Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the South African Veterinary Association
On-line version ISSN 2224-9435
Print version ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.81 n.1 Pretoria Jan. 2010
ARTICLE ARTIKEL
Dose-related effects of cerulein short infusions on proximal small bowel motility in sheep
K W Romański
Laboratory of Clinical Physiology, Department of Biostructure and Physiology, Veterinary School, Wrocdaw University of Environmental and Life Sciences, Norwida 31, PL-50-375 Wrocdaw, Poland. E-mail: krzysztof.romanski@up.wroc.pl
ABSTRACT
The effect of cholecystokinin (CCK) upon the intestinal motility has not been entirely explored in ruminants. The aim of this study was to examine the precise effects of CCK amphibian analogue, cerulein, on small-intestinal myoelectric activity in rams in the course of chronic experiments. Five rams underwent implantation of bipolar platinum electrodes to the duodenal bulb, the distal duodenum and jejunum. During continuous myoelectrical and motor recordings, 0.15 M NaCl or the various doses of cerulein were administered intravenously. Short infusions of the smallest dose of cerulein exerted a slight and mostly insignificant effect on the duodenal bulb and the duodenal myoelectric activity index (MAI) values. In the duodenal bulb, the effects of cerulein on myoelectric activity were dose-dependent and closely related to the phase of the MMC. In the duodenum, the higher doses of the hormone evoked short stimulatory response followed by longer inhibitory biphasic effects on MAI. These effects were inversely related to the duration of hormone injection. Infusions of hormones at the higher doses caused a less pronounced biphasic effect. It is concluded that cerulein exerts an inhibitory effect upon the myoelectric activity of the duodenal bulb and a strong stimulatory and inhibitory (biphasic) effect on duodenal motility in sheep.
Keywords: cerulein, duodenal bulb, duodenum, migrating myoelectric complex, myoelectric activity, sheep
INTRODUCTION
Duodenal motility is a very important intestinal phenomenon, crucial for normal digestion and absorption, and its motor functions are controlled by many neuro-hormonal mechanisms. Cholecystokinin (CCK) is one of the principal gastrointestinal hormones involved in this control. The role of the hormone is not limited to motility and it also participates in the control of other physiological functions.54 In humans and monogastric animals, CCK affects the motor activity of the whole gastrointestinal tract in vivo and in vitro.2,11,56 The recognised effect of CCK on small-intestinal motility is clear. The hormone inhibits the arrival of the migrating motor complex (MMC) in the upper small bowel.19 Furthermore, it evokes the specific spike burst pattern and may increase spiking activity as well as hasten intestinal transit time.29,36,56 Its amphibian analogue, cerulein, is also active in mammals. In sheep, CCK peptides are also active in modulation of gastrointestinal motility, inhibit the arrival of the MMC in the upper small intestine and affect contractile activity, but their influence on intestinal motility has not been fully elucidated although several studies have been under taken.38,45,46,53 The dual effect of CCK on small-intestinal motility can also be expected to occur in sheep. The duodenal spiking activity response to CCK and whether there is a difference in this response between the duodenal bulb and the duodenum in conscious sheep have still to be elucidated.
MATERIALS AND METHODS
Animal preparation
Five healthy adult rams of the Polish Merino breed weighing 38-43 kg each were used. The rams were fed with good-quality hay, 1 kg daily, and a grain mixture (Dolpasz, Wroc»aw). They were fasted for 24 h before surgery, but allowed unlimited access to drinking water. The experimental procedure was approved by the Ethical Committee of the Veterinary School, Wrocdaw University of Environmental and Life Sciences, Poland.
After general and local anaesthesia45, right lateral laparotomy (diagonal incision) was performed and 2 bipolar platinum electrodes were implanted, one on the serosal side of the duodenal bulb, 5.5-6 cm distal to the pyloric ring and the other on the distal duodenum 50 cm below the bulbar electrode. A strain gauge force transducer (RB Products, Madison), calibrated individually before surgery, was attached near the duodenal electrode to verify the myoelectric activity tracings. Details of this procedure have been described elsewhere.43,45 Marked wires were externalised through the stab incision, soldered to the plug and fixed to the wool. Within 3 days the animals returned to normal feeding. The skin sutures were removed 10 days after surgery.
