Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.81 n.4 Pretoria Dec. 2010
CLINICAL COMMUNICATION KLINIESE MEDEDELING
Steroid-sparing effect of mycophenolate mofetil in the treatment of a subepidermal blistering autoimmune disease in a dog
P J GinelI,*; B Blanco,; R LucenaI; C R JiménezII; C Peinado-GuitartIII; E MozosII
IDepartamento de Medicina y Cirugía Animal, Universidad de Córdoba, Campus de Rabanales, 14014 Córdoba, Spain
IIDepartamento de Anatomía y Anatomía Patológica Comparadas, Universidad de Córdoba, Cordoba, Spain
IIIClínica Veterinaria Fray Isidoro de Sevilla, Seville, Spain
ABSTRACT
A 7-year-old female Cocker spaniel-cross was referred with an 8-month history of mucocutaneous erosive dermatitis. On physical examination, skin lesions affected the eyelids and periocular area, lips and vulva. Lesions were symmetrical with small diffuse superficial ulcers, haemorrhagic crusts, adherent purulent exudation in haired skin, and alopecia with hyperpigmentation and scarring. Histopathologic evaluation showed multiple, non-intact dermoepidermal junction vesicles and ulceration associated with a dermal lichenoid infiltrate. Immunohistochemistry showed strong to moderate reactivity in the dermoepidermal junction for the antibodies directed against canine IgG, human IgG lambda light chains and C3, respectively. A diagnosis of autoimmune subepidermal blistering dermatosis was made. Treatment with oral prednisone at 2 mg/kg and mycophenolate mofetil (MMF) at 20 mg/kg twice daily was initiated and after 4 weeks the ulcers and erosions were cured. During the rest of treatment, MMF was maintained at 10 mg/kg twice daily and prednisone could be tapered to 0.25 mg/kg once every other day without recurrences. In conclusion, this case report shows that MMF was well tolerated and might be effective as steroid-sparing agent in the long-term treatment of this autoimmune subepidermal blistering disease.
Keywords: blistering dermatosis, canine, mycophenolate mofetil.
INTRODUCTION
Bullous skin diseases are characterised by the formation of clefts, leading to vesicles or bullae. Separation most commonly occurs between the dermis and epidermis and results from loss of structural integrity of the basement membrane zone. Subepidermal bullous diseases (SEBD) have been described mostly in the dog. Although their clinical presentation is variable, it is not possible histopathologically to identify a specific disease beyond the term 'subepidermal blistering or bullous'. Immunological studies for detection of in situ and circulating autoantibodies directed against basement membrane specific antigens are required for a definitive diagnosis6.
Bullous pemphigoid (BP), mucous membrane pemphigoid (MMP), epidermolysis bullosa acquisita (EBA), linear IgA disease and bullous drug eruptions are SEBD described in the dog6. However, because of the lack of appropriate immunologic techniques, SEBD in dogs were all diagnosed as BP until 1998, when the 1st case of canine EBA was reported14. Soon, linear IgA disease was described in 2 dogs11, and MMP was established as the most common autoimmune disease of the basement membrane zone in the dog and cat12,13.
Conventional therapy of SEBD in humans and dogs is troublesome. Corticosteroids are combined with immunosuppressants such as azathioprine and cyclophosphamide but treatments may be unsuccessful and side effects associated with high corticosteroid doses are common. In humans, there are no randomized, controlled, double-blind studies comparing the use of various therapeutic agents. Treatment decision still relies heavily on the experience of individual clinicians5. Mycophenolate mofetil (MMF) is an immunosuppressive drug that selectively inhibits purine synthesis primarily in lymphocytes (T and B-cells) and has been used for human MMP and other autoimmune diseases9.A retrospective study evaluated the effectiveness and toxicity of immunosuppressive therapies in the treatment of 338 human ocular MMP cases. The therapies based on MMF were the second-most successful after those based on cyclophosphamide and the best tolerated with the least side effects of all therapies used16.
