Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.81 n.1 Pretoria Jan. 2010
ARTICLE ARTIKEL
Occurrence of Theileria parva and other haemoprotozoa in cattle at the edge of Hluhluwe-iMfolozi Park, KwaZulu-Natal, South Africa
S B A S YusufmiaI; N E CollinsI; R NkunaI; M TroskieI; P Van den BosscheI,II; B L PenzhornaI,*
IDepartment of Veterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort, 0110 South Africa
IIDepartment of Animal Health, Institute of Tropical Medicine, Antwerp, Belgium
ABSTRACT
Theileria parva, the most important bovine theilerial species in sub-Saharan Africa, causes widespread mortality and morbidity in endemic areas. A survey was conducted using buffy-coat specimens from 60 apparently healthy adult communally herded Nguni-type cattle at the northeastern edge of the Hluhluwe-iMfolozi Park to determine, by means of PCR and Reverse Line Blot (RLB) hybridisation, the occurrence of Theileria and Babesia species. The presence of Trypanosoma species was determined using PCR-RFLP. Results showed that 6.7 % of the specimens were positive for Theileria parva. This significant finding suggests that cattle in South Africa, and not only African buffaloes (Syncerus caffer), may be subclinical carriers of T. parva. Other species identified were T. mutans (83.3 %), T. velifera (70.0 %), Theileria sp. (sable) (46.8 %) and T. taurotragi (1.7 %). Two specimens (3.3 %) were positive for Babesia bovis and single specimens (1.7 %) positive for B. bigemina and B. rossi, respectively. Mixed infections, of up to 4 species, were common (65.0 %). Only 1 specimen was found to be positive for Trypanosoma vivax, and 2 for T. theileri, of which only the first species is pathogenic.
Keywords: Babesia bigemina, Babesia bovis, Babesia rossi, cattle, Hluhluwe-iMfolozi Park, Theileria mutans, Theileria parva, Theileria taurotragi, Theileria velifera, Theileria sp. (sable), Trypanosoma theileri, Trypanosoma vivax, wildlife-livestock interface.
INTRODUCTION
Theileriosis, a tick-transmitted protozoan disease, is a major constraint for cattle production in the tropics and subtropics12. Theileria parva is the most important species in sub-Saharan Africa, while T. annulata occurs further northwards and eastwards. Three subspecies of T. parva were previously recognised: T. parva parva, causing classical East Coast fever (ECF)16; T. parva lawrencei, causing Corridor disease17; and T. parva bovis, causing Zimbabwe theileriosis or January disease15. This subdivision has been abandoned34. East Coast fever is an acute, usually fatal, disease that is transmitted between infected cattle and susceptible cattle by the 3-host ixodid tick, Rhipicephalus appendiculatus16. Corridor disease is an acute disease transmitted principally from asymptomatic carrier African buffalo (Syncerus caffer) to susceptible cattle by R. appendiculatus as well as by R. zambeziensis and possibly by R. duttoni17. Zimbabwe theileriosis is usually a milder form of the disease, with transmission by R. appendiculatus between infected and susceptible cattle15.
ECF was introduced to South Africa during 1901-1903 by cattle imported from Kenya and Tanzania for restocking after the ravages of rinderpest and the Anglo-Boer War14. It is estimated that 1.25 million of the 4 million cattle in the affected area had died of ECF by 1914. The disease was eradicated from southern Africa during a 50-year campaign consisting of movement control, tick control, destocking of infected pastures and slaughtering of infected herds16. The vector was not eradicated, however, and the original buffalo-associated T. parva remained endemic in South African and Zimbabwean buffalo populations40.
Corridor disease is an acute, usually fatal disease of cattle resembling ECF17.It is caused by infection with T. parva strains from African buffaloes, the only wild ruminant species that is a carrier of the causative organism36. The first outbreak in South Africa occurred in the corridor between the then Hluhluwe and Umfolozi Game Reserves in KwaZulu-Natal (now incorporated in the Hluhluwe-iMfolozi Park), shortly after ECF had been eradicated27. The disease occurs sporadically wherever cattle are in contact with infected African buffaloes and in the presence of the vector ticks. In areas where cattle are raised, acceptance of buffaloes for conservation or recreation is a major constraint because of Corridor disease17. Buffaloderived T. parva is universally distributed in wild buffaloes in southern Africa, except in the Addo Elephant National Park and some other game reserves in non-endemic areas where T. parva-free animals have been translocated30.In contrast to the situation in buffaloes, erythrocytic piroplasms in cattle are usually absent or too scanty to infect ticks, so the disease is usually self-limiting in cattle. The distinct seasonality of R. appendiculatus, which has a 2-year life cycle in southern Africa38, and transstadial transmission of T. parva also play a major role in this regard. The possibility of T. parva from buffalo transforming under natural conditions in South Africa to T. parva causing ECF has always been a highly contentious topic, as it places the movement of buffaloes under severe constraints36.
