Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the South African Veterinary Association
versão On-line ISSN 2224-9435
versão impressa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.81 no.1 Pretoria Jan. 2010
ARTICLE ARTIKEL
The efficacy of a topically applied combination of cyphenothrin and pyriproxyfen against the southern African yellow dog tick, Haemaphysalis elliptica, and the cat flea, Ctenocephalides felis, on dogs
J J FourieI,*; L J FourieI,II; I G HorakII; M G SnymanIII
IClinVet International, PO Box 11186, Universitas, 9321 South Africa
IIDepartment of Zoology and Entomology, University of the Free State, PO Box 339, Bloemfontein, 9300 South Africa
IIIVetrem Developments, PO Box 908-477, Montana, 0151 South Africa
ABSTRACT
The objective of this study was to determine the therapeutic and residual efficacy of a topically applied combination of cyphenothrin (40 %) and pyriproxyfen (2 %) against the tick Haemaphysalis elliptica and the flea Ctenocephalides felis on dogs. Twelve dogs were infested with 50 ticks 2 days before they were treated and with approximately 100 fleas 6 days before treatment and again 2 days before treatment and with 50 ticks and approximately 100 fleas at weekly intervals thereafter. They were ranked according to their flea counts and sex 5 days before treatment and randomly allocated to an untreated control group of 6 dogs and a treated group of 6 dogs. Ticks and fleas were collected from the dogs 48 h after treatment and 48 h after each infestation and live and dead ticks and live fleas were counted. The counts of ticks and fleas were transformed to geometric means, and efficacy was calculated by comparing these means. The product had a therapeutic efficacy of 83.1 % against H. elliptica and 97.5 % against C. felis 2 days after treatment. The residual period of protection during which efficacy was > 90% was 5 weeks for both H. elliptica and C. felis.
Keywords: Ctenocephalides felis, cyphenothrin, dogs, efficacy study, Haemaphysalis elliptica, pyriproxyfen, topical application.
INTRODUCTION
Ticks and fleas are without doubt the most important ectoparasites of domestic dogs. Ticks are important because they can give rise to 'tick worry', transmit diseases to dogs, and may also bite and transmit diseases to the owners of the dogs8,20. Fleas are of veterinary importance because they cause intense irritation in healthy dogs, flea-bite allergies in sensitised dogs and are the intermediate hosts of the larval stage of the dog tapeworm Dipylidium caninum. This tapeworm may also infect humans, and especially children17,18.
At least 22 ixodid tick species have been collected from dogs in South Africa7,9,10,12. The most commonly encountered of these are the southern African yellow dog tick, Haemaphysalis elliptica, the kennel tick, Rhipicephalus sanguineus, and the glossy brown tick Rhipicephalus simus. In the western Free State Province and the southwestern Western Cape Province R. simus is replaced by Rhipicephalus gertrudae10,12. The 2 most important of the last 4 tick species are H. elliptica, the only proven vector of Babesia canis rossi14, the cause of virulent babesiosis in domestic dogs, and R. sanguineus the vector of Ehrlichia canis, the cause of ehrlichiosis in dogs4. Both ticks are also capable of transmitting Rickettsia conorii, the cause of tick-bite fever or tick typhus in humans3,13,16.
Haemaphysalis elliptica is an old South African taxon and has recently been re-established as such, as opposed to Haemaphysalis leachi, a tick that occurs north of South Africa and with which it had long been confused2. In our experience H. leachi does not occur in South Africa and where it is mentioned in the pre-2007 South African literature it should be read as H. elliptica.
Haemaphysalis elliptica is widespread in South Africa11 and is likely to occur wherever there are adequate rodent and dog populations to sustain it17.Itisa 3-host tick, of which the larvae and nymphs use murid rodents as their hosts of choice15. The adults infest domestic dogs and cats and the larger wild felids and some canids6,10. Adult ticks are most abundant on dogs in the Eastern Cape Province from June to February9, during the period May to September in the Western Cape Province10, during the period October to February in Free State Province12 and from January to April in northeastern KwaZulu-Natal Province, South Africa7.
