Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the South African Veterinary Association
versión On-line ISSN 2224-9435
versión impresa ISSN 1019-9128
J. S. Afr. Vet. Assoc. vol.81 no.1 Pretoria ene. 2010
ARTICLE ARTIKEL
Vaccination against GnRH may suppress aggressive behaviour and musth in African elephant (Loxodonta africana) bulls - a pilot study
H M De NysI; H J BertschingerI,*; J A TurkstraII; B ColenbranderIII; R PalmeIV; A M HumanI
ISection of Reproduction, Department of Production Animal Studies, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort, 0110 South Africa
IIPepscan Therapeutics, Zuidersluisweg 2, 8243 RC Lelystad, Netherlands
IIIFaculty of Veterinary Medicine, Utrecht University Yalelaan 1, 3584 CL Utrecht, Netherlands
IVDepartment of Biomedical Sciences/Biochemistry, University of Veterinary Medicine, 1210 Vienna, Austria
ABSTRACT
Aggressive behaviour and musth are constant problems in captive and sometimes in free-ranging African elephant bulls. Aggressive bulls are difficult and musth bulls almost impossible to manage without severely restricting their movement either by leg-chaining or using tranquillisers. This study investigated the relationship between faecal androgen metabolites (FAM) and faecal cortisol metabolites (FCM) concentrations and aggressive behaviour and tested a GnRH vaccine as a means of down-regulating aggressive behaviour and musth in 1 free-ranging and 5 captive elephant bulls. The bulls were non-aggressive (n = 3), aggressive (n = 2) or in musth (n = 1) at the onset of the study. The bulls were injected with a GnRH vaccine-adjuvant combination 3 or 4 times at 3-to 7-week intervals. Behaviour, FAM and FCM concentrations were measured during every week prior to vaccination until 4 months after the last vaccination. FAM concentrations were positively correlated with aggressive behaviour before the 1st vaccination. Androgen production, as reflected by FAM concentrations, was down-regulated in 3 of the 6 immunised bulls. At least 2 bulls and possibly a 3rd showed behavioural improvement following GnRH vaccination and in all 3 temporal gland secretion ceased. No further aggressive behaviour was observed until the end of the study in any of the bulls. The results of this 1st GnRH immunisation study suggest that it could be a useful method to control aggressive behaviour and musth in African elephant bulls.
Keywords: aggressive behaviour, cortisol,down-regulation, faeces, GnRH vaccine, musth, testosterone.
INTRODUCTION
Musth, a condition exhibited periodically by adult male elephants43, is associated with increased aggressive behaviour11,41,43 and creates serious problems in the management of captive and free-ranging elephant bulls. Such bulls may even endanger the lives of both animals and humans. The characteristics of musth in Asian (Elephas maximus)11,21,22 and African (Loxodonta africana)20,41 elephant bulls are remarkably similar43. Apart from increased aggressive behaviour, the main signs of musth are heavy and continuous temporal gland secretion (TGS) and continuous urine dribbling20,22,41. Musth has been shown to be associated with increased androgen levels in the blood8,15,16,18,30,43,44, urine5,42 and faeces17,18. Faecal androgen metabolites (FAM) and faecal cortisol metabolites (FCM), measured with an epiandrosterone and an 11-oxoaetiocholanolone enzyme immunoassay (EIA) have been validated as a tool for non-invasive monitoring of endogenous secretion rates of testosterone and cortisol, respectively, in African elephant bulls15,16,18.
Young free-ranging bulls often become problematic in smaller game reserves where there is a lack of natural hierarchical social structure and no or too few adult bulls to control them47. They generally enter musth at an earlier age and for longer periods than normal. Often the removal of the bull constitutes the only solution. The early appearance of long-lasting musth episodes also occurs in captive or domesticated bulls due to the decreased intensity of dominant relationships43,45, good nutrition and a reduction in environmental stressors8,43,44. Bulls become less responsive to commands and difficult to control12,22,25. Generally they have to be restrained to such an extent that it becomes an animal welfare issue25,30,43,50. Often food and water supply are reduced30,50 and tranquillisers may be employed to allow basic management procedures to continue50. In some instances, bulls have to be removed from working programmes or even euthanased.