Experimental design
A total of 105 experiments lasting 5-6 h each were conducted. Myoelectric and motor activities were continuously recorded using a multichannel electroencephalograph (Reega Duplex TR XVI, Alvar Electronics, Montreuil) also adapted for mechanical activity recordings. Twenty-four hours before each experiment, food was removed from the cage. At least 2 consecutive phases of the MMC including 1 full normal cycle of the MMC were recorded each time. During control recordings, slow injections of 5 m 0.15 M NaCl were administered over 30 s into the jugular vein through a thin polyethylene catheter introduced before the experiment commenced. The saline injections were administered during the course of phases 1 (5 min after its start in the duodenum), 2a (5 min after its start in the duodenum), or phase 2b (5 min after its start in the duodenum) of the MMC. In the course of basic experiments, slow intravenous injections of cerulein (Farmitalia Carlo Erba, Milan, Italy) at small, moderate, and high doses, i.e. 1, 10 and 100 ng/kg were applied. The small dose of cerulein was administered over 30 s, the moderate dose over 30 or 60 s, and the high dose over 30, 60 or 120 s. Each dose was given in separate randomised experiments at the same periods as the saline injections. After saline or cerulein administration, the myoelectric and motor activities were recorded until the arrival of the 1st organised phase 3 of the MMC. After cessation of all the experiments, the animals were sacrificed humanely and the positions of the electrodes and the strain gauge force transducer were confirmed during autopsy.
0.15 M NaCl were administered over 30 s into the jugular vein through a thin polyethylene catheter introduced before the experiment commenced. The saline injections were administered during the course of phases 1 (5 min after its start in the duodenum), 2a (5 min after its start in the duodenum), or phase 2b (5 min after its start in the duodenum) of the MMC. In the course of basic experiments, slow intravenous injections of cerulein (Farmitalia Carlo Erba, Milan, Italy) at small, moderate, and high doses, i.e. 1, 10 and 100 ng/kg were applied. The small dose of cerulein was administered over 30 s, the moderate dose over 30 or 60 s, and the high dose over 30, 60 or 120 s. Each dose was given in separate randomised experiments at the same periods as the saline injections. After saline or cerulein administration, the myoelectric and motor activities were recorded until the arrival of the 1st organised phase 3 of the MMC. After cessation of all the experiments, the animals were sacrificed humanely and the positions of the electrodes and the strain gauge force transducer were confirmed during autopsy.
Analysis of data
The MMC cycles and their phases were identified in the duodenum according to the criteria proposed by Code & Marlett5 with a slight modification.41 The division of phase 2 into phases 2a and 2b of the MMC, proposed earlier by Dent et al.,7 was performed according to more precise criteria.45 The myoelectric and motor recordings were visually analysed and the myoelectric activity index (MAI) values were calculated as describe previously43). The MAI values were calculated by multiplying the average amplitude of each spike burst within the period examined, as described previously44, by the duration of this spike burst and expressed as the sum of the areas of all the spike bursts during 1 minute (µV/s/min). The spike bursts with amplitudes below 3 µV were omitted. The duration of the periods was equal to 1 minute. In the recordings obtained from the experiments with saline and cerulein injections, the MAI values were calculated in 4 periods (each lasting 1 min), i.e. 1 period before the injection and 10 similar periods after the injection. On the tracings, the measurements were performed using a calliper with an accuracy of about 0.3 mm.
All the values were grouped and the means and standard deviations were calculated. Statistical significance, i.e. when P < 0.05, was calculated using the Student's t-test for paired and unpaired values, where appropriate preceded by 1-way analysis of variance.51
RESULTS
Short infusions of saline evoked no effect and these data are not shown here.
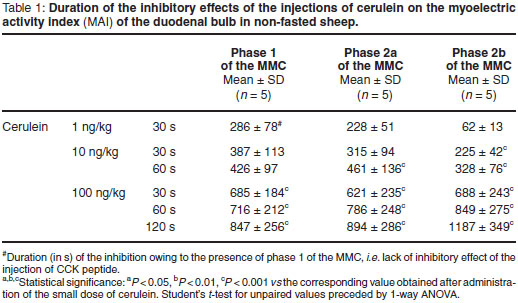
Short infusions of the small dose of cerulein administered during phase 1 of the MMC induced no changes, while short infusions during phases 2a and 2b of the MMC induced the weakest alterations in the spike bursts in the duodenal bulb (Table 1). Infusions of moderate doses of cerulein inhibited spike bursts in the duodenal bulb for longer periods than after infusion of the small doses although only the value obtained during phase 2b was significant when the hormone was infused over 30 s and during phase 2a and 2b when the hormone was infused over 60 s (Table 1). In these experiments no excitatory changes were observed except the 'small' spike bursts (Fig. 1). When the high dose of cerulein was infused, duration of the inhibition of spiking activity was significant regardless of the duration of infusion and MMC phase (Table 1).

