Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the South African Veterinary Association
versión On-line ISSN 2224-9435
versión impresa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.81 no.3 Pretoria sep. 2010
ARTICLE ARTIKEL
Diversity and seasonal occurrence of Eimeria species in a mixed flock of communally reared sheep and goats in Mafikeng in the North West Province, South Africa
F R Bakunzi; S N Thwane; L E Motsei*; B M Dzoma
Centre for Animal Health Studies, North West University (Mafikeng), Private Bag X2046, Mmabatho, 2735 South Africa
ABSTRACT
Diversity and seasonal occurrence of coccidia in a communally reared mixed flock of sheep and goats at Mafikeng, North West Province, South Africa, was determined between March 2008 and February 2009. Faecal specimens were collected directly from the rectum of the animals and the number of oocysts per gram of faeces (opg) determined. The mean monthly opg for goats was significantly higher than that for sheep. Higher oocyst counts were observed during the hot, rainy season than during the cold, dry season. The highest mean values for both the sheep (862.5 opg) and goats (1200 opg) were recorded during March. Six species (Eimeria crandallis, E. bakuensis, E. weybridgensis, E. ahsata, E. intricata, and E. ovinoidalis) were recovered from sheep, with E. crandallis and E. bakuensis occurring most frequently. The last 2 species, together with E. ahsata, are considered among the most pathogenic species in sheep. In goats, 7 species (E. arloingi, E. jolchijevi, E. caprina, E. alijevi, E. caprovina, E. christenseni and E. hirci) were recovered, with E. arloingi and E. jolchijevi occurring most frequently. Up to 5 Eimeria species were recovered from individual specimens in goats while up to 4 were recovered in sheep. No cross-infections between goats and sheep were recorded and no clinical coccidiosis was noted during the study. It is increasingly becoming evident that the pathogenic E. arloingi is one of the most commonly occurring Eimeria species in goats in South Africa.
Keywords: communal sheep, diversity, Eimeria species, goats, seasonal occurrence.
INTRODUCTION
Coccidiosis in small ruminants, caused by host-specific Eimeria species10,isof economic and medical importance. Infections of sheep and goats, involving both normal and diseased individuals5,9,22, have been observed in almost all rearing systems worldwide. Clinical coccidiosis is a major contributor to enteric disease of sheep and goats, occurs mainly in young animals, and has higher prevalence under conditions of intensive husbandry and various stress factors3,20. Even though coccidiosis may prove fatal, its greater economic importance lies in the unthriftiness and lowered productivity that it causes17. Fifteen and 16 Eimeria species have been described from sheep and goats, respectively15,20. Knowledge of the prevalence of Eimeria species in a flock helps to minimise economic losses, and to evaluate infection potential and control programmes24. Coccidial infections of small stock have been reported from several African countries, including Botswana14, Kenya7,11, Nigeria23, South Africa5,6, Tanzania9 and Zimbabwe2. Except for a few reports5,6,16, there is a general paucity of information on the species and prevalence of coccidiosis in sheep and goats in South Africa, however, more so the communally reared ones. The objectives of this study were therefore to determine the diversity and seasonal occurrence of coccidial oocysts in a communal, mixed flock of sheep and goats in Mafikeng.
MATERIALS AND METHODS
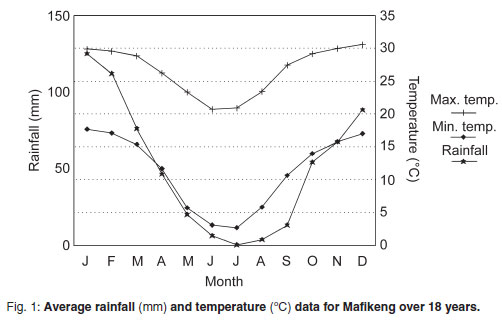
The study was conducted at the North West University teaching farm in Mafikeng (25º49'S, 25º36'E), in the North West Province of South Africa from March 2008 to February 2009. Mafikeng has a typical semi-arid savanna climate, with a long dry season extending from May to October. The mean monthly minimum temperatures vary from 2.7 ºC in July to 17.7 ºC in January, while mean maximum temperatures vary from 20.7 ºC in June to 30.6 ºC in December. The mean monthly meteorological data for Mafikeng over the last 18 years are presented in Fig. 1.
Thirty-two each of adult (>1 year) Dorper sheep and Boer goats were used in this study. The animals were housed together in a partially roofed enclosure (15 × 15 m ) that had a dirt floor. The animals were kraaled at night and let out by day to graze on communal rangelands where they mixed freely with indigenous goats, sheep and cattle.
Faecal samples from goats and sheep were collected directly from the rectum of the animals at monthly intervals. The faecal samples were transported to the laboratory on ice and if not analysed immediately were stored at 4 ºC. The modified McMaster technique was performed to determined the number of oocysts per gram (opg) of faeces18. For species identification, faecal samples were collected directly from the rectum of 10 each of sheep and goats, and immediately sent to the laboratory for processing. At the laboratory, the samples were allowed to sporulate, after which they were processed and species identification performed as previously described6.