Consequently, there is an urgent need to develop methods to control musth and aggressive behaviour in order to improve the well-being of the bulls and the safety of people and other animals. Reducing testosterone secretion could be a way to control musth. Surgical castration has been used13,14,38but is impractical, expensive and irreversible. The use of anti-androgens36, GnRH agonists6,10 and GnRH antagonists6 has been investigated but with limited or no success. The use of gonadotrophin releasing hormone (GnRH) vaccines to down-regulate the hypothalamic-pituitary-gonadal axis could be a useful way to control musth and aggressive behaviour. Immunisation with GnRH has been used successfully in many domestic as well as some non-domestic species9,23,34,51 to control reproduction and androgen associated behaviour. It is reversible23,28,29,34 and no adverse side effects have been recorded23,26,27. The aims of this study were to investigate a possible relationship between concentrations of faecal androgen and glucocorticoid metabolites and aggressive behaviour and to test a GnRH vaccine as a means of controlling aggressive behaviour and musth in African elephant bulls.
MATERIALS AND METHODS
Elephant bulls
Six elephant bulls were used in the study and individually named (see also Table 1).
Kinkel: intractable and hands-off management. Aggressive towards the dominant cow and had pushed her into the moat surrounding the elephant enclosure on a few occasions.
Thembo: wild and free-ranging on Tshukudu Game Reserve (Limpopo Province) but accustomed to the presence of people. Episodes of aggression towards people and other animals had been noted. The bull was captured on Day 66 (day of the 2nd booster) of the study because he was damaging fences and lodges adjacent to the reserve. He was translocated to Elephants for Africa for Ever, which trains elephants, at Moketsi in the Limpopo Province.
Toto, Chaka and Makuvhuzi: Imire Game Park (Zimbabwe): all 3 trained bulls were non-aggressive at the onset of the trial but had shown periods of aggression previously.
Grootvoet: ±40 years old; wild and free-ranging on Shambala Private Game Reserve (Limpopo Province). His musth cycle had started 3 months earlier but due to TGS and urine dribbling he was judged to be in full musth at the time of the 1st vaccination. He was considered to be the only sexually mature bull as he was older than 35 years of age3.
GnRH vaccine and adjuvants
The GnRH vaccine used in this study was previously described by Oonk et al.39 and was provided by Pepscan Systems (Lelystad, The Netherlands). It is a modified GnRH-tandem-dimer-ovalbumin conjugate in which the GnRH molecules are modified by substituting L-glycine in the 6-position with D-lysine to enable conjugation to ovalbumin. The vaccine was originally developed for the immunocastration of male piglets39. Two different adjuvants were used: Montanide® ISA 51 (Seppic, Paris, France), consisting of manide oleate in mineral oil, and CovaccineTM (Covaccine B.V., Utrecht, The Netherlands), which is a proprietary product. Preparation of the vaccine with the Montanide® ISA 51 adjuvant was as follows: 1.5 m ISA 51 was added to 1.5 m
ISA 51 was added to 1.5 m vaccine containing 2 mg peptide conjugate in PBS buffer. The mixture was emulsified using 2 syringes and a connector. In the case of the Covaccine 1.5 m
vaccine containing 2 mg peptide conjugate in PBS buffer. The mixture was emulsified using 2 syringes and a connector. In the case of the Covaccine 1.5 m adjuvant was simply added to 1.5 m
adjuvant was simply added to 1.5 m vaccine and shaken briefly.
vaccine and shaken briefly.
Vaccination protocol
All 6 bulls were immunised with the GnRH vaccine (Table 1). The bulls were vaccinated 3 times at intervals of 3-7 weeks. Despite clear instructions, Toto's 2nd booster was only administered 147 days after the 1st booster instead of the 21 days used for his stable mates. As a result of incomplete dart delivery of the ISA 51-GnRH emulsion during the 1st 3 vaccinations, Kinkel was given a 3rd booster, this time using the Covaccine adjuvant. The vaccine was administered deep intramuscularly into the semimem-branosus-semitendinosus muscle mass by hand (25 mm 18-guage needle) or by means of a dart (5 m Dan-Inject® dart fitted with 60 mm barbless needle; Dan-Inject ApS, Børkop, Denmark).