In the duodenum, infusion of cerulein at the small dose during phase 1 of the MMC evoked a transient excitatory effect in the 1st minute, lasting 5-15 s after cessation of the peptide infusion (Table 2). The application of a moderate dose of cerulein during phase 1 of the MMC caused more evident, but also short alterations in the duodenal myoelectric activity when the hormone was injected either over 30 s or 60 s. Administration of cerulein at the high dose in the course of phase 1 of the MMC induced an evident (the greatest) stimulatory response in the duodenal MAI during the 1st minute and then after 1-3 minutes of the attenuated response the stimulation became significant again. This effect was partially related to the duration of hormone injection (Table 2 ).
Infusion of the small cerulein dose during phase 2a of the MMC produced a transient, non-significant response in the next and 2 last minutes of observation (Table 3). Application of the moderate dose over 30 s increased MAI significantly in the next minute, while administration of cerulein over 60 s increased MAI significantly for 3 minutes. The increase of MAI in the last 2 minutes was observed but it did not reach statistical significance. Representative examples of the stimulatory and inhibitory effect of a moderate dose of cerulein are shown in Fig. 1. Following infusion of the high dose of cerulein in the course of phase 2a of the MMC, a significant increase in the duodenal MAI values was observed for 3 consecutive minutes and then a decrease in the MAI values, lasting 1-2 min, occurred. However, this inhibition was not significant. No stimulatory response was observed in the last minutes (Table 3). The correlation between the spike bursts and contractions is shown in Fig. 1.
Infusion of the small dose of cerulein during phase 2b of the MMC induced no changes in MAI although a stimulatory tendency (not significant) was observed in the 1st minute following hormone infusion (Table 4). The moderate dose of cerulein caused clear stimulatory effect in the 1st minute followed by inhibition of MAI lasting 5-6 min. When cerulein was infused at the high doses in the course of phase 2b of the MMC, initially partial then strong inhibition of MAI followed again by partial inhibition was observed. The total duration of this inhibitory period in the duodenum lasted 12-25 min, while complete inhibition began 1-7 min after cessation of the peptide injection and lasted 3-8 min. No stimulatory effect in all 3 rates of the high dose of cerulein was observed (Table 4).
DISCUSSION
Infusions of cerulein elicited marked excitatory and inhibitory effects in the myoelectric and motor activity of the ovine upper small bowel. As found in the present study, it has also been demonstrated that the effect of CCK and cerulein may be different in the various regions of the small bowel.9,45 The role of duodenal bulb motility in the transport of digesta relies mainly on gastric emptying because of the importance of the pressure gradient between the antrum and duodenum.47 Thus, the duodenal bulb motility differs from duodenal motor function in sheep, i.e. fewer phasic contractions and spike bursts can be observed in the duodenum and phase 3 of the MMC is often of shorter duration or absent.42 In the present study cerulein evoked weaker and almost non-detectable excitation as well as pronounced inhibition of the normal (stronger) spike bursts in the duodenal bulb. These spike bursts apparently originated from the circular smooth muscle layer. Furthermore, stimulatory changes on low-amplitude spike bursts, most probably originating from the longitudinal muscle, were recorded. It has already been reported that cholecystokinin octapeptide (CCK-OP) exerts a direct contractile effect on the longitudinal muscle and inhibits circular muscle motility.6 However, reported data on the matter are scanty.
In the duodenum, moderate doses of CCK peptides had a clear dual effect. A prompt increase in spiking and contractile activity followed by inhibitory alterations were seen. Biphasic and even triphasic responses of gastric and duodenal motility to CCK-OP and other peptides acting through CCK receptors have already been observed.14,31 These findings suggest that the mechanism of CCK action on duodenal motility is complex and may exhibit adaptive features.
