Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.81 n.1 Pretoria Jan. 2010
CLINICAL COMMUNICATION KLINIESE MEDEDELING
Description of the pathology of a gazelle that died during a major outbreak of foot-and-mouth disease in Israel
A BerkowitzI; T WanerII; R KingIII; H YadinIV; S PerlV
IKoret School of Veterinary Medicine, The Hebrew University of Jerusalem, PO Box 12, Rehovot, Israel
IIIsrael Institute for Biological Research, PO Box 19, Ness Ziona, Israel
IIIIsrael Nature & National Parks Protection Authority, Jerusalem, Israel
IVVirology Department, Kimron Veterinary Institute, Beit Dagan, Israel
VPathology Department, Kimron Veterinary Institute, Beit Dagan, Israel
ABSTRACT
Naturally occurring foot-and-mouth disease (FMD) in wildlife is a relatively mild condition but occasionally it can be devastating as has been documented in impala in South Africa and in mountain gazelles in Israel. This report describes pathological changes in an adult male gazelle with FMD from an outbreak in the Nature Reserve of Ramot-Issachar region and the lower Galilee in Israel. The outbreak was characterised by the malignant form of the disease, which is uncommon among domestic animals. Lesions observed included, ulceration in the oral cavity, oesophagus and ruminal pillars, coronitis, multifocal cardiac necrosis and pancreatic necrosis and inflammation. Pneumonia, caused by Muellerius capillaries was an incidental finding.
Keywords: foot-and-mouth disease, Gazella gazelle gazelle, mountain gazelle.
INTRODUCTION
Foot-and-mouth disease (FMD) is a highly contagious viral disease known since the 16th century1. The disease affects a wide variety of cloven-hoofed animals. The disease is present in Israel and sporadic outbreaks are dependent on regional epidemiological circumstances9. In most domesticated livestock 2 major clinical manifestations are encountered: a relatively benign form characterised by mucosal vesicles and ulcerations, accompanied by a reduction in milk yield and loss of appetite. This form is further characterised by high morbidity and relatively low mortality involving adult animals. The other major manifestation is a malignant form that usually affects young animals, is characterised by extensive necrosis in various tissues and is accompanied by high mortality3.
Among wildlife, as is often the case in domestic livestock, naturally occurring FMD is a relatively mild disease from which animals recover in 1-2 weeks10. However FMD disease can occasionally be devastating to wildlife as has been documented in South Africa in impala (Aepyceros melampus)5 and in mountain gazelles (Gazella gazelle gazelle) in Israel6. During the spring and summer of 2007 a major outbreak of FMD occurred among mountain gazelles in northern Israel. Both young and adult animals were affected. In this outbreak the disease manifested as the malignant clinical form, similar to a previous outbreak in mountain gazelles described in 19886. The present article describes the pathological findings in a gazelle that succumbed to FMD during the outbreak in 2007.
CASE HISTORY
A male mountain gazelle was brought to the wild animal quarantine clinic (Israel Wildlife Hospital, Afek, Rosh Ha'ayin) in a moribund condition and died a few hours later. The FMD outbreak in which this animal was involved occurred in a nature reserve in the Ramot-Issachar region and the lower Galilee highlands. A total of 81 mountain gazelles (constituting about 10-15 % of the population) with suspected FMD were found; 46 of these were dead and 35 were sick. Most of the latter also succumbed.
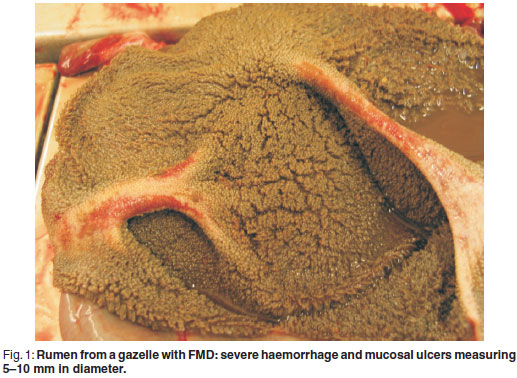
At necropsy, ulcers of 2-5 mm in diameter were found on the lips, gums, tongue and oesophageal lumen. Severe hyperaemia and examples of sloughing of the skin were seen on the coronary bands. On the ruminal pillars, severe haemorrhage and mucosal ulcers measuring 5-10 mm in diameter were observed (Fig. 1). Chronic cranio-ventral pneumonia was evident with multifocal granulomatous foci. Multifocal epicardial petechiae with multifocal wide pale myocardial striation (tiger heart) were present.