In South Africa, the Kruger National Park and Hluhluwe-iMfolozi Park are regarded as endemic areas for buffaloderived T. parva. The South African Animal Disease Act (Act 35 of 1984) stipulates the following controlled veterinary acts to be performed in outbreaks of Corridor disease: Susceptible animals: Contact between cattle and African buffaloes shall be prevented; all cattle in a controlled area shall be dipped or sprayed regularly by the responsible person with an efficient remedy; no animal shall be chemotherapeutically treated without the written authorisation of the director. Contact animals shall be isolated and dipped or sprayed with an efficient remedy under the supervision of an officer or an authorised person in the manner and at the intervals determined by the responsible state veterinarian. Infected animals shall be isolated and dipped or sprayed with an efficient remedy under the supervision of an officer or an authorised person in the manner and at the intervals determined by the responsible state veterinarian. In contrast to the regulations pertaining to ECF, slaughter of infected animals is therefore not enforced, on the assumption that infected animals, which may not be treated, will succumb to the disease and will not become subclinical carriers.
The campaign to control the ECF epidemic in South Africa by intensive regulatory control of R. appendiculatus had a major effect on single-host ticks, including Rhipicephalus (Boophilus) spp., ensuring that bovine babesiosis remained of secondary importance for many decades. In the latter part of the 20th century, relaxation or breakdown of tick-control measures and the advent of acaricide resistance in Rhipicephalus (Boophilus) spp., caused bovine babesiosis to increase in prevalence and significance to the point where it became recognised as one of the most important livestock diseases in South Africa7,8.
Trypanosomosis is a complex, debilitating and often fatal disease caused by infection with one or more of the pathogenic tsetse fly-transmitted protozoan parasites of the genus Trypanosoma5. The most important species responsible for the disease complex, commonly known as 'nagana' in livestock, include Trypanosoma brucei, T. congolense and T. vivax1.Two tsetse fly species, Glossina austeni and G. brevipalpis, persisted in KwaZulu-Natal despite eradication attempts13.
A number of molecular, biochemical and immunological tools are available that enable differentiation between Theileria species4. Early molecular detection techniques involved the use of probes to detect repetitive regions in parasite genomic deoxyribonucleic acid (DNA). The polymerase chain reaction (PCR) assay has largely superseded DNA probes. PCR has a higher sensitivity and specificity for detecting parasite DNA in blood. False positives may occur, however, due to contamination. As it is not always possible to detect mixed infections with PCR, a reverse line blot (RLB) hybridisation assay targeting the 18S ribosomal ribonucleic acid (rRNA) gene has been developed for simultaneous identification and differentiation of distinct piroplasm species present in the same sample11. The RLB has proven to be a very powerful tool in detecting subclinical infections. It also gives an indication of new species or genotypes possibly being present in the sample32.
Bovine trypanosomosis has been shown to be prevalent in anaemic cattle in the vicinity of the Hluhluwe-iMfolozi Park41. In a subsequent survey, blood specimens were taken randomly from healthy cattle in the same area to determine the occurrence of Trypanosoma species. This offered the opportunity to determine the occurrence of other haemoparasites, especially Theileria parva, in these cattle. The RLB was used for detection of Theileria species in these buffy-coat specimens and PCR-RFLP was used to determine occurrence of Trypanosoma species.
MATERIALS AND METHODS
Specimen collection
Specimens were collected during March 2006 from 60 randomly selected, adult, apparently healthy, communallygrazed Nguni-type cattle from various herds adjacent to the northeastern edge of the Hluhluwe-iMfolozi Park. The total number of cattle in the area was not known. Jugular blood was collected in Vacutainer® tubes coated with ethylenediamine- tetra-acetic acid (EDTA) as anticoagulant. Micro-capillary tubes were filled with blood from the Vacutainer tubes and centrifuged for 5 min at 9000 r.p.m. A diamond-tipped pen was used to cut the capillary tubes immediately above the buffy coat, and the buffy coat was extruded onto filter paper (Whatman no. 3). The buffy-coat-spotted filter paper was dried, sealed in a plastic bag containing silica gel and stored at -20 ºC until processed further at the Molecular Biology Laboratory, Department of Veterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria.