Cerulein action therefore appears to be similar to CCK-OP effect. Cerulein is considered as a CCK-OP analogue because of very similar structure.25 The latter is known as one of existing forms of CCK in the body that has been confirmed in genetic studies. However, CCK-33, CCK-39, and CCK-59 appear to be synthesised in the 1st order.18 It is nevertheless known that CCK, as some other regulatory peptides, is processed to its biologically active forms by cleavage at single basic amino acids. The preprocholecystokinin is natural precursor of all forms of CCK.32 Synthesised CCK is released into the local environment directly or indirectly, via luminal CCK-releasing factor34 and is bound to a specific receptor within a target cell. A more specific CCK-A receptor is located within the gastrointestinal tract where a CCK-B/gastrin receptor is also present. These receptors are cloned from a specific gene and are linked with G-protein within the cellular membrane.21,39 These receptors were found in the smooth muscle cells, endocrine cells, and vagal nerve.8,15,28 Binding of CCK (or cerulein) to the receptor evokes a cascade of intracellular processes involving second messenger systems, activation of protein kinase C, and protein phosphorylation.26,39 Thus, CCK can affect gastrointestinal motility owing to its direct action on the smooth muscles27, through the peptidergic route3,40,50,55, as well as in cooperation with other regulatory peptides.12,17,20,22,48,49 In sheep, the mode and mechanism of CCK action seems to be similar to those in monogastric species. CCK can modulate the interdigestive motor activity via CCK-A receptors37 although its action via CCK-B/gastrin receptors cannot be excluded.57 CCK can affect gastrointestinal function peripherally, but also centrally.16,23,24 Furthermore, direct or indirect actions of CCK in cooperation with other peptides are also possible.33,52,57 The composite mechanism of CCK action on ovine gastrointestinal motility explains its dual effect.
The observed stimulatory effect of a moderate dose of CCK on duodenal motility seems to be primary35, and this conclusion can also be inferred from the present study. Similar effects have previously been reported for cerulein.1,36,38,43 Other reports indicate that the effect of CCK on duodenal motility can be inhibitory or no effect can be observed, while some authors observed a simultaneous stimulatory effect in the jejunum.4,10,13,30
The small doses of CCK-OP and cerulein induced no effect in the duodenal bulb, although discernible inhibitory changes were observed after moderate doses. As discussed earlier45, the moderate doses of CCK-OP and cerulein as well as the highest doses administered over 120 s remained within the physiological range. Thus the only reasonable inference that can be drawn from these findings is that endogenous CCK may also inhibit the myoelectric activity in the duodenal bulb and that the inhibitory effect of CCK represents the primary response in this region. In the duodenum, the small doses of CCK peptides elicited transient stimulation in the myoelectric and motor activities and the moderate doses evoked the dual effect. These observations may suggest that in the duodenum the action of CCK can be physiological and comprise both excitatory and inhibitory responses, although the excitatory response can be regarded as the primary effect.
Finally, it is concluded that cerulein evokes an inhibitory effect in ovine duodenal bulb myoelectric activity, while its primary short-term effect on the duodenum is stimulatory and followed by longer inhibition. Thus, the mechanisms of cerulein action on these 2 parts of the duodenum are partially different.
