kokkonen U M, Eriksson L 1987 Cardiovascular and allied actions of xylazine and atropine in unanaesthetized goats. Journal of Veterinary Pharmacology and Therapeutics 10: 11-16 [ Links ]
27. Kumar A, Thurmon J C 1979 Cardiopulmonary, haematocytologic and biochemical effects of xylazine in goats. Laboratory Animal Science 29: 486-491 [ Links ]
28. Kuusela E, Raekallio M, Anttila M, Falck I, Molsa S, Vainio O 2000 Clinical effects of medetomidine and its enantiomers in dogs. Journal of Veterinary Pharmacology and Therapeutics 23: 15-20 [ Links ]
29. Lebowitz P, Cote M, Daniels A 1982 Comparative cardiovascular effects of midazolam in healthy patients. Anaesthesia and Analgesia 61: 771-775 [ Links ]
30. Lin H C, Purohit R C, Powe T A 1997 Anaesthesia in sheep with propofol or with xylazine ketamine followed by halothane. Veterinary Surgery 26: 247-252 [ Links ]
31. Lumb W V, Jones E W 1984 Veterinary anaesthesia ( 2nd edn). Lea and Febiger, Philadelphia [ Links ]
32. Luna S P L, Taylor P M, Dick C J 1993 Midazolam and ketamine induction before halothane anaesthesia in ponies: cardiorespiratory, endocrine and metabolic changes. Journal of Veterinary Anaesthesia 20: 49 [ Links ]
33. MacDonald E, Virtanen R 1992 Review of the pharmacology of medetomidine and detomidine: two chemically alpha-2 adrenoceptor agonists used as veterinary sedatives. In Short C E, Van Poznak A V (eds) Animal pain, Churchill Livingstone, New York: 181-191 [ Links ]
34. MacDonald E, Scheinin H, Scheinin M 1988 Behavioural and neurochemical effects of medetomidine, a novel veterinary sedative. European Journal of Pharmacology 158: 119-127 [ Links ]
35. Malik V, Singh B 2008 Effect of midazolam supplementation on ketamine anaesthesia in butorphanol-xylazine premedicated horses. Indian Journal of Animal Sciences 78: 486-488 [ Links ]
36. Marshall B E, Wollman H 1980 General anaesthetics. In Gilman A G, Goodman L S, Gilman A (Eds) Goodman and Gillman's pharmacological basis of therapeutics (6th edn). Macmillan Publishing, New York: 276-299 [ Links ]
37. Mirakhur K K, Khanna A K, Prasad B 1984 Diazepam as a sedative in calves. Agriculture Practice 5: 29-32 [ Links ]
38. Mohammad F K, Zangana I K, Abdul-Latif A R 1993 Medetomidine sedation in sheep. Journal of Veterinary Medicine Series A 40: 328-331 [ Links ]
39. Muge D K, Chambers J P, Livingston A 1994 Analgesic effect of medetomidine in sheep. Veterinary Record 135:43-44 [ Links ]
40. Orr H E, Roughan J V, Flecknell P A 2005 Assessment of ketamine and medetomidine anaesthesia in the domestic rabbits. Veterinary Anaesthesia and Analgesia 32: 271-279 [ Links ]
41. Pawde A M, Amarpal, Singh G R, Kumar N 1996 Clinicophysiological effects of medetomidine in female goats. Small Ruminant Research 20: 95-98 [ Links ]
42. Peshin P K, Nigam J M, Mirakhur K K 1985 Cardiovascular and respiratory changes in sheep during thiopentone and halothane anaesthesia. Indian Journal of Animal Sciences 55: 91-97 [ Links ]
43. Ponder S W, Clarke W G 1980 Prolonged depression of thermoregulation after xylazine administration to cats. Journal of Veterinary Pharmacology and Therapeutics 3: 203-207 [ Links ]
44. Pratap K, Amarpal, Aithal H P, Singh G R 1997 Clinical evaluation of different preanaesthetics in goats: An experimental study. Indian Veterinary Journal 74: 897-898 [ Links ]
45. Pypendop B H, Verstegen J P 1998 Haemodynamic effects of medetomidine in the dog: a dose titration study. Veterinary Surgery 27: 612-622 [ Links ]
46. Reid J, Nolan A M, Welch E 1993 Propofol as an induction agent in the goat: a pharmacokinetic study. Journal of Veterinary Pharmacology and Therapeutics 16:488-493 [ Links ]
47. Riazuddin M, William B J, Ameerjan K 2004 Studies on halothane, isoflurane anaesthesia in dorsal and lateral recumbency in cattle. Indian Journal of Veterinary Surgery 25: 75-76 [ Links ]