In this case report the use of MMF as steroid sparing agent in a dog diagnosed with SEBD is described. The combined use of MMF and prednisone allowed good control of the cutaneous lesions for more than 1 year follow-up with a minimal dose of oral prednisone (0.25 mg/kg e.o.d.) and MMF (10 mg/kg b.i.d.).
CASE HISTORY
A 7-year-old entire female Cocker spaniel-cross with an 8-month history of mucocutaneous erosive dermatitis was referred for dermatological examination. There was no previous history of systemic or dermatological diseases. The dog lived indoors and there were no other pets in the house. Onset of clinical signs was not associated with deworming agents, vaccines or any other medication. Initial lesions consisted of painful and mildly pruritic, small bilateral ulcers and erosions affecting eyelids and vulva. Previous diagnostic tests had included haemogram, serum biochemistry and Leishmania serum titres were all within the normal reference range. The dog had previously been treated with a rather low dose of oral generic cephalexin (15 mg/kg b.i.d.) for 3 weeks with no response, followed by topical betamethasone administration that provided some improvement of the vulvar lesions, but new deeper ulcerative lesions developed affecting the medial canthus of both eyelids. The dog was then treated with several courses of prednisone (1 mg/kg b.i.d.) for the previous 4 months, with partial improvement of vulvar lesions while the periocular lesions continued to worsen. Finally, prednisone was tapered to 0.5 mg/kg s.i.d. before the patient was referred to the University hospital.
On physical examination the dog had a normal body condition and, apart from skin lesions, appeared to be in good health. Detailed dermatological examination showed skin lesions associated with mucocutaneous sites consisting of erosions and small ulcers affecting especially the eyelids and periocular area, lips and vulva. Facial lesions were symmetrical and from the lower eyelid extended rostrally affecting the periocular haired skin, which showed haemorrhagic crusts, small scattered superficial ulcers and a arciform band of alopecic and hyperpigmented skin that had apparently healed. This atrophic area was bordered by a similarly shaped area that was inflamed, hypopigmented and covered by a strongly adherent purulent exudate. The dog also had bilateral keratoconjuctivitis and severe blepharospasm that was diagnosed as keratoconjuctivitis sicca (KCS). Differential diagnosis included demodicosis, dermatophytosis, mucocutaneous pyoderma, allergic dermatitis, pemphigus foliaceus and skin blistering diseases such as pemphigus vulgaris, BP, MMP, EBA and linear IgA disease. Skin scrapings were negative for demodex mites and fungi. Cytology from the centre and borders of the ulcerated lesions showed non-degenerated neutrophils and mononuclear cells. As the owner was reluctant to stop treatment, prednisone (0.5 mg/kg s.i.d.) was maintained and a new visit scheduled in 2 weeks. At that time, lesions had worsened and the area of facial alopecia was greatly extended. The periocular and adjacent facial skin were severely inflamed and showed multiple discrete haemorrhagic crusts and punctate ulcers (Fig. 1).