REFERENCES
1. Bertaccini G, de Caro G, Endean R, Erspamer V, Impicciatore M 1968. The actions of caerulein on the smooth muscle of the gastrointestinal tract and the gall bladder. British Journal of Pharmacology 34:291-310 [ Links ]
2. Botella A, Delvaux M, Berry P, Frexinos J, Bueno L 1992. Cholecystokinin and gastrin induce cell contraction in pig ileum by interacting with different receptor subtypes. Gastroenterology 102,779-786 [ Links ]
3. Bucinskaite V, Kurosawa M, Lundeberg T 2000. Exogenous cholecystokinin-8 reduces vagal afferent nerve activity in rats through CCK(A) receptors. British Journal of Pharmacology 129:1649-1654 [ Links ]
4. Bueno L, Praddaude F 1979. Electrical activity of the gallbladder and biliary tract in sheep and its relationships with antral and duodenal motility. Annals of Biology, Animal Biochemistry and Biophysics 19:1109-1121 [ Links ]
5. Code C F, Marlett J A 1975. The interdigestive myoelectric complex of the stomach and small bowel of dogs. Journal of Physiology (London) 246:289-309 [ Links ]
6. D'Amato M, Stamford I F, Bennett A 1989 Inhibition of CCK-induced contraction by non-peptide antagonists in human alimentary tract muscle. Pharmacological Research 21:659-660 [ Links ]
7. Dent J, Dodds W J, Sekiguchi T, Hogan W J, Arndorfer R C 1983 Interdigestive phasic contractions of the human lower esophageal sphincter. Gastroenterology 84:453-460 [ Links ]
8. Devalle J, Chiba T, Park J, Yamada T 1993 Distinct receptors for cholecystokinin and gastrin on canine fundic D-cells. American Journal of Physiology 264:G811-G815 [ Links ]
9. Fargeas M J, Bassotti G, Fioramonti J, Bueno L 1989 Involvement of different mechanisms in the stimulatory effects of cholecystokinin octapeptide on gastrointestinal and colonic motility in dogs. Canadian Journal of Physiology and Pharmacology 67:1205-1212 [ Links ]
10. Fleckenstein P, Öigaard A 1977 Effects of cholecystokinin on the motility of the distal duodenum and the proximal jejunum in man. Scandanavian Journal of Gastroenterology 12:375-378 [ Links ]
11. Fornai M, Coluzzi R, Antonioli L, Baschiera F, Ghisu N, Tuccori M, Gori G, Blandizzi C, Del Tacca M 2006 CCK (2) receptors mediate inhibitory effects of cholecystokinin on the motor activity of guinea-pig distal colon. European Journal of Pharmacology 557:212-220 [ Links ]
12. Fung L, Pokol-Daniel S, Greenberg G R 1994 Cholecystokinin type A receptors mediated intestinal fat-induced inhibition of acid secretion through somatostatin-14 in dogs. European Journal of Pharmacology 134:2376-2382 [ Links ]
13. Giralt M, Vergara P 2000 Inhibition by CCK of ascending contraction elicited by mucosal stimulation in the duodenum of the cat. European Journal of Pharmacology 12:173-180 [ Links ]
14. Giuliani S, Lippe I.T, Maggi C A, Meli A 1990 Dual effects of cholecystokinin-octapeptide on duodenal motility of urethaneanesthetized rats. Journal of Pharmacology and Experimental Therapeutics 252:1312-1317 [ Links ]
15. Grider J R, Makhlouf G M 1990 Distinct receptors for cholecystokinin and gastrin on muscle cells of stomach and gallbladder. American Journal of Physiology 259:G184-G190 [ Links ]
16. Guevara-Guzmán R, Lévy F, Jean A, Nowak R 2005 Electrophysiological responses of nucleus tractus solitarius neurons to CCK and gastric distension in newborn lambs. Cellular and Molecular Neurobiology 25:393-406 [ Links ]
17. Guilmeau S, Buyse M, Tsocas A, Laigneau J P, Bado A 2003 Duodenal leptin stimulates cholecystokinin secretion: evidence of a positive leptin-cholecystokinin feedback loop. Diabetes 52:1664-1672 [ Links ]
18. Haun R S, Minth C D, Andrews P C, Dixon J E 1989 Molecular biology of gut peptides. In Schultz S G (ed.) Handbook of physiology. The gastrointestinal system Vol. 2. American Physiological Society, Bethesda, MD: 1-43 [ Links ]
19. Heppell J, Blinks S, Kelly K A, GoVLW 1982 Inhibition of small intestinal interdigestive motility by cholecystokinin octapeptide (CCK-OP). In Wienbeck M (ed.) Motility of the digestive tract. Raven Press, New York: 207-214 [ Links ]