Collection and storage of faecal and serum samples
Faecal samples were collected on 4 to 5 consecutive days prior to the primary vaccination (Stage 1); 2 weeks after each vaccination (Stages 2, 3, 4 and 5; Stage 5, Kinkel only) and 2 (Stage 6; Thembo only) and 4 months (Stage 7) after the last vaccination. Sampling during Stages 4 and 7 was not possible for the Imire elephants and Grootvoet, respectively. An additional sample was collected from Grootvoet 3 months before the lst vaccination. Immediately after defaecation a 50 g aliquot was taken from the centre of a faecal ball, transferred to a labelled plastic zip-lock bag and transported on ice until freezing (-20 ºC) 30 min to 4 hours later. In the case of Kinkel (Johannesburg Zoo), however, samples could only be collected in the morning once the bull had left his night room.
Blood samples were collected from 2 bulls while immobilised (Thembo and Grootvoet) and from the 3 tractable Imire bulls (Makavhuzi, Chaka and Toto) when feasible (Table 2). As Kinkel was neither tractable nor immobilised during the trial, no serum samples could be collected from him. Once separated, the serum was stored at -20 ºC until analysed.
Faecal steroid analyses
The extraction procedure used for both steroid groups has been described33,35. Briefly, 0.5 g of thawed wet faeces was extracted with 80 % aqueous methanol. Following centrifugation, 1 m of the supernatant was transferred into a new vial and 5 m
of the supernatant was transferred into a new vial and 5 m diethylether plus 0.2 m
diethylether plus 0.2 m 5% NaHCO3 added. This mixture was vortexed for 10 s, centrifuged (3000 × g for 15 min) and then frozen for 30 min at -70 ºC. The diethylether supernatant was transferred into a new vial and dried down under a flow of nitrogen at 45 ºC. The dried extracts were redisolved in 0.5 m
5% NaHCO3 added. This mixture was vortexed for 10 s, centrifuged (3000 × g for 15 min) and then frozen for 30 min at -70 ºC. The diethylether supernatant was transferred into a new vial and dried down under a flow of nitrogen at 45 ºC. The dried extracts were redisolved in 0.5 m assay buffer.
assay buffer.
The epiandrosterone EIA40 used in this study has been validated for African elephants15. The study demonstrated that concentrations of measured FAM are a reliable indicator of circulating blood testosterone concentrations in African elephant bulls. The 11-oxoaetiocholanolone EIA (measuring cortisol metabolites with a 3α-hydroxy-11-oxo-structure) was validated for African elephants and the measured FCM were demonstrated to be a reliable indicator of blood cortisol concentrations16. This EIA has been described in detail35.
Serum testosterone
Total serum testosterone concentrations were analysed using a direct radioimmunoassay (RIA) kit (Coat-a-Count® Total Testosterone, Diagnostic Products Corporation, Los Angeles, CA). This assay has been validated in domestic animals1,46 and used for wildlife species such as cheetah and African wild dogs2.
Collection of behavioural data
Frequency of aggressive behaviour was assessed daily during Stages 1, 2, 3, 4, 5 and 7 (Kinkel); Stages 1, 4 and 7 (Thembo) and during Stages 1, 2 and 7 (Imire bulls) using behavioural traits relating to aggression, musth and dominance as previously described19,43,45.In total, 38 different behavioural traits related to aggression were used. Some of the more important ones were: head high, chin tucked in, shaking head, ear-flapping, waving, forward trunk swing, pushing, kicking, charging/advance towards, grabbing, throwing or destroying objects, tusking, rumbling and urine dribbling. The monitoring frequency of the Imire bulls and Thembo was lower because of distance constraints. Grootvoet was free-ranging and mostly solitary so that behaviour towards other elephants could not be monitored. Observations allowed the classification of bulls as being in musth or non-musth, compare frequencies of aggressive behaviour before and after immunisation, determining the dominance hierarchy between bulls and detection of other possible behavioural and physical changes. A male in full musth exhibits temporal gland secretion and urine dribbling, or evidence of recent urine discharge43.