DNA extraction
DNA was extracted from 60 buffy coat filter papers (at least 3 disks, 3 mm in diameter per sample) using QIAamp DNA Mini kit (QIAGEN®), according to the manufacturer's instructions. DNA was eluted in 150 µl of AE buffer and stored at 4 ºC.
Polymerase Chain Reaction (PCR) for Theileria and Babesia species
Primers RLB F2 (5'-GAC ACA GGG AGG TAG TGA CAA G-3') and biotinlabeled RLB R2 (5'-Biotin-CTA AGA ATT TCA CCT CTA ACA GT-3)' were used for amplification of the V4 hypervariable region of Theileria and Babesia 18S rRNA genes11. Reactions were performed in a final volume of 25 µ with Platinum Quantitative PCR Super mix-UDG (Invitrogen), 0.25 µ
with Platinum Quantitative PCR Super mix-UDG (Invitrogen), 0.25 µ of each primer (20 pmol) and 2.5 µ
of each primer (20 pmol) and 2.5 µ of purified DNA. A touchdown PCR programme was conducted in the Gene Amp PCR system 9700. The cycling conditions were as follows: 3 min at 37 ºC; 10 min at 94 ºC; and 10 cycles of 94 ºC for 20 s, 67 ºC for 30 s, 72 ºC for 30 s, with decreasing annealing temperature after every second cycle by 2 ºCfor 5 times until the annealing temperature reached 57 ºC. Finally 40 cycles of 94 ºC for 20 s, 57 ºC for 30 s and 72 ºC for 30 s were performed. Babesia bovis was used as positive control, while bovine DNA was used as negative control.
of purified DNA. A touchdown PCR programme was conducted in the Gene Amp PCR system 9700. The cycling conditions were as follows: 3 min at 37 ºC; 10 min at 94 ºC; and 10 cycles of 94 ºC for 20 s, 67 ºC for 30 s, 72 ºC for 30 s, with decreasing annealing temperature after every second cycle by 2 ºCfor 5 times until the annealing temperature reached 57 ºC. Finally 40 cycles of 94 ºC for 20 s, 57 ºC for 30 s and 72 ºC for 30 s were performed. Babesia bovis was used as positive control, while bovine DNA was used as negative control.
Reverse Line Blot (RLB) hybridisation
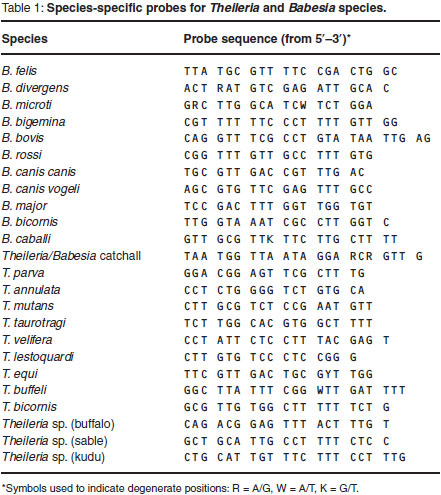
The PCR products were analysed using the RLB hybridisation technique11,28,29.A Theileria and Babesia genus-specific oligonucleotide probe and 23 species-specific probes (Table 1) were included on the membrane.
PCR for Trypanosoma species
The primers used for the amplification of Trypanosoma species were as described10. The first amplification was done using the forward primer 18ST nF2 (5'-CAA CGA TGA CAC CCA TGA ATT GGG GA-3') and 18ST nR3 (5'-TGC GCG ACC AAT AAT TGC AAT AC-3') as reverse primer. A semi-nested second amplification was done using 18ST nF2 with the reverse primer 18ST nR2 (5'GTG TCT TGT TCT CAC TGA CAT TGT AGT G-3').