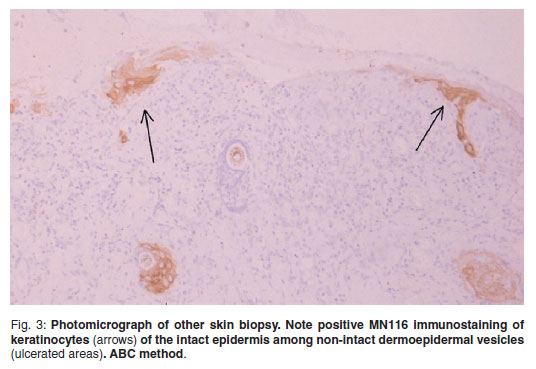
Four 8 mm diameter samples of periocular skin, 2 from each side, were taken by punch biopsy, fixed in 10 % neutral buffered formalin and processed routinely to paraffin wax by standard techniques. Serial sections, 3-4 micrometres thick, were stained with haematoxylin and eosin, Giemsa and periodic acid-Schiff (PAS). Histopathologic evaluation showed predominantly chronic lesions consisting of dermoepidermal clefts and multiple non-intact dermoepidermal junction vesicles and secondary ulceration (Fig. 2). Reactive hyperplastic epidermis was observed among the clefts or ulcerated areas. The outer dermis was covered by cell debris, erythrocytes and fibrinosuppurative exudate. An infiltrate composed of neutrophils, abundant plasma cells, lymphocytes, macrophages and occasional eosinophils was present at the dermis and was arranged in a lichenoid pattern closely associated with the dermoepidermal junction (Figs2 & 3). There was a dermal fibrovascular reaction consisting of marked hyperaemia and moderate fibroplasia in chronic ulcerated lesions. A morphological diagnosis of vesiculobullous subepidermal autoimmune disease was made. Differential diagnoses included BP, MMP, EBA and linear IgA dermatosis. To confirmthe histopathologicical diagnosis of SEBD, immunohistochemical stains were performed by a routine avidin-biotin-peroxidase complex method (Vector) previously described elsewhere15. The primary antibodies used, their dilutions and sources were the following: rabbit anti-human-IgG lambda light chains, (catalogue number A0194, polyclonal antibody; dilution: 1/800, Dako; Glostrup, Denmark); sheep anti canine IgG (catalogue number: ARB-55850, polyclonal, dilution 1/200, Dako; Glostrup, Denmark) mouse anti-human C3 (catalogue number F0201, polyclonal antibody; dilution: 1/100, Sigma; Madrid, Spain) and mouse anti human pan-cytokeratins, clone MNF116 (catalogue number M0821, monoclonal antibody recognizing cytokeratins5, 6, 8, 17; dilution 1/50, Dako; Glostrup, Denmark). Tissue sections were incubated with either normal rabbit serum for the polyclonal antibodies or mouse ascites fluid for monoclonal antibodies as negative controls. Normal canine skin and human lymph nodes were used as positive controls. The immunohistochemical study showed that monoclonal antibody MNF116 reacted with the keratinocytes of the intact epidermis, delineating areas of early non-intact vesicles (Fig. 3) that produced multiple small ulcers. These findings supported the morphological diagnosis of subepidermal vesiculation. Moreover, strong to moderate reactivity was found for the antibodies directed against canine IgG, human IgG lambda light chains and C3 respectively, associated with the dermoepidermal junction of the vesicles, as well as the adjacent dermal superficial microvasculature, plasma cells and macrophages (Fig. 4A, B). Unfortunately, further immunological testing, including identification of targeted antigens to differentiate among SEBD, could not be performed.


Once the diagnosis of autoimmune SEBD was confirmed, treatment with oral prednisone (2 mg/kg b.i.d.), and MMF (20 mg/kg b.i.d.; Cellcept, Roche Farma) was prescribed to control the progression of facial scarring. After 4 weeks there was visible improvement of the lesions. The ulcers and erosions had healed almost completely, leaving a large area of cicatricial skin.
The dosage of prednisone was reduced to 1 mg/kg b.i.d. and, to avoid the onset of diarrhoea and haemorrhagic enteritis, the dosage of MMF was also reduced to 10 mg/kg b.i.d.. No side effects associated with MMF treatment were observed. Blood cell counts and serum biochemical analysis performed after this 1st month of MMF administration only showed typical corticosteroid-associated changes. During the following months MMF was maintained at the same dosage and, after 6 months of treatment, the prednisone dosage had been tapered to 0.25 mg/kg e.o.d. On 2 occasions, several months apart, the dog developed new skin lesions but they were solitary, very localised and healed without changing the therapy. By contrast to the dermatological lesions, the KCS was refractory to its specific treatment, which included ophthalmic cyclosporine (Optimmune; Schering-Plough, Spain), artificial tears and several ophthalmic glucocorticoid solutions. After 12 months of follow up, the skin lesions seemed to be well controlled, although the area of periocular and facial scarring was evident (Fig. 5). The dog was lost to follow up 4 months later, 16 months after initiating the treatment and after 10 months on the low prednisose dose. MMF therapy continued to be well tolerated, no new lesions had been observed and due to owner's reluctance and lack of corticosteroid side effects no further reduction in the prednisone dosage was attempted.