20. Herzig K-H, Louie D S, Owyang C 1994 Somatostatin inhibits CCK release by inhibiting secretion and action of CCK-releasing peptide. American Journal of Physiology 266:G1156-G1161 [ Links ]
21. Holicky E L, Hadac E M, Ding X-Q, Miller L J 2001 Molecular characterization and organ distribution of type A and B cholecystokinin receptors in cynomolgus monkey. American Journal of Physiology 281:G507-G514 [ Links ]
22. Jansen J B M J, De Jong A J L, Singer M V, Niebel W, Rovati L C, Lamers C B H W 1990 Role of cholecystokinin in bombesin-and meal-stimulated pancreatic polypeptide secretion in dogs. Digestive Diseases and Sciences 35:1073-1077 [ Links ]
23. Kania B F, Brikas P, Bueno L, Fioramonti J, Zaremba-Rutkowska M 1999 The evaluation of the role of CCK in the opioid modulation of the motility of the gastrointestinal tract in sheep. Journal of Veterinary Pharmacological Theory 22:153-160 [ Links ]
24. Kania B F, Kania K, Romanowicz K, Tomaszewska D, Sutiak V, Wrońska-Fortuna D 2005 Centrally administered PD 140.548 N-methyl-d-glucamine prevents the autonomic responses to duodenal pain in sheep. Research in Veterinary Sciences 81:109-118
25. Kominami G, Okabe H, Imoda K, Mizojiri K 1994 Combined high-performance liquid chromatography and radioimmunoassay for ceruletide and its metabolites in dog plasma and urine. Journal of Pharmacy and Biomedical Analytics 12:413-418 [ Links ]
26. Lankisch T O, Tsunoda Y, Lu Y, Owyang C 2002 Characterization of CCKA receptor affinity states and Ca2+ signal transduction in vagal nodose ganglia. American Journal of Physiology 282:G1002-G1008 [ Links ]
27. Li W, Zheng T Z, Qu S Y 2000 Effect of cholecystokinin and secretin on contractile activity of isolated gastric muscle strips in guinea pigs. World Journal of Gastroenterology 6:93-95 [ Links ]
28. Lin C W, Miller T R 1992 CCK-A and CCK-B/gastrin receptors are present on rabbit vagus nerve. American Journal of Physiology 263:R591-R595 [ Links ]
29. Lin H C, Zaidel O, Hum S 2002 Intestinal transit of fat depends on accelerating effect of cholecystokinin and slowing effect of an opioid pathway. Digestive Diseases and Sciences 47:2217-2221 [ Links ]
30. Martins S R, Oliveira R B, Ballejo G 2006 Activation of neural cholecystokinin-1 receptors induces relaxation of the isolated rat duodenum which is reduced by nitric oxide synthase inhibitors. Brazilian Journal of Medical and Biological Research 39:271-275 [ Links ]
31. McLeay L M, Wong M H 1989 Excitatory and inhibitory effects of gastrin peptides on gastric motility in sheep. American Journal of Physiology 257:R388-R395 [ Links ]
32. Merchant J L, Dickinson C J, Yamada T 1994. Molecular biology of gut peptides. Model of gastrointestinal hormones. In Johnson L R (ed.) Physiology of the gastrointestinal tract. Raven Press, New York: 295-350 [ Links ]
33. Mineo H, Iwaki N, Onaga T, Kato S 1994 Effects of intravenous infusions of cholecystokinin-8 and pentagastrin on plasma concentrations of insulin and glucagon in sheep. Research in Veterinary Sciences 56:298-302 [ Links ]
34. Miyasaka K, Funakoshi A 1998 Luminal feedback regulation monitor peptide, CCK-releasing peptide, and CCK receptors. Pancreas 16:277-283 [ Links ]
35. Mizumoto A, Ueki S, Ohtawa M, Itoh Z 1992 Endogenous CCK is not involved in the regulation of interdigestive gastrointestinal and gallbladder motility in conscious dogs. Regulatory Peptides 41:249-256 [ Links ]
36. Niederau C, Karaus M 1991 Effects of CCK receptor blockade on intestinal motor activity in conscious dogs. American Journal of Physiology 260:G315-G324 [ Links ]
37. Onaga T, Mineo H, Kato S 1997 Effect of L364718 on interdigestive pancreatic exocrine secretion and gastroduodenal motility in conscious sheep. Regulatory Peptides 68:139-146 [ Links ]
38. Ormas P, Belloli C, Sagrada A, Arioli F, Tanzi G B, Beretta C 1984 Possible mechanisms of action of caerulein on intestinal motility of sheep. Annales de la Recherches Vétérinaire 15:557-562 [ Links ]