RFLP for differentiation of Trypanosoma species
The RFLP analysis of the 18S rRNA PCR products was performed10. Briefly, a 6 µ aliquot of amplified DNA was digested with Msp1 and Eco571 restriction enzymes in buffer Y+/Tango with S-adenosylmethionine according to the manufacturer's specifications (Gibco, UK) using 0.6 U of each enzyme per µ
aliquot of amplified DNA was digested with Msp1 and Eco571 restriction enzymes in buffer Y+/Tango with S-adenosylmethionine according to the manufacturer's specifications (Gibco, UK) using 0.6 U of each enzyme per µ of PCR product in 15 µ
of PCR product in 15 µ total volume. The digestion was incubated overnight in a water bath at 37 ºC. Restriction products were resolved on a 10 % polyacrylamide gel together with a 100 bp DNA ladder (MBI Fermentas, Lithuania) for fragment size determination. DNA fragments were separated by horizontal electrophoresis in 0.5× TBE buffer at 100 V for 2.5 h. The gel was stained using Sybr Green I nucleic acid gel stain (Roche diagnostics)10.
total volume. The digestion was incubated overnight in a water bath at 37 ºC. Restriction products were resolved on a 10 % polyacrylamide gel together with a 100 bp DNA ladder (MBI Fermentas, Lithuania) for fragment size determination. DNA fragments were separated by horizontal electrophoresis in 0.5× TBE buffer at 100 V for 2.5 h. The gel was stained using Sybr Green I nucleic acid gel stain (Roche diagnostics)10.
RESULTS
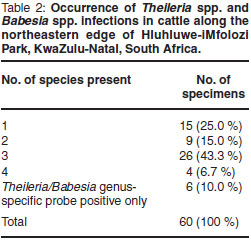
Haemoprotozoa DNA could be extracted from all specimens. Occurrence of Theileria spp., Babesia spp. and Trypanosoma spp. is summarised in Fig. 1. Theileria parva occurred in 4 specimens (6.7 %). Theileria mutans (83.3 %) and T. velifera (70.0 %) were the most prevalent Theileria species. These were also the only haemoparasites occurring as single-species infections: 10 specimens (16.7 %) were infected with T. mutans only, and 5 specimens (8.3 %) with T. velifera only. Occurrence of singlespecies and multiple-species infections is shown in Table 2. Confirmed multiplespecies infections occurred in 39 (65 %) of the specimens. Six specimens (10 %) were positive for the Theileria/Babesia genus-specific probe only; no hybridisation occurred to species-specific probes on the RLB membrane (Table 2).
DISCUSSION
Four specimens (6.7 %) were positive for T. parva. As ECF no longer occurs in South Africa, we assume that this result indicates the presence of buffalo-associated T. parva. Specimens were obtained from apparently healthy cattle only, which strongly suggests that these cattle are subclinical carriers of buffalo-associated T. parva.
Corridor disease has been regarded as self-limiting in cattle because they usually die before the parasite develops to the piroplasm stage, which is infective to ticks30. Experimental evidence suggests that some bovines survive the disease, however, and may serve as reservoirs of infection15. Furthermore, South Africa is considered free of T. parva, except in designated Corridor disease-infected areas including and bordering the Kruger National Park and Hluhluwe-iMfolozi Park. Therefore the national herd is essentially naïve and completely susceptible to T. parva39.
These results and those of a study on another cattle population39, suggest that cattle could be subclinical carriers of T. parva parasites in South Africa. A study should be carried out to determine whether these parasites could serve as a source of infection to disease-transmitting ticks. These findings would be very important, as they could indicate that current control regulations in South Africa may have to be revised. The assumption that only buffaloes are carriers of the parasite, and thus merely preventing contact with buffaloes is an appropriate management strategy, may no longer be tenable. Therapeutic treatment of Corridor disease is not allowed in South Africa as recovered intact cattle could develop a carrier status and could become infective to ticks35. If carriers are present and cattle-to-cattle transmission occurs, it could lead to the selection of a subpopulation of T. parva parasites that are better adapted to cattle as host. If this were to happen in South Africa, as is speculated is the case in East Africa2, it would impact negatively on the cattle industry.