The most commonly encountered flea on dogs in South Africa is the cat flea, Ctenocephalides felis19. The numbers of Ctenocephalides spp., amongst which C. felis was predominant, increased on kennelled dogs, on which no insecticide was used, from very low numbers in winter to reach a peak during late summer, decreasing again during autumn5. Effective control of C. felis will reduce irritation in healthy dogs, while absolute control is necessary when dogs display flea-bite allergies. Control of both C. felis and D. caninum is necessary to reduce the likelihood of infection in dogs and humans with this tapeworm.
The present paper describes an efficacy study in kennelled dogs artificially infested with adult H. elliptica and C. felis and to which a combination of the insecticides cyphenothrin (40 %) and pyriproxyfen (2 %) (Sergeant's Gold®: Sergeant's Pet Care Products, Omaha, USA) was applied in a spot-on formulation.
MATERIALS AND METHODS
The study was a parallel group design, blinded, randomised, controlled efficacy study. Although it was not a good clinical practice investigation, it was carried out in line with the VICH GL9 guideline on good clinical practice1. The study was conducted on 2 groups of dogs, each consisting of 6 animals. Each group comprised 3 male and 3 female dogs, all more than 6 months old, and weighing between 10.6 kg and 16.4 kg, with hair-lengths varying between 18.7 mm and 60.0 mm. Each dog was fitted with an electronic transponder with unique alphanumeric numbers and harboured no ticks or fleas at the commencement of the study. The dogs had not been dipped 4 in an acaricide/insecticide during the 8 weeks prior to the day of treatment, or treated with an acaricide/insecticide spot-on/spray during the preceding 12 weeks. They were individually housed in pens with concrete floors and a sleep bench in an indoors animal unit. No contact between dogs was possible. The animals were fed a commercially available animal feed once daily according to the manufacturer's recommendation. Potable water was available ad libitum. The accommodation was in compliance with the 'National Code for Animal Use in Research, Education, Diagnosis and Testing of Drugs and Related Substances in South Africa' (Director General of Agriculture, Pretoria, South Africa).
A laboratory-bred strain of H. elliptica, of which the larvae and nymphs were fed on rabbits, was used for artificial infestation of the dogs. The adult ticks used for these infestations were unfed, at least 1 week old and of a balanced sex ratio. Each dog was artificially infested with approximately 50 ticks on the days indicated in the experimental design summarised in Table 1. A laboratory-bred strain of C. felis (routinely fed on cats) was used for all infestations. Fleas were unfed and of mixed sex. Each dog was infested with approximately 100 fleas on the days indicated in Table 1. After treatment had been administered to the dogs, subsequent batches of fleas were not placed on or near the site at which the medication had been applied.
The study followed a randomised block design and the Day 5 flea counts of each dog were used for ranking and group allocation purposes. The 12 dogs were ranked, within sex, in descending order of individual flea counts. Within each sex animals were subsequently blocked into 2 blocks of 3 dogs each, and within each block dogs were randomly allocated to Groups 1 and 2. The groups were coded to blind the persons performing the posttreatment assessments. The dogs in Group 1 served as negative controls and received no treatment, whereas the dogs in Group 2 were treated with the product at a label recommended dosage of 3 m /dog.
/dog.
Ticks were collected from the dogs 48 h after treatment and 48 h after each infestation by direct observation following parting of the hair and palpation. All ticks were removed and the condition of each tick was recorded using the parameters listed in Table 2. Fleas were collected from the dogs 48 h after treatment and again after each infestation by combing the hair coat with a fine-toothed flea comb. Combing was performed by making several strokes with the comb in each area of the animal, each time following the lie of the hair. Movement, from one part of the animal's body surface to the next was via strokes overlapping each other, so that no site was left uncombed.
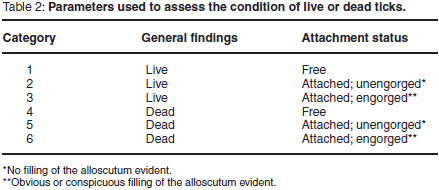
The primary criterion for the assessment of efficacy was based on the number of live ticks and fleas collected from dogs in the control group compared with the treated group on those days on which counts were conducted.
Percentage therapeutic efficacy against ticks was calculated as follows:
Therapeutic efficacy (%) = 100 × (Gmc- Gmt) / Gmc,
where Gmc = geometric mean number of live ticks (categories 1-3, Table 2) on dogs in the control group on Day 2 after treatment of the dogs in the treated group, and Gmt = geometric mean number of live ticks (categories 1-3) on dogs in the treated group on Day 2 after treatment.