RESULTS
Over the 12-month period, 768 faecal specimens were collected, 384 from sheep and 384 from goats. Six species, namely E. crandallis (100 %), E. bakuensis (100 %), E. weybridgensis (60 %), E. ahsata (40 %), E. intricata (20 %) and E. ovinoidalis (20 %) were recovered from the sheep. Seven species were recovered from goats, namely E. arloingi (80 %), E. jolchijevi (80 %), E. caprina (40 %), E. alijevi (40 %), E. christenseni (20 %), E. caprovina (20 %) and E. hirci (20 %). Up to 5 Eimeria species were recovered from individual specimens in goats, while up to 4 were recovered from sheep. No cross-infections between goats and sheep were recorded.
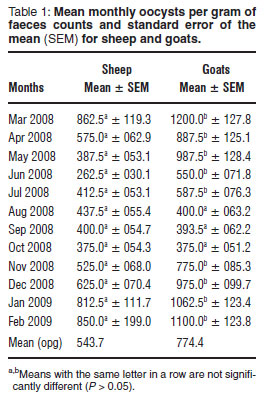
Overall mean opg counts in sheep and goats were 544 and 774, respectively (Table 1). Statistical analysis revealed significantly (P < 0.05) higher levels in goats than in sheep. The highest individual count for sheep was 9000 opg, compared to 13 200 opg for goats. Except for the preiod August-October 2008, when opg counts were generally low, monthly mean opg counts for goats were significantly (P < 0.005) higher than those of sheep. In both sheep and goats, the opg counts followed a seasonal pattern. Opg decreased from March till August and remained low until October. Counts then started rising gradually until February (Table 1). The lowest mean values for sheep (263 opg) and goats (375 opg) were recorded in June and October, respectively, while the highest mean counts for both species were recorded in March, at 863 opg and 1200 opg, respectively. No evidence of clinical coccidiosis was noted during the study.
DISCUSSION
Six and 7 Eimeria species were recovered from sheep and goats, respectively, with no cross-infections occurring. This was not surprising since Eimeria species are known to be host specific10. Fifteen and 16 species of Eimeria have been recorded in sheep and goats, respectively4,20, of which 6 and 7 species, respectively, were identified in this study. The most frequently occurring species in sheep were E. crandallis and E. bakuensis, which together with
E. ahsata, are also the most pathogenic species in sheep8,19. This implies higher risk of coccidiosis in sheep around Mafikeng when other predisposing factors exist. In a study on coccidiosis of sheep on commercial farms in South Africa16, an additional 3 species to those identified around Mafikeng, namely E. parva, E. faurei, and E. granulosa were identified. The current results also compare well with those recorded in Tanzania9 and Jordan1, with most of the species being similar. In goats, 7 species were identified, with E. arloingi and E. jolchijevi being the most frequently occurring. Eimeria arloingi is regarded as one of the most pathogenic species in goats together with E. christenseni and E. ninakohlyakimovae10,13, again indicating the possible high risks for goats around Mafikeng. In a similar study in an area about 300 km east of Mafikeng, E. arloingi was also the most frequently occurring species followed by E. hirci, which occurred less frequently in this study6. Another study in South Africa identified all the species in the current study except E. caprovina16. Based on the current study and others conducted in South Africa6,16, it appears E. arloingi is among the most commonly occurring Eimeria species of goats in South Africa.
Sheep excreted significantly fewer oocysts than goats in this study. This was in agreement with studies in Kenya, other parts of South Africa and Tanzania, where the mean opg counts for goats were higher than those of sheep5,9,11. In both sheep and goats, higher oocyst counts were observed during the months of January to March, peaking in March (Table 1), most likely owing to the heavy rains and higher temperatures which occur in these months (Fig. 1) that favour oocyst sporulation18,21. A study performed in southern Botswana, however, revealed no obvious seasonal patterns in the faecal levels of coccidial oocysts in goats14.
No evidence of clinical coccidiosis was found in this study. Previous studies have shown that Eimeria oocysts are widely present in the faeces of both normal and diseased individuals9.
In conclusion, goats had higher oocyst counts than sheep, while the highest counts for both species of small stock occurred during the hot, wet season than during the dry, cold season. Six different species of Eimeria were recovered from sheep, while 7 species were recovered from goats. Up to 5 Eimeria species were recovered from individual specimens in goats while up to 4 were recovered in sheep. No cross-infections between goats and sheep were recorded for all Eimeria species. It is increasingly becoming evident that E. arloingi is one of the most common Eimeria species in goats in South Africa.
ACKNOWLEDGEMENTS
The authors are grateful to the National Research Foundation (NRF-South Africa) and the North-West University for financing this study. Technical staff at the Centre for Animal Health Studies are thanked for their assistance during sample collection and analysis.