The high prevalence of T. mutans infection (83.3 %) was not really surprising, as it has been shown that virtually all calves in endemic areas may be infected by the time they are 6 months old26. African buffalo also harbour T. mutans3,33 and there is field and experimental evidence suggesting that strains of T. mutans from buffalo may be more pathogenic to cattle than those derived from cattle20. The prevalence of T. velifera, which is also known to infect buffalo37, was also quite high (70.0 %). This piroplasm is generally regarded as apathogenic in cattle21. Theileria taurotragi, which is mildly pathogenic in cattle but has been associated with cerebral theileriosis (so-called turning disease) was initially described from eland Taurotragus oryx9,18,19,22. The high prevalence of Theileria sp. (sable) (46.8 %) is interesting. This species, which has been reported from cattle in Tanzania, is incriminated in causing mortalities in sable antelope (Hippotragus niger) and possibly roan antelope (Hippotragus equinus)29, neither of which occurs in Hluhluwe-iMfolozi Park. It has also been reported from African buffalo, blue wildebeest (Connochaetes taurinus), klipspringer (Oreotragus oreotragus) and reedbuck (Redunca arundinum)29, all of which occur in Hluhluwe-iMfolozi Park. A closely related Theileria species has been reported from dogs in KwaZulu-Natal25.
Two of the specimens were positive for B. bovis, indicating a degree of the additional challenge the herd may be exposed to. A third sample was positive for B. bigemina. This extremely low prevalence of Babesia spp. indicates effective tick control in the area.
One of the specimens was positive for B. rossi. This parasite had previously only been found in canids23,24,31 and thus this result may have been due to contamination. With PCR product contamination, however, more than 1 false positive result would have been expected. Accidental transmission of parasites to aberrant hosts is possible. These parasites may survive and be detected, although it is unlikely that the parasite would be able to reproduce and cause disease in the accidental host. It is possible that accidental transmission could have produced a positive result. Theileria equi, which had not been described previously as a parasite of canids, has been found in asymptomatic dogs6. It was concluded that infection of dogs with T. equi is not unlikely to happen and that molecular methods can lead to a correct identification of piroplasmids in accidental hosts. Similarly, in this study, accidental transmission of B. rossi in cattle probably occurred.
A fairly large percentage (10 %) of the specimens were positive for the Theileria/Babesia genus-specific probe only, but no hybridisation occurred to species-specific probes on the RLB membrane. This could indicate that there may be novel Theileria or Babesia species in cattle or variants of species present in these specimens. Alternatively, it could indicate the presence of a known species for which no probe was included on the blot. Not all probes that are specific for all the Theileria and Babesia species that could occur in cattle were used in this study; for example, B. occultans was not included. Alternatively, the concentration of species-specific DNA may have been too low to be detected in the hybridisation reaction. Sequence analysis could be carried out on these specimens that hybridised only to the Theileria and Babesia genus-specific probe to determine the nature of these specimens.
Two of the 60 specimens were positive for Trypanosoma theileri and 1 was positive for T. vivax. Results of a previous survey of the same area showed that bovine trypanosomosis is prevalent in the vicinity of the Hluhluwe-iMfolozi Park41. According to that survey, in which anaemic animals had been selected, 60.5 % of the specimens were positive for Trypanosoma congolense41. Trypanosoma vivax is one of the species responsible for causing nagana, while Trypanosoma theileri (subgenus Megatrypanum) is generally regarded as non-pathogenic.
In summary, we determined occurrence of haemoprotozoa in 60 cattle at the wildlife/livestock interface in northern KwaZulu-Natal. The most important finding was that 4 cattle (6.7 %) were infected with T. parva. The presence of subclinical T. parva carriers, which were also recently found in another cattle population39 in KwaZulu-Natal, would suggest that the premises on which current Corridordisease-control strategies in South Africa are based may have to be reassessed.
This paper emanates from project V015/08, approved by the Research Committee of the Faculty of Veterinary Science and the Animal Use and Care Committee of the University of Pretoria and funded by National Research Foundation grant GUN 44403 to BL Penzhorn.
ACKNOWLEDGEMENTS
Dr Dirk Geysen is thanked for supplying specimens and Ms Raksha Bhoora is thanked for her assistance in the laboratory.