DISCUSSION
In this report a case of SEBD associated with keratoconjunctivitis and severe facial scarring in a dog and its successful treatment with prednisone and MMF as steroid sparing agent is described. After slowly tapering prednisone, over 6 months, to a very low dose on alternate days, the skin lesions remained well controlled for another 10 months when the dog was lost to follow-up. During that time, prednisone therapy could probably have been discontinued if the keratoconjuctivitis, which was of much concern to the owner, had responded to its specific treatment or if clinical signs of iatrogenic hyperadrenocorticism had been present.
Clinical differential diagnoses for SEBD in the dog include BP, MMP, EBA, linear IgA dermatosis and bullous drug eruptions6. Precise differentiation among these dermatoses requires demonstration of circulating autoantibodies and their targeted antigens in the basement membrane zone. Other vesiculobullous dermatosis such as pemphigus foliaceus, pemphigus vulgaris, systemic lupus erythematosus and vesicular cutaneous lupus erythematosus of Collies and Shetland sheepdogs could be ruled out clinically or histopathologically. Clinically, EBA was unlikely since lesions never involved footpads or other skin pressure points7. Linear IgA disease is a very rare condition and in the 2 cases that have been described previously, lesions were more generalised11. In the case reported here, BP and MMP are the 2 SEBD more likely involved.
Bullous pemphigoid is a very rare vesiculobullous subepidermal autoimmune skin and mucosal disease of both dogs and cats. The wide variation in clinical presentation of formerly reported cases probably indicates that multiple SEBD were identified incorrectly as BP6. In fact, BP has been confirmed immunologically in only 7 patients10. In these dogs, lesions of BP consisted of macules, vesicles or bullae, ulcers and crusts that first occurred on the head, ears and trunk. Paw pads were rarely affected and although mucosal or mucocutaneous junctional sites were involved in 4 dogs (57 %), oral lesions were less common and none of these dogs had periocular involvement. In the case described here, oral and paw pad lesions were not detected as is common in BP, but eyelid and periocular involvement and the distribution of lesions restricted or close to mucocutaneous junctions clinically makes a diagnosis of BP less likely and are more suggestive of MMP.
Mucous membrane pemphigoid is the most common autoimmune disease of the basement membrane zone in the dog12, 13. In humans and dogs with MMP, vesicles progress to ulcers that often heal with scars, and lesions predominate on mucosae and at mucocutaneous junctions, while the haired skin is affected only occasionally5, 13. In this case, skin lesions initiated at mucocutaneous junctions were erosive and ulcerative when they involved haired skin and produced hypopigmented macules and distinctive adherent crusts12, 13. In previously reported cases of MMP, cicatricial lesions were observed in some but not all dogs affected12. By contrast, facial atrophic scarring was prominent in this case, probably worsened by the chronicity and seemed to progress very slowly despite adequate control of inflammation, as has been described in human MMP16.
In human MMP, the oral mucosa is the most common site affected, followed by ocular disease with the possibility of progressive corneal scarring and subsequent blindness5. After the onset of the skin lesions, this dog developed severe bilateral KCS refractory to specific ophthalmic treatment in spite of optimal owner compliance. Although corneal involvement has not been described in previous cases of canine MMP, ocular MMP is a frequent form of the disease in human patients. However, because a conjunctival biopsy could not be obtained, the possible association between cutaneous and ocular lesions could not be ascertained. If this had been the case, response to the MMF treatment would not have been as good as has been reported in human ocular MMP16.