Data analysis
For each animal, the samples and observations were grouped in Stage 1 (before primary vaccination), Stage 2 (after primary vaccination), Stage 3 (after lst booster), Stage 4 (after 2nd booster), Stage 5 (after 3rd booster), Stage 6 (2 months after last booster) and Stage 7 (4 months after last booster). FAM were analysed by repeated measures ANOVA. For each bull, differences in concentrations between different stages were analysed by means of 1-way ANOVA with post hoc comparison using the Tukey-Kramer multiple comparison test. Mean FAM values for aggressive and non-aggressive non-musth bulls during Stage 1 were grouped and compared using the 2-sample t-test. The non-parametric Spearman rank order correlation test was used to analyse the correlation between FAM and FCM for each individual bull. The α-level of significance was set at <0.05 for all the tests.
RESULTS
Vaccination
None of the bulls showed injection-site reactions or signs of lameness at any stage after vaccination. Reaction of bulls to darting was brief and varied from mild surprise to brief confusion which ceased within 10 min. Hand-injection of the 3 tractable bulls was well tolerated. As mentioned under 'vaccination protocol', Toto's 2nd booster was overlooked and only administered 147 days (21 weeks) after the 1st booster instead of the 3-7 weeks required by the protocol. Vaccination using the Dan-Inject® darting system was found to be a practical means of administering the vaccine with the Covaccine adjuvant. The Montanide ISA 51 adjuvant was unsuitable for dart delivery because the emulsion was too viscous, resulting in incomplete injection of the vaccine. After this vaccine-adjuvant combination was once again unsuccessful during the 2nd booster vaccination of Kinkel, ISA 51 was abandoned as adjuvant. Hand injection was suitable to vaccinate trained bulls.
Behaviour and GnRH vaccination
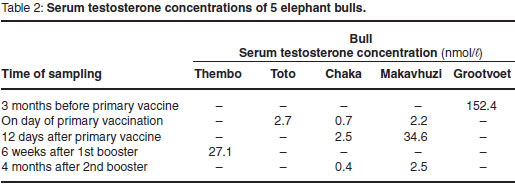
The frequencies of aggressive behaviour in relation to the stages of the study are shown in Fig. 1. Grootvoet was the only bull in musth at the time and before the primary vaccination. He went out of musth 10 days after the 1st vaccination and his aggressive behaviour ceased completely. Kinkel and Thembo showed both aggressive behaviour and TGS without urine dribbling before the primary vaccination. In Kinkel, TGS ceased and aggressive behaviour was reduced after the 4th vaccination. The bull remained docile until the end of the observation period. Thembo's aggressive behaviour and TGS ceased after the 2nd booster vaccination and no further aggressive behaviour or irritability was observed. Toto, Chaka and Makavhuzi did not show aggressive behaviour prior to vaccination. No changes in their behaviour were observed throughout the study. Chaka, however, came into full musth 10 months after the last vaccination, which was 6 months after the end of the trial.
Faecal androgen metabolites (FAM)
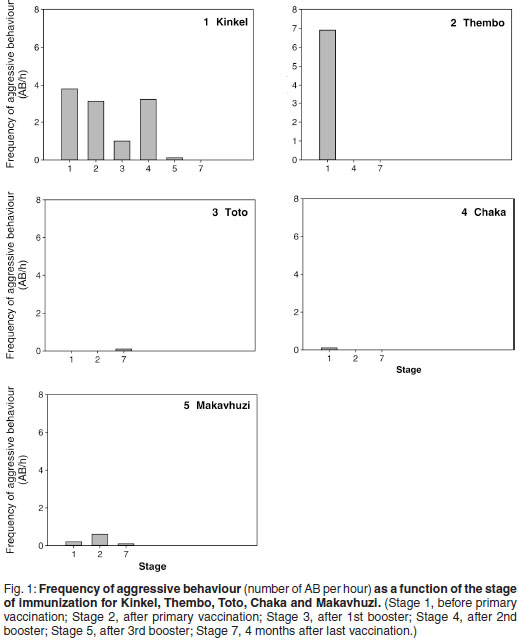
The relationship between behaviour and FAM concentrations during Stage 1 is shown in Fig. 2. The 2 aggressive bulls Kinkel and Thembo (179 ± 8 ng/g) had significantly (P < 0.05) higher concentrations than the 3 non-aggressive bulls Makavhuzi, Chaka and Toto (97 ± 31 ng/g).