Microscopical pathological findings
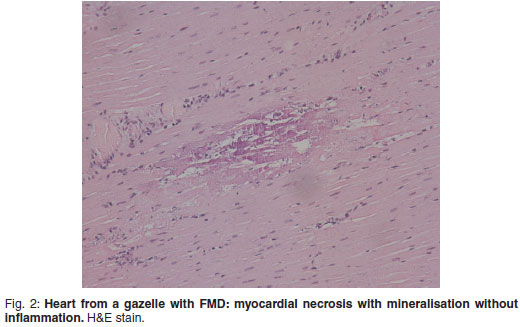
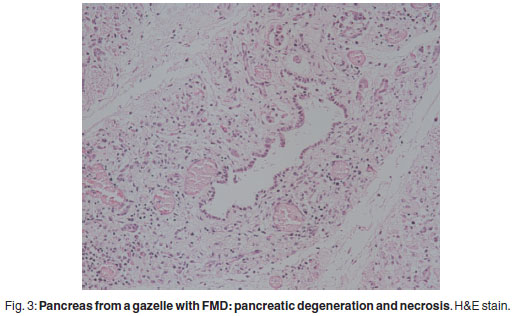
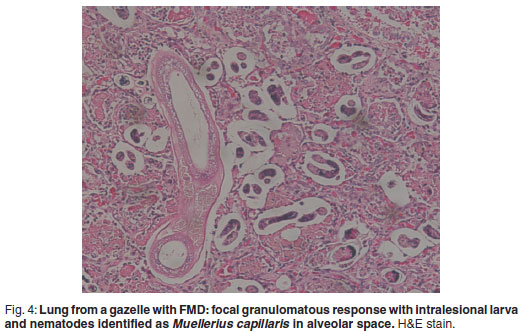
There was prominent multifocal necrosis and mineralisation of the cardiac muscle fibres with foci of mineralisation (Fig. 2). A few neutrophils were found scattered in the necrotic foci. Degenerative changes were present at the periphery of the necrotic foci. In the pancreas there was multilobular necrosis of exocrine acini as well as necrosis of endocrine cells (Fig. 3). In most lobules only acinar ducts could still be visualised. In the remaining acini the exocrine cells appeared to be smaller than normal with scant cytoplasm and loss of zymogen granules. Small to moderate numbers of histiocytes, plasma cells and lymphocytes and small numbers of neutrophils were seen scattered throughout the pancreatic tissue. Mild fibrosis and multifocal petechial haemorrhages were also evident. Moderate amounts of fibrin and oedema fluid were present in acini and between lobules. There were scattered foci of peripancreatic fat necrosis and saponification. Bronchioalveolar pneumonia was characterised by diffuse infiltration of neutrophils and macrophages. There was also diffuse alveolar oedema with moderate active congestion in the affected areas of the lungs. Intralesional adult nematodes (Muellerius capillaris) and their larvae and eggs were present in the alveoli (Fig. 4).
The serotype of the FMD virus responsible for the present outbreak was identified and classified by the FMD laboratory of the Virology division of the Kimron Veterinary Institute, Beit Dagan, Israel. Tongue epithelium homogenate from the necropsied mountain gazelle was cultured for FMD virus on secondary pig kidney cells and injected into newborn mice. The virus type was identified by the ELISA test as FMD type O18.
DISCUSSION
FMD is a disease affecting all cloven-hoofed animals3. It is caused by a virus that belongs to the genus Aphthovirus in the family Picornaviridae. The virus can be spread aerogenously, by fomites or by animal products (especially bone marrow, lymph nodes and viscera). It may survive on hay and other fomites for several weeks. Cattle, sheep, goats and the African buffalo (Syncerus caffer) can remain carriers for up to 2 years. Pigs are not carriers of the virus.
The virus enters and multiplies in the pharynx and lungs, followed by viraemic dissemination to surface epithelium with subsequent lesion development at sites of mechanical or physiological stress such as oral and pedal epithelium and the teats of lactating animals. The virus probably gains entry to these areas via Langerhans cells with replication in a contiguous group of cells in the stratum spinosum3. The result is cellular degeneration, lysis and the formation of epidermal vesicles. Characteristic lesions develop on the oral mucosa, tongue, interdigital cleft and teats. In cattle loss of weight and hypersalivation are also common signs. Other clinical features include buccal hyperaemia and mild catahrral stomatitis, vesicle formation on the lips, cheeks, gums, hard palate, dental pad and rostral dorsum of the tongue. Similar lesions were seen in the case presented here. Primary vesicles coalesce to produce 5-6 mm vesicles that eventually rupture, ulcerate and may be complicated by secondary infection.