REFERENCES
1. Anene B M, Onah D N, Nawa Y 2001 Drug resistance in pathogenic African trypanosomes: What hopes for the future? Veterinary Parasitology 96:83-100 [ Links ]
2. Barnett S F, Brocklesby D W 1966 The passage of Theileria lawrencei (Kenya) through cattle. British Veterinary Journal 122:396-409 [ Links ]
3. Brocklesby D W 1965 A new theilerial parasite of the African buffalo (Syncerus caffer). Bulletin of Epizootic Diseases of Africa 13:325-330 [ Links ]
4. Collins N E, Allsopp M T E P, Allsopp B A 2002 Molecular diagnosis of theileriosis and heartwater in bovines in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 96:217-224 [ Links ]
5. Connor R J, Van den Bossche P 2004 African animal trypanosomoses. In Coetzer J A W, Tustin R C (eds) Infectious diseases of livestock (2nd edn). Oxford University Press Southern Africa, Cape Town: 251-296 [ Links ]
6. Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero J C 2003 Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Part 1. Epizootiological aspects. Veterinary Parasitology 113:189-201 [ Links ]
7. de Vos A J 1979 Epidemiology and control of bovine babesiosis in South Africa. Journal of the South African Veterinary Association 50:357-362 [ Links ]
8. de Vos A J, de Waal D T, Jackson L A 2004 Bovine babesiosis. In Coetzer J A W,Tustin R C (eds) Infectious diseases of livestock (2nd edn). Oxford University Press Southern Africa, Cape Town: 406-424 [ Links ]
9. Flanagan H O, le Roux J M W 1957 Bovine cerebral theileriosis: a report on two cases occurring in the Union. Onderstepoort Journal of Veterinary Research 27:453-461 [ Links ]
10. Geysen D, Delespaux V, Geerts S 2003 PCR-RFLP using SSU-rDNA amplification as an easy method for species-specific diagnosis of Trypanosoma species in cattle. Veterinary Parasitology 110:171-180 [ Links ]
11. Gubbels J M, de Vos A P, van der Weide M, Viseras J, Schouls L M,de Vries E, Jongejan F 1999 Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. Journal of Clinical Microbiology 37:1782-1789 [ Links ]
12. Jongejan F, Uilenberg G 1994 Ticks and control methods. Revue Scientifique et Technique, Office International des Épizooties 13:1201-1226 [ Links ]
13. Kappmeier K, Nevill E M, Bagnall R J 1998 Review of tsetse and trypanosomosis in South Africa. Onderstepoort Journal of Veterinary Research 65:195-203 [ Links ]
14. Lawrence J A 1992 History of bovine theileriosis in southern Africa. In Norval R A I, Perry B D, Young A S (eds) The epidemiology of theileriosis in Africa. Academic Press, London: 1-31 [ Links ]
15. Lawrence J A, de Vos A J, Irvin A D 1994 Zimbabwe theileriosis. In Coetzer J A W, Thompson G R, Tustin R C (eds) Infectious diseases of livestock with special reference to southern Africa, Vol. 1. Oxford University Press, Cape Town: 326-328 [ Links ]
16. Lawrence J A, Perry B D, Williamson S M 2004 East Coast fever. In Coetzer J A W, Tustin R C (eds) Infectious diseases of livestock (2nd edn). Oxford University Press Southern Africa, Cape Town: 448-463 [ Links ]
17. Lawrence J A, Perry B D, Williamson S M 2004 Corridor disease. In Coetzer J A W, Tustin R C (eds) Infectious diseases of livestock (2nd edn). Oxford University Press Southern Africa, Cape Town: 468-471 [ Links ]
18. Lawrence J A, Williamson S M 2004 Turning sickness. In Coetzer J A W, Tustin R C (eds) Infectious diseases of livestock (2nd edn). Oxford University Press Southern Africa, Cape Town: 475-477 [ Links ]
19. Lawrence J A, Williamson S M 2004 Theileria taurotragi infection. In Coetzer J A W, Tustin R C (eds) Infectious diseases of livestock (2nd edn). Oxford University Press Southern Africa, Cape Town: 478-479 [ Links ]
20. Lawrence J A, Williamson S M 2004 Theileria mutans infection. In Coetzer J A W, Tustin R C (eds) Infectious diseases of livestock (2nd edn). Oxford University Press Southern Africa, Cape Town: 480-482 [ Links ]
21. Lawrence J A, Williamson S M 2004 Theileria velifera infection. In Coetzer J A W, Tustin R C (eds) Infectious diseases of livestock (2nd edn). Oxford University Press Southern Africa, Cape Town: 483-484 [ Links ]