Figure 3 shows the within bull effects of a GnRH vaccination on FAM concentrations. Significant differences between stages were observed in Kinkel (P < 0.05), Thembo (P < 0.001), Toto (P < 0.05) and Chaka (P < 0.05). Only Stages 1, 5 and 7 were taken into consideration for Kinkel due to the lack of complete administration when Montanide ISA 51 was used for the first 3 vaccinations. No significant differences were found in Makavhuzi and Grootvoet. The faecal sample collected from Grootvoet 3 months before the 1st vaccination (in full musth), however, had a FAM concentration (209 ng/g) approximately 3 times higher than the samples collected during Stages 1, 2, 3 and 4 (62 ± 24 ng/g).
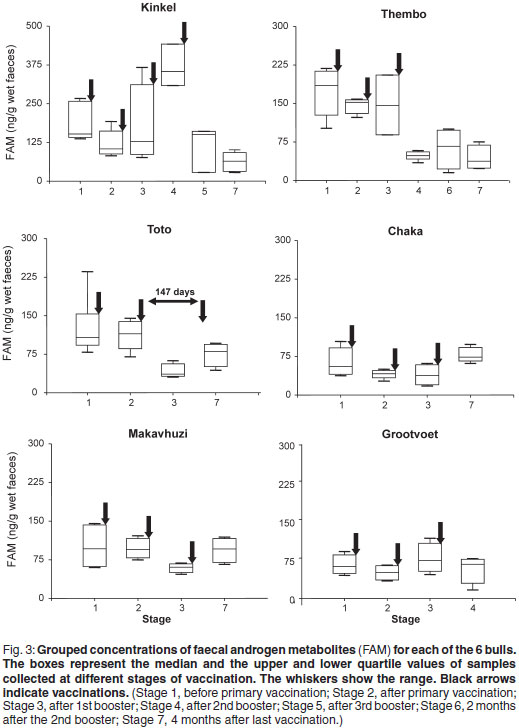
Serum testosterone
The results of the serum testosterone assays are shown in Table 2. They were significantly correlated with FAM concentrations collected during the corresponding periods (r = 0.83; P < 0.005).
Faecal cortisol metabolites (FCM)
FCM concentrations in relation to the stages are shown in Fig. 4. Again, only Stages 1, 5 and 7 were taken into consideration for Kinkel. Significant differences between stages were observed in Kinkel (P = 0.05), Thembo (P = 0.05), Chaka (P < 0.01), Makavhuzi (P < 0.05) and Grootvoet (P < 0.001). Wide variations were observed throughout in Toto and there were no significant differences between stages.
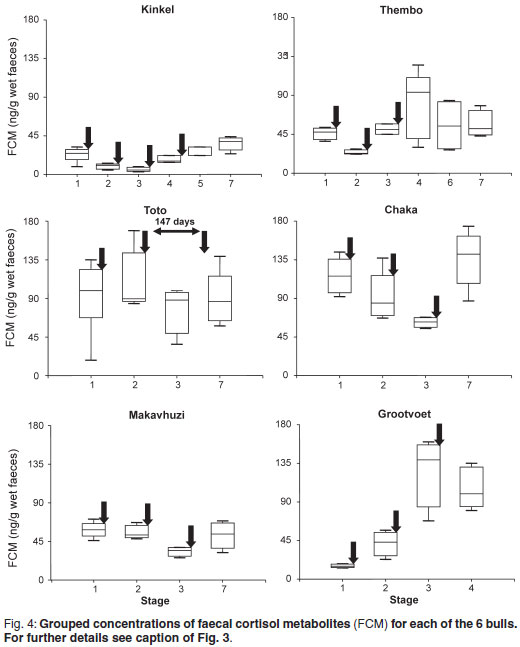
There were no significant correlations between FAM and FCM in Kinkel, Thembo, Toto and Grootvoet whereas positive correlations were found for Chaka (r = 0.51; P < 0.05) and Makavhuzi (r = 0.48; P < 0.05).