Percentage residual efficacy was calculated as follows:
Residual efficacy (%) against ticks = 100 × (Gmc- Gmr) / Gmc,
where Gmc = geometric mean number of live ticks (categories 1-3, Table 2) on dogs in the control group on Days 9, 16, 23, 30, 37 and 44 after treatment of the dogs in the treated group, and Gmr = geometric mean number of live ticks (categories 1-3, and 6) on dogs in the treated group on Days 9, 16, 23, 30, 37 and 44 after treatment. Even though category 6 ticks (Table 2) were dead on the day of examination, they had managed to engorge before dying and were therefore included with the live ticks.
Efficacy against fleas was calculated as follows:
Efficacy (%) = 100 × (mc- mt) / mc,
where mc = geometric mean number of live fleas on the control group of dogs (Group 1), and mt = geometric mean number of live fleas on the treated group (Group 2).
The groups were compared using an ANOVA with a treatment effect after logarithmic transformation of the tick and flea counts. The level of significance of the tests was set at 5 %. The residual period of protection against ticks and fleas was prescribed as the number of weeks post-treatment during which efficacy was >90 %.
RESULTS
Mean tick and flea counts and the efficacy of the product against H. elliptica and C. felis on the various days of assessment are summarised in Tables 3 and 4. Therapeutic efficacy against H. elliptica based on geometric means was 83.1 %, and the residual period of protection against ticks during which efficacy was >90 % was 5 weeks. Therapeutic efficacy against C. felis was 97.5 %, and the residual period of protection was 5 weeks.
DISCUSSION
It must be born in mind that a therapeutic efficacy of 83.1 % for the product investigated was obtained against H. elliptica that had already been attached for 2 days. On the other hand, its residual efficacy, which was >90 % for 5 weeks after treatment, was measured against ticks that were placed on dogs after the animals had been treated. Efficacy thus appears to be greater against unattached ticks than against attached ticks. This pattern of efficacy makes it a suitable parasiticide for use at the commencement of the tick season to prevent a rapid seasonal increase in the numbers of H. elliptica. Initial treatment should be administered during July in the Eastern and Western Cape Provinces9,10, during November in Free State Province12, during January in northern KwaZulu-Natal7, and according to the seasonal occurrence of ticks at other localities. Repeated application thereafter will ensure that tick numbers remain low during the remainder of the tick season.
The investigated product had a therapeutic efficacy of 97.5 % against the flea, C. felis. Its residual efficacy increased slightly to 100 % 9 days after treatment and remained at a high level up to and including 37 days after treatment. Its therapeutic and residual efficacy ensures its appropriateness for use during the commencement of the flea season when the numbers of C. felis are rapidly increasing and thereafter to keep numbers down. This would be during November or December in northern Gauteng Province5 and probably also at other localities in South Africa with a similar climate.
ACKNOWLEDGEMENTS
We express our sincere thanks to the technical staff of Clinvet Laboratories for their assistance in maintaining the tick and flea colonies and with the infestation and collection of ticks and fleas from the dogs. Dr H. Luus of Clinvet Laboratories was responsible for the statistical processing of the data.