48. Ruffolo R R, Nichols A J, Stadel J M 1993 Pharmacological and therapeutic applications of alpha-2 adrenoceptor subtypes. Annual Review of Pharmacology and Toxicology 32: 243-279 [ Links ]
49. Rugh K S, Zinn G M, Paterson J A, Thome J G 1985 Inhalant anaesthetics in cattle. Laboratory Animal Science 35: 178-181 [ Links ]
50. Ruskoaho H 1986 Subtypes and functions of alpha adrenoceptors. Acta Veterinaria Scandinavica 82: 17-28 [ Links ]
51. Sakaguchi M, Nishimura R, Sasaki N, Ishiguro T, Tamura H, Takeuchi A 1995 Chemical restraint by medetomidine ketamine and its cardiopulmonary effects in pigs. Journal of Veterinary Medicine A 42: 293-299 [ Links ]
52. Short C E, Keats A S, Liotta D, Hall C W 1968 Anaesthesia for cardiac surgery in calves. American Journal of Veterinary Research 29: 2287-2294 [ Links ]
53. Siegel S, Castellan J J 1988 Non-parameteric statistics for behavioural sciences. Mc Graw Hill, Singapore [ Links ]
54. Sinclair M D 2003 A review of the physiological effects of alpha-2 agonists related to the clinical use of medetomidine in small animal practice. Canadian Veterinary Journal 44: 885-897 [ Links ]
55. Singh H 1988 Studies on diazepam, chloral hydrate and halothane anaesthesia in relation to different surgical operations in bovine. M.V.Sc. thesis, Punjab Agriculture university, Ludhiana, India [ Links ]
56. Singh J, Mirakhur K K, Sobti V K, Kohli R N 1980 Haemodynamics, blood gas and metabolic alterations during thiopentone anaesthesia in buffaloes. Zentralblatt für Veterinärmedizin 27A: 730-739 [ Links ]
57. Singh R, Peshin P K, Patil D B, Sharda R, Singh J, Singh A P, Sharifi D 1994 Evaluation of halothane as an anaesthetic in camels. Journal of Veterinary Medicine Series A 41: 359-368 [ Links ]
58. Singh S 2007 Evaluation of thiopentalketamine/propofol with premedicants i.e. Atropine, diazepam/midazolam-butorphanol in canine orthopaedic patients. MVSc thesis, Deemed University, I.V.R.I., Izatnagar, (UP), India [ Links ]
59. Singh V, Amarpal, Kinjavdekar P, Aithal H P, Pratap K 2005 Medetomidine with ketamine and bupivacaine for epidural analgesia in buffaloes. Veterinary Research Communications 29: 1-18 [ Links ]
60. Snedecor G W, Cochran W G 1980 Statistical methods (9th edn). Iowa State University, Press, Ames [ Links ]
61. Steffey E P, Howland D 1979 Halothane anaesthesia in calves. American Journal of Veterinary Research 40: 372-376 [ Links ]
62. Steffey E P, Howland D 1978 Cardiovascular effect of halothane in horses. American Journal of Veterinary Research 39: 611-615 [ Links ]
63. Stegmann G F 1999 Observations on the use of some cardiopulmonary effects of midazolam, xylazine and midazolam-ketamine combination in the goats. Journal of the South African Veterinary Association 70: 122-126 [ Links ]
64. Stegmann G F, Bester L 2001 Some clinical effects of midazolam premedication in propofol-induced and isoflurane-maintained anaesthesia in dogs. Journal of the South African Veterinary Association 72: 214-216 [ Links ]
65. Stoelting P K 1991 Inhaled anaesthetics. In Pharmacology and physiology in anaesthetic practice (2nd edn). Lippincott, Philadelphia: 33-69 [ Links ]
66. Tverskoy M, Fleyshman G, Bradley E L, Kissin L 1988 Midazolam-thiopental anaesthetic interaction in patients. Anaesthesia and Analgesia 67: 342-345 [ Links ]
67. Vainio O 1991 Propofol infusion anaesthesia in dogs premedicated with medetomidine. Journal of Veterinary Anaesthesia 18: 35-37 [ Links ]
68. White R J, Bark H, Bali S 1986 Halothane anaesthesia in the dromedary camel. Veterinary Record 119: 615-617 [ Links ]
Received: May 2010.
Accepted: December 2010
* Author for correspondence. E-mail: vickeyvet@gmail.com