DISCUSSION
This is the 1st study that reports the use of a GnRH vaccine to attempt down-regulation of musth or aggressive behaviour in African elephant bulls. Working with wildlife species like the African elephant often makes accessing a suitable sample size for research projects difficult, particularly when a new unproven drug is being tested. Another constraint is the accessibility of individual animals. It is not always possible to carry out treatments and sampling as required in a research protocol. The safety of vaccine-adjuvant administration was clearly demonstrated in the bulls of this trial.
When non-musth bulls were divided into aggressive (Thembo and Kinkel) and non-aggressive groups (Toto, Chaka and Makavhuzi) during Stage 1, behaviour was well correlated to FAM concentration. The mean FAM concentration of the non-aggressive bulls was significantly lower than the mean of the aggressive bulls. During this stage both aggressive bulls had permanent mucoid TGS without urine dribbling which is equivalent to the 1st and last stage of full musth (bulls entering or leaving full musth17,18). Kinkel's behaviour only improved after the 3rd booster vaccination which was the 1st time vaccine delivery was complete. This was accompanied by cessation of TGS and a significant lowering of FAM concentrations during Stages 5 and 7. Thembo's behaviour, accompanied by a cessation of TGS, improved after the 1st booster. A significant decrease in FAM concentration was also seen. At the same time he was taken into captivity where he has remained for the last 6years. He is revaccinated (since 2006 with the commercially available GnRH vaccine Improvac, Pfizer Animal Health, Sandton, South Africa) every 6 to 8 months, is tractable and used for education purposes and elephant-back rides.
Of the 3 non-aggressive bulls, Toto also showed a significant decrease in FAM after the 1st booster (Stage 3). The other 2 other bulls (Chaka and Makavhuzi) showed no significant changes, with concentrations having been low from the onset of the trial. They remained non-aggressive for the remainder of the observation period and for another 6 months thereafter when Chaka came into musth. At the time, he and the 2 other bulls had not been revaccinated since the 2nd booster.
The results of Grootvoet are somewhat of an enigma. From a behavioural point of view, (presence of TGS and urine dribbling) he was in full musth during Stage 1. However, FAM concentrations during Stage 1 reflected those of nonmusth, non-aggressive bulls. A faecal sample taken 3 months previously during full musth (same musth cycle) had a 3 times higher FAM concentration (209 ng/g), typically found in musth bulls. This was corroborated by a serum testosterone concentration of 152 nmol/ in a blood sample collected on the same day. Musth behaviour ceased 10 days after primary vaccination. In our opinion, given his lower FAM concentrations during Stage 1 and the fact that he had already been in full musth for at least 3 months, he was at the end of his musth cycle. The vaccine may or may not have assisted in ending musth. Certainly 10 days were sufficient to have allowed an initial rise in antibody titre.
in a blood sample collected on the same day. Musth behaviour ceased 10 days after primary vaccination. In our opinion, given his lower FAM concentrations during Stage 1 and the fact that he had already been in full musth for at least 3 months, he was at the end of his musth cycle. The vaccine may or may not have assisted in ending musth. Certainly 10 days were sufficient to have allowed an initial rise in antibody titre.