REFERENCES
1. Abo-Shehada M N, Abo-Farieha H A 2003 Prevalence of Eimeria species among goats in northern Jordan. Small Ruminant Research 49: 109-113 [ Links ]
2. Chhabra R C, Pandey V S 1991 Coccidia of goats in Zimbabwe. Veterinary Parasitology 39: 199-205 [ Links ]
3. Craig T M 1986 Epidemiology and control of coccidia in goats. Veterinary Clinics of North America. Food Animal Practice 2: 389-395 [ Links ]
4. Foreyt W J 1986 Epidemiology and control of coccidia in sheep. Veterinary Clinics of North America. Food Animal Practice 2: 283-388 [ Links ]
5. Harper C K, Penzhorn B L 1998 Seasonal occurrence of coccidia in a mixed herd of sheep and goats at Nebo, Northern Province of South Africa. Journal of the South African Veterinary Association 69: 93-94 [ Links ]
6. Harper C K, Penzhorn B L 1999 Occurrence and diversity of coccidia in indigenous Saanen and crossbred goats in South Africa. Veterinary Parasitology 82: 1-9 [ Links ]
7. Kanyari P W N 1993 The relationship between coccidial and helminth infections in sheep and goats in Kenya. Veterinary Parasitology 51: 137-141 [ Links ]
8. Kaufmann J 1996 Parasitic infections of domestic animals: a diagnostic manual. Birkhäuser, Basel [ Links ]
9. Kusiluka L J M, Kambarage D M, Matthewman R W, HarrisonLJS, Daborn C J 1996 Coccidiosis of small ruminants in Tanzania. Small Ruminant Research 21: 127-131 [ Links ]
10. Levine N D 1986. The protozoan phylum Apicomplexa Vol. 2. CRC Press, Boca Raton, Florida [ Links ]
11. Maingi N, Munyua W K 1994 The prevalence and intensity of infection with Eimeria species in sheep in the Nyandarua district of Kenya. Veterinary Research Communications 18: 19-25 [ Links ]
12. Matjila P T, Penzhorn B L 2002. Occurrence and diversity of bovine coccidia at 3 localities in South Africa. Veterinary Parasitology 104: 93-102 [ Links ]
13. MoA (Ministry of Agriculture, Fisheries and Food) 1986 Manual of veterinary parasitological laboratory techniques (3rd edn). Reference Book 418. Her Majesty's Stationery Office, London [ Links ]
14. Nsoso S J, Machete J B, Molatole M, Ndebele R T, Lebani N N, Chabo R G, Kalake A M, Jacyna L, Segadimo B W, Mine O M 2001 The impact of traditional management on seasonal internal parasite burdens and productivity of indigenous Tswana goats in southern Botswana. Onderstepoort Journal of Veterinary Research 68: 101-104 [ Links ]
15. Platzer B, Prsol H, Cieslicki M, Joachim A 2005 Epidemiology of Eimeria infections in an Austrian milking sheep flock and control with diclazuril. Veterinary Parasitology 129: 1-9 [ Links ]
16. Qwalela B P, Jooste R, Page P C 2009 Prevalence of Eimeria species in domestic bovines, ovines and caprines on commercial farms in South Africa. World Association for the Advancement of Veterinary Parasitology, Calgary, Canada, 9-14 August 2009: 190 [ Links ]
17. Radostits O M, Blood D C, Gray C C 1994 Veterinary medicine (6th edn). Baillière Tindall, London [ Links ]
18. Reinecke R K 1983 Veterinary helminthology. Butterworths, Durban/Pretoria [ Links ]
19. Rommel M 2000 Protozoeninfektionen der Wiederkäuer (Rind, Schaf, Ziege), Eimeriose (Coccidiose). In Rommel M, Eckert J, Kutzer E, Körting W, Schnieder T (eds) Veterinärmedizinische Parasitologie (5th edn). Paul Parey, Berlin: 133-149 [ Links ]
20. Smith M C, Sherman D M 1994 Goat medicine. Lea & Febiger, Philadelphia [ Links ]
21. Soulsby E J L 1982 Helminths, arthropods and protozoa of domesticated animals (7th edn). Baillière Tindall and Cassell, London [ Links ]
22. Taylor M A, Catchpole J 1994 Coccidiosis of domestic ruminants. Applied Parasitology 35: 73-86 [ Links ]
23. Who A Y, Little D A, Ikwuegbu O A 1994 Prevalence of coccidial infections in the West African Dwarf goat in the subhumid zone of Nigeria. Tropical Animal Health and Production 26: 1-6 [ Links ]
24. Yakhchali M, Golami E 2008 Eimeria infection (Coccidia: Eimeriidae) in sheep of different age groups in Sanandaj city, Iran. Veterinarski Archiv 78: 57-64 [ Links ]
Received: November 2009.
Accepted: July 2010.
* Author for correspondence. E-mail: lebogang.motsei@nwu.ac.za