The diagnosis of SEBD is based on histopathology and immunofluorescence. Direct immunofluorescent (DIF) testing is both sensitive and specific for the diagnosis of MMP and is considered the gold standard for diagnosing MMP. The presence of immunoreactants, most commonly IgG, IgA or C3, deposited in a linear fashion along the epithelial/subepithelial basement membrane zone of a mucous membrane, is thought to be diagnostic for MMP5. Multiple vesicles and bullae at the dermoepidermal junction, with an intact epidermis as in BP, or with retention of the irregular or peg-like contour of the epidermis are the main histopathological features of early lesions in MMP6. Moreover, intact vesicles frequently appear devoid of inflammatory cells12.Inthe case reported here, non-intact vesicles and bullae were always observed in the dermoepidermal junction and the superficial dermis was covered by a variable quantity of cell debris, plasma exudates and inflammatory cells. The immunohistochemical study showed IgG, IgGlambda chains and C3 deposition along the superficial dermis which was consistent previously reported results using DIF11. Using the antibody MNF116, the normal epidermis delineating vesicles already non-intact could be clearly demonstrated. In our opinion, because the skin samples were taken 6-8 months after the onset of the 1st lesions, the histopathological pattern was more chronic and complex compared with what has been described in earlier stages of the disease6, 12, and did not allow a clear distinction between MMP or BP. As in all the SEBD, further immunological testing including identification of targeted antigens are required for differentiation. Unfortunately, further biopsies and dog serum were not available to identify the specificity of the autoantibodies.
Immunossupressive drugs are used as steroid sparing agents in the management of blistering autoimmune diseases. In the dog reported here, the combined use of MMF and prednisone allowed good control of the cutaneous lesions and the dosage of oral prednisone could be reduced to 0.25 mg/kg e.o.d. with no reccurrences over 10 months. Thus MMF might be a useful steroid-sparing agent for the long-term treatment of canine SEBD. Mycophenolate mofetil is a relatively new immunosuppressant of low toxicity which is being increasingly utilised in veterinary medicine. It was developed as an organ transplant drug, with toxicity studies performed originally on dogs. The active metabolite is mycophenolic acid, which inhibits de novo synthesis of purines (guanine). As B and T lymphocytes depend exclusively upon guanosine synthesis, while other cells can use the salvage guanosine synthesis pathway, the cellular inhibitory effect is specific. Thus, apart from T and B lymphocytes, MMF does not target other cell populations2, 8. The therapeutic benefit of MMF has been demonstrated in various human skin disorders, particularly in psoriasis and pemphigus vulgaris, and some authors consider MMF to be the most important candidate for the replacement of azathioprine in transplantation medicine2.
In dogs, the major side effect associated with MMF administration is dose-dependent diarrhoea, which is usually prevented by decreasing the initial 20 mg/kg twice daily dosage by 50 % after the 1st month of treatment. However, some dogs may exhibit soft stools with traces of blood as early as 6 days after receiving MMF4. In this case, MMF was well tolerated and no gastrointestinal or haematological signs were seen during the 1st month of treatment or after the 50 % decrease in the initial MMF dosage. In human patients, the more common side effects are nausea, gastritis and abdominal cramping. Haematological complications have been reported but occur less often than with azathioprine2, and MMF is considered to lack significant myelosuppression, nephrotoxicity or hepatotoxicity, even in children1.
One concern with this new drug may be its cost. For a 10-kg dog, the cost of treatment is about 2.4 euros daily during the 1st month and half that amount for the rest of the treatment, a cost similar to alternative therapies such as cyclosporin.
In conclusion, this case report shows that MMF can be recommended as a steroid-sparing agent for the long-term treatment of a canine SEBD, most probably BP or MMP. The drug was well tolerated in trials and, depending on the dog's size, the cost of the treatment is not high, making MMF a suitable alternative to other immunosuppressive drugs used in veterinary dermatology.