39. Rao R V, Holicky E L, Kuntz S M, Miller L J 2000 CCK receptor phosphorylation exposes regulatory domains affecting phosphorylation and receptor trafficking. American Journal of Physiology 279: C1986-C1992 [ Links ]
40. Rodriguez-Membrilla A, Vergara P 1997 Endogenous CCK disrupts the MMC pattern via capsaicin-sensitive vagal afferent fibers in the rat. American Journal of Physiology 272: G100-G105 [ Links ]
41. Romanski K W 2002 Characteristics and cholinergic control of the 'minute rhythm' in ovine antrum, small bowel and gallbladder. Journal of Veterinary Medicine A 49:313-320 [ Links ]
42. Roman ski K W 2003 Character and cholinergic control of myoelectric activity in ovine duodenal bulb: relationships to adjacent regions. Veterinarski Arhiv 73:1-16 [ Links ]
43. Romanski K W 2004 Ovine model for clear-cut study on the role of cholecystokinin in antral, small intestinal and gallbladder motility. Polish Journal of Pharmacology 56:247-256 [ Links ]
44. Romanski K W 2006 Changes in amplitude and duration of the spike bursts within phase 3 of the migrating myoelectric complex in the small bowel of fasted, non-fasted and fed sheep. Bulletin of the Veterinary Institute in Pulawy 50:239-245 [ Links ]
45. Romanski K W 2007 Regional differences in the effects of various doses of cerulein upon the small-intestinal migrating motor complex in fasted and non-fasted sheep. Journal of Animal Physiology and Animal Nutrition 91:29-39 [ Links ]
46. Romanski K W 2009 Cholecystokinindependent selective inhibitory effect on 'minute rhythm' in the ovine small intestine. Animal 3:275-286 [ Links ]
47. Ruckebusch Y 1989 Gastrointestinal motor functions in ruminants. In Schultz S G (ed.) Handbook of physiology. The gastrointestinal system Vol. 1. American Physiological Society, Bethesda, MD: 1225-1282 [ Links ]
48. Schmid R, Schusdziarra E, Schulte-Frohlinde E, Maier V, Classen M 1989 Effect of CCK on insulin, glucagon, and pancreatic polypeptide levels in humans. Pancreas 4:653-661 [ Links ]
49. Schmidt W E, Creutzfeldt W, Höcker M, Nustede R, Choudhury A R, Schleser A, Rovati L C, Fölsch U R 1991 Cholecystokinin receptor antagonist loxiglumide modulates plasma levels of gastro-enteropancreatic hormones in man. European Journal of Clinical Investigation 21:501-511 [ Links ]
50. Simasko S, Ritter R C 2003 Cholecystokinin activates both A-and C-type vagal afferent neurons. American Journal of Physiology 285: G1204-G1213 [ Links ]
51. Snedecor G W, Cochran W G 1971 Statistical methods. The Iowa State University Press, Ames, IO, USA [ Links ]
52. Spencer G S, Berry C, Johnston S 1991 Neuroendocrine regulation of growth hormone secretion in sheep. IV. Central and peripheral cholecystokinin. Domestic Animal Endocrinology 8:555-563 [ Links ]
53. Titchen D A 1984 Gastrointestinal peptide hormone distribution, release, and action in ruminants. In Milligan L P, Growum W L, Dobson A (ed.) Control of digestion and metabolism in ruminants. Prentice-Hall, Englewood Cliffs: 227-248 [ Links ]
54. Walsh J H 1994 Gastrointestinal hormones. In Johnson L R (ed.) Physiology of the gastrointestinal tract. Raven Press, New York: 1-128 [ Links ]
55. Xu M Y, Lu H M, Wang S Z, Shi W Y, Wang X C, Yang D X, Yang C X., Yang L Z 1998 Effect of devazepide reversed antagonism of CCK-8 against morphine on electrical and mechanical activities of rat duodenum in vitro. World Journal of Gastroenterology 4:524-526 [ Links ]
56. Xu M Y, Yang D X, Wang S Z, Jin H B, Zhou X H, Yang X P, Han J S 1998 Antagonistic effect of CCK-8 on morphine-inhibited electrical and contractile activities of rat jejunum in vitro. Sheng Li Xue Bao 50:469-473 [ Links ]
57. Zavros Y, Shulkes A 1997 Cholecystokinin (CCK) regulates somatostatin secretion through both the CCK-A and CCK-B/gastrin receptors in sheep. Journal of Physiology (London) 505:811-821 [ Links ]
Received: March 2009.
Accepted: December 2009.