22. Martin H, Brocklesby D W 1960 A new parasite of the eland. Veterinary Record 72:331-332 [ Links ]
23. Matjila P T, Leisewitz A L, Jongejan F, Bertschinger H J, Penzhorn B L 2008 Molecular detection of Babesia rossi and Hepatozoon sp. in African wild dogs (Lycaon pictus) in South Africa. Veterinary Parasitology 157: 123-127 [ Links ]
24. Matjila P T, Leisewitz A L, Jongejan F, Penzhorn B L 2008 Molecular detection of tick-borne protozoal and ehrlichial infections in domestic dogs in South Africa. Veterinary Parasitology 155:152-157 [ Links ]
25. Matjila P T, Leisewitz A L, Oosthuizen M C, Jongejan F, Penzhorn B L 2008 Detection of a Theileria species in dogs in South Africa. Veterinary Parasitology 157:34-40 [ Links ]
26. Moll G, Lohding A, Young A S 1984 Epidemiology of theileriosis in the Trans-Mara Division, Kenya. Husbandry and disease background and preliminary investigations on theileriosis in calves. Preventive Veterinary Medicine 2:801-832 [ Links ]
27. Neitz W O 1955 Corridor disease: a fatal form of bovine theileriosis encountered in Zululand. Bulletin of Epizootic Diseases of Africa 3:121-123 [ Links ]
28. Nijhof A M, Penzhorn B L, Lynen G, Mollel J O, Bekker C, Jongejan F 2003 Babesia bicornis sp. n. and Theileria bicornis sp. n.: tick-borne parasites associated with mortality in the black rhinoceros (Diceros bicornis). Journal of Clinical Microbiology 41:2249-2254 [ Links ]
29. Nijhof A M, Pillay V, Steyl J, Prozesky L, Stoltsz W H, Lawrence J A, Penzhorn B L, Jongejan F 2005 Molecular characterization of Theileria species associated with mortality in four species of African antelopes. Journal of Clinical Microbiology 43:5907-5911 [ Links ]
30. Norval R A I, Perry B D, Young A S 1992 The epidemiology of theileriosis in Africa. Academic Press, London [ Links ]
31. Nuttall G F H 1910 On haematozoa occurring in wild animals in Africa. 1. Piroplasma rossi N. Sp. and Haemogregarinia canis adusti n. Sp. found in the jackal. Parasitology 3:108-116 [ Links ]
32. Oura C A L, Bishop R P, Wampande E M, Lubega G W, Tait A 2004 Application of a reverse line blot assay to the study of haemoparasites in cattle in Uganda. International Journal of Parasitology 34:603-613 [ Links ]
33. Paling R W, Grootenhuis J G, Young A S 1981 Isolation of Theileria mutans from Kenyan buffalo, and transmission by Amblyomma gemma. Veterinary Parasitology 8:31-37 [ Links ]
34. Perry B D, Young A S 1993 The naming game: the changing fortunes of East Coast fever and Theileria parva. Veterinary Record 133:613-616 [ Links ]
35. Potgieter F T, Roos J A, de Vos A J 1985 Implications of chemotherapy of Theileria lawrencei infections (Corridor disease) in cattle. South African Journal of Science 81:44 [ Links ]
36. Potgieter F T, Stoltsz W H, Blouin E F, Roos J A 1988 Corridor disease in South Africa: A review of the current status. Journal of the South African Veterinary Association 59:155-160 [ Links ]
37. Schreuder B E C, Uilenberg G, Tondeur W 1977 Studies on Theileriidae (Sporozoa) in Tanzania. VIII. Experiments with African buffalo (Syncerus caffer). Tropenmedizin und Parasitologie 28:367-371 [ Links ]
38. Short N J, Norval R A I 1981 Seasonal activity of Rhipicephalus appendiculatus Neumann 1901 (Acari: Ixodidae) in the highveld of Zimbabwe Rhodesia. Journal of Parasitology 67:77-84 [ Links ]
39. Thompson B E, Latif A A, Oosthuizen M C, Troskie M, Penzhorn B L 2008 Occurrence of Theileria parva infection in cattle on a farm in the Ladysmith district, KwaZulu-Natal, South Africa. Journal of the South African Veterinary Association 79:31-35 [ Links ]
40. Uilenberg G 1999 Immunization against diseases caused by Theileria parva: a review. Tropical Medicine and International Health 4(9): A12-A20 [ Links ]
41. Van den Bossche P, Esterhuizen J, Nkuna R, Matjila T, Penzhorn B, Geerts S, Marcotty T 2006 An update of the bovine trypanosomosis situation at the edge of the Hluhluwe-iMfolozi Park, KwaZulu-Natal Province, South Africa. Onderstepoort Journal of Veterinary Research 73:77-79 [ Links ]
Received: October 2009.
Accepted: February 2010.
* Author for correspondence. E-mail: banie.penzhorn@up.ac.za