REFERENCES
1. Alkali A S, Bharati A, Yesudian P D, Parslew R A G, Field A 2007 Childhood mucous membrane pemphigoid: successful control with mycophenolate mofetil monotherapy. British Journal of Dermatology 156: 1406 [ Links ]
2. Assmann T, Ruzicka T 2002 New immunosuppressive drugs in dermatology (mycophenolate mofetil, tacrolimus): unapproved uses, dosages, or indications. Clinics in Dermatology 20: 505-514 [ Links ]
3. Chan L S 2001 Mucous membrane pemphigoid. Clinics in Dermatology 19: 703-711 [ Links ]
4. Chanda S M, Sellin J H, Torres C M, Yee J P 2002 Comparative gastrointestinal effects of mycophenolate mofetil capsules and enteric-coated tablets of sodium-mycophenolic acid in beagle dogs. Transplantation Proceedings 34: 3387-3392 [ Links ]
5. Daniel E, Thorne J E 2008 Recent advances in mucous membrane pemphigoid. Current Opinion in Ophthalmology 19: 292-297 [ Links ]
6. Gross T L, Ihrke P J, Walder E J, Affolter V K (eds) 2005 Bullous and acantholytic disease of the epidermis and the dermal-epidermal junction. In Skin diseases of the dog and cat. Clinical and histopathologic diagnosis. Blackwell Science, Oxford: 27-48 [ Links ]
7. Hill P B, Boyer P, Lau P, Rybnicek J, Hargreaves J, Olivry T 2008 Epidermolysis bullosa acquisita in a great Dane. Journal of Small Animal Practice 49: 89-94 [ Links ]
8. Marzano A V, Dassoni F, Caputo R 2006 Treatment of refractory blistering autoimmune diseases with mycophenolic acid. Journal of Dermatology Treatments 17: 370-376 [ Links ]
9. Megahed M, Schmiedeberg S, Becker J, Ruzicka T 2001 Treatment of cicatricial pemphigoid with mycophenolate mofetil as a steroid-sparing agent. Journal of the American Academy of Dermatology 45: 256-259 [ Links ]
10. Olivry T, Dunston S M, Fahey M, Nguyen N, Marinkovich M P 2000 Autoantibodies against the processed ectodomain of collagen XVII (BPAG2, BP180) define a canine homologue of linear IgA disease of humans. Veterinary Pathology 2000, 37, 302-309 [ Links ]
11. Olivry T, Dunston S M, Schachter M, Luting X, Nguyen N, Marinkovich P, Chan L S 2001 A spontaneous canine model of mucose membrane (cicatricial) pemphigoid, an autoimmune blistering disease affecting mucosae and mucocutaenous junctions. Journal of Autoimmunity 16: 411-421 [ Links ]
12. Olivry T, Dunston S M, Zhang G, Ghohestani R F 2002 Laminin-5 is targeted by autoantibodies in feline mucous membrane (cicatricial) pemphigoid. Veterinary Immunology and Immunopathology 88: 123-129 [ Links ]
13. Olivry T, Fine J, Dunston S M, Chase D, Tenorio A, Monteiro-Riviere N A, Chen M, Woodley D T 1998 Canine epidermolysis bullosa acquisita: circulating autoantibodies target the aminoterminal non-collagenous (NC1) domain of collagen VII in anchoring fibrils. Veterinary Dermatology 9: 19-31 [ Links ]
14. Olivry T 2003 Natural bullous pemphigoid in companion animals. In Chan L S (ed.) Animal models of immune dermatoses. CRC Press, Boca Raton: 201-211 [ Links ]
15. Pérez J, Méndez A, de Lara F C, MartÍn M P, Mozos E 1997 Ovine squamous cell carcinoma: immunocharacterisation of neoplastic cells and peritumoural celular infiltrate. Research in Veterinary Science 63: 43-47 [ Links ]
16. Saw V P, Dart J K, Rauz S, Ramsay A, Bunce C, Xing W, Maddison P G, Phillips M 2008 Immunosuppressive therapy for ocular mucous membrane pemphigoid strategies and outcomes. Opthalmology 115: 253-261 [ Links ]
Received: July 2010.
Accepted: October 2010.
* Author for correspondence. E-mail: pginel@uco.es