REFERENCES
1. Anon. 2000 International Cooperation on Harmonization of Technical Requirements for the registration of veterinary medicinal products, Guideline 9, Good Clinical Practice, July 2000. Online at: http://www.vichsec.org/pdf/2000/G109_st7.pdf (accessed 24 March 2010) [ Links ]
2. Apanaskevich D A, Horak I G, Camicas J-L 2007 Redescription of Haemaphysalis (Rhipistoma) elliptica (Koch, 1844), an old taxon of the Haemaphysalis (Rhipistoma) leachi group from East and southern Africa, and of Haemaphysalis (Rhipistoma) leachi (Audouin, 1826) (Ixodida, Ixodidae). Onderstepoort Journal of Veterinary Research 74:181-207 [ Links ]
3. Beati L, Kelly P J, Matthewman L A, Raoult D 1995 Prevalence of rickettsia-like organisms in ticks collected around Zimbabwe. Journal of Medical Entomology 32:787-792 [ Links ]
4. Groves M G, Dennis G L, Amyx H L, Huxsoll D L 1975 Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). American Journal of Veterinary Research 36:937-940 [ Links ]
5. Horak I G 1982 Parasites of domestic and wild animals in South Africa. XIV. The seasonal prevalence of Rhipicephalus sanguineus and Ctenocephalides spp. on kennelled dogs in Pretoria North. Onderstepoort Journal of Veterinary Research, 49:63-68 [ Links ]
6. Horak I G, Braack L E O, Fourie L J, Walker J B 2000 Parasites of domestic and wild animals in South Africa. XXXVIII. Ixodid ticks collected from 23 wild carnivore species. Onderstepoort Journal of Veterinary Research 67:239-250 [ Links ]
7. Horak I G, Emslie F R, Spickett A M 2001 Parasites of domestic and wild animals in South Africa. XL. Ticks on dogs belonging to people in rural communities and carnivore ticks on the vegetation. Onderstepoort Journal of Veterinary Research 68:135-141 [ Links ]
8. Horak I G, Fourie L J, Heyne H, Walker J B, Needham G R 2002 Ixodid ticks feeding on humans in South Africa: with notes on preferred hosts, geographic distribution, seasonal occurrence and transmission of pathogens. Experimental and Applied Acarology 27:113-136 [ Links ]
9. Horak I G, Jacot Guillarmod A, Moolman L C, De Vos V 1987 Parasites of domestic and wild animals in South Africa. XXII. Ixodid ticks on domestic dogs and on wild carnivores. Onderstepoort Journal of Veterinary Research 54:573-580 [ Links ]
10. Horak I G, Matthee S 2003 Parasites of domestic and wild animals in South Africa. XLIII. Ixodid ticks of domestic dogs and cats in the Western Cape Province. Onderstepoort Journal of Veterinary Research 70:187-195 [ Links ]
11. Howell C J, Walker J B, Nevill E M 1978 Ticks, mites and insects infesting domestic animals in South Africa. Part 1. Descriptions and biology. Department of Agricultural Technical Services, Republic of South Africa, Science Bulletin, No. 393 [ Links ]
12. Jacobs P A H, Fourie L J, Kok D J, Horak I G 2001 Diversity, seasonality and sites of attachment of adult ixodid ticks on dogs in the central region of the Free State Province, South Africa. Onderstepoort Journal of Veterinary Research 68:281-290 [ Links ]
13. Kelly P J 2001 Amblyomma hebraeum is a vector of Rickettsia africae and not R. conorii. Journal of the South African Veterinary Association 72:182 [ Links ]
14. Lewis B D, Penzhorn B L, Lopez-Rebollar L M, De Waal D T 1996 Isolation of a South African vector-specific strain of Babesia canis. Veterinary Parasitology 63:9-16 [ Links ]
15. Matthee S, Horak I G, Beacournu J-C, Durden L A, Ueckermann E A, McGeoch M A 2007 Epifaunistic arthropod parasites of the four-striped mouse, Rhabdomys pumilio, in the Western Cape Province, South Africa. Journal of Parasitology 93:47-59 [ Links ]
16. Neitz W O, Alexander R A, Mason J H 1941 The transmission of tick-bite fever by the dog tick Rhipicephalus sanguineus, Latr. Onderstepoort Journal of Veterinary Science and Animal Industry 16:9-17 [ Links ]
17. Norval R A I 1984 The ticks of Zimbabwe. IX. Haemaphysalis leachi and Haemaphysalis spinulosa. Zimbabwe Veterinary Journal 15:9-17 [ Links ]
18. Reinecke R K 1983 Veterinary helminthology. Butterworths, Durban/Pretoria [ Links ]
19. Segerman J 1995 Siphonaptera of southern Africa. Handbook for the identification of fleas. Publications of the South African Institute for Medical Research, Johannesburg, No. 57 [ Links ]
20. Walker J B, Keirans J E, Horak I G 2000 The genus Rhipicephalus (Acari, Ixodidae): a guide to the brown ticks of the world. Cambridge University Press, Cambridge [ Links ]
Received: March 2009.
Accepted: February 2010.
* Author for correspondence. E-mail: josephus@clinvet.com