In young animals, and occasionally in adults, there is a tendency to develop the malignant lethal form of the disease. This includes necrotising myocarditis that appears as pale, poorly-defined foci within ventricular muscle (referred to as tiger heart). Chronic lesions include inflammation and scarring of the myocardium.
Pancreatitic acinar necrosis, inflammation and regeneration is another manifestation of the malignant form of FMD and diabetes mellitus has been observed in both experimental and natural disease1. The virus is known to replicate in pancreatic cells of mice4,7.
This outbreak of FMD in 2007 was the 2nd documented in mountain gazelles. As in the 1st outbreak adults commonly developed the malignant form of the disease, which is uncommon among domestic and other wild animals6. Findings in the 1st outbreak included myocardial necrosis, interdigital and oral vesiculation, ruminal pillar ulceration and pancreatic necrosis. The mortality rate was high. The heart lesions seen in the present case are typical of the malignant form of the disease, frequently encountered in young animals. The same applies to the muco-vesiculo-ulcerative lesions which are frequently encountered in adults with the malignant form. It is less common to see both forms appear simultaneously, especially in adults. The changes seen in the pancreas correlate with the fact that the virus has been demonstrated to replicate in pancreatic cells and its presence has been demonstrated in experimentally-inoculated bovines2. The infestation with lung worms is quite frequently encountered among wild ruminants and is usually acknowledged as an incidental finding.
Outbreaks causing high mortality rates in adult mountain gazelles, while the virus exists endemically among domestic animals, suggests that mountain gazelles may be an especially vulnerable species to FMD virus and may serve as a useful indicator of the status of the virus in endemic areas.
REFERENCES
1. Barker I K, Van Dreumel A A, Palmer N. Foot-and-mouth disease In Jubb K V F, Kennedy P C, Palmer N (eds) 1992 Pathology of domestic animals. Academic Press, San Diego: 141-144 [ Links ]
2. Bergmann I E, Malirat V, Auge de Mello P, Gomes I 1996 Detection of foot-and-mouth disease viral sequences in various fluids and tissues during persistence of the virus in cattle. American Journal of Veterinary Research 57:134-137 [ Links ]
3. Brown C C, Baker D C, Barker I K 2007 Alimentary system. In Maxie M G (ed.) Jubb, Kennedy and Palmer's pathology of domestic animals (5th edn). Saunders, Edinburgh: 135-137 [ Links ]
4. Jones A L 1965 Growth of foot and mouth disease virus in organ cultures of mouse pancreas. Nature 207:665-666 [ Links ]
5. Keet D F, Hunter P, Bengis R G, Bastos A, Thomson G R 1996 The 1992 foot-andmouth disease epizootic in the Kruger National Park. Journal of the South Africa Veterinary Association 67:83-87. [ Links ]
6. Perl S, Yadin H, Yakobson B, Zuckerman E, Orgad U 1989 Pathological changes in mountain gazelles challenged with FMD virus, with special reference to pancreatic lesions. Revue Scientifique et Technique 8:765-769 [ Links ]
7. Portiansky E L, Gonzalez P H 1995 Protective effect of lidocaine in the experimental foot-and-mouth disease pancreatitis. Experientia 51:1060-1062 [ Links ]
8. Roeder P L, Le Blanc Smith P M 1987 The detection and typing of foot-and-mouth disease virus by enzyme-linked immunosorbent assay: a sensitive, rapid and reliable technique for primary diagnosis. Research in Veterinary Science 43:225-232 [ Links ]
9. Shimshony A, Orgad U, Baharav D, Prudovsky S, Yakobson B, Bar Moshe B, Dagan D 1986 Malignant foot-and-mouth disease in mountain gazelles. Veterinary Record 119:175-176 [ Links ]
10. Thomson G R, Vosloo W, Bastos A D 2003 Foot and mouth disease in wildlife. Virus Research 91:145-161 [ Links ]
Received: June 2009.
Accepted: February 2010.
* Author for correspondence. E-mail: wanertnt@gmail.com