It should also be mentioned that immobilisation of Thembo may have had a temporary influence on androgen secretion. At the end of Stage 3 when his FAM concentrations were still elevated and serum testosterone was above baseline concentrations (27 nmol/ ) he was captured using etorphine and xylazine for relocation into captivity. Opioids and tranquillisers are known to temporarily decrease pulsatile release of GnRH4,48. Brown et al.6 found reduced testosterone concentrations in a musth bull 2 days after capture but sensitivity to anaesthesia and the effects of capture seemed to be more specific in musth than non-musth bulls. On the other hand, repeated immobilisations of a captive Asian bull in musth did not suppress signs of musth, although androgens were not monitored in this bull25. Another factor that needs to be considered is the possible effect of stress in the case of Thembo. Chronic stress is known to decrease pulsatile release of GnRH and thus androgen secretion24,31,37. Prior to capture (Stages 1, 2 and 3) Thembo's faecal FCM concentrations were low (
) he was captured using etorphine and xylazine for relocation into captivity. Opioids and tranquillisers are known to temporarily decrease pulsatile release of GnRH4,48. Brown et al.6 found reduced testosterone concentrations in a musth bull 2 days after capture but sensitivity to anaesthesia and the effects of capture seemed to be more specific in musth than non-musth bulls. On the other hand, repeated immobilisations of a captive Asian bull in musth did not suppress signs of musth, although androgens were not monitored in this bull25. Another factor that needs to be considered is the possible effect of stress in the case of Thembo. Chronic stress is known to decrease pulsatile release of GnRH and thus androgen secretion24,31,37. Prior to capture (Stages 1, 2 and 3) Thembo's faecal FCM concentrations were low ( 45 ng/g) with small variations. Following capture they were significantly higher during Stage 4 with a much larger variation (Fig-. 4), but very similar to those of Toto and Chaka and Grootvoet following the 1st booster. Thembo was calm and tractable from the outset in captivity but the wider range during Stage 4 may reflect anxiety resulting from new experiences in captivity. The ranges narrowed progressively during Stages 6 and 7. A possible role of chronic stress can therefore not be excluded.
45 ng/g) with small variations. Following capture they were significantly higher during Stage 4 with a much larger variation (Fig-. 4), but very similar to those of Toto and Chaka and Grootvoet following the 1st booster. Thembo was calm and tractable from the outset in captivity but the wider range during Stage 4 may reflect anxiety resulting from new experiences in captivity. The ranges narrowed progressively during Stages 6 and 7. A possible role of chronic stress can therefore not be excluded.
Previous attempts to control musth and aggressive behaviour, without resorting to castration, have yielded limited success and have only been tested on 1 or 2 bulls. Anti-androgens36, GnRH super-agonists6,10 or antagonists6 were employed and the GnRH super-agonists produced more favourable results. Brown et al.6 showed that the GnRH agonist leuprolide disrupts normal pituitary-gonadal function in free-ranging African elephant bulls. Initially testosterone was reduced to baseline concentrations. The testes, however, became hyper-responsive to gonadotrophic stimulation. Similar results were obtained by one of the authors (HJB) with deslorelin implants in a donkey jack. The implants initially suppressed testosterone release but a rebound occurred after 10 days and testosterone concentrations actually increased in relation to pre-treatment concentrations. One study showed that administration of leuprolide acetate to a 52-year old Asian bull for several years decreased testosterone concentrations and musth could be prevented when the drug was administered during the 1st pre-musth manifestations10. Whether or not the GnRH super-agonists are reliable, the formulations used cannot be administered remotely by means of darting.
There appeared to no positive relationship between aggressive behaviour and faecal FCM concentrations. Kinkel, who showed a high frequency of aggressive behaviour during Stages 1-4 had consistently low concentrations of FCM. Thembo's concentrations were also low during Stage 1 when he exhibited aggressive behaviour. Mean concentrations and ranges increased after cessation of aggressive behaviour. In Grootvoet mean concentrations and ranges increased once musth ceased. Two of the non-aggressive bulls (Toto and Chaka) had high means and ranges of FCM concentrations almost throughout the study whereas FCM concentration in the 3rd non-aggressive bull was consistently lower throughout. It has been reported that high androgen concentrations in African elephants seemed to suppress cortisol secretion17,18. The mechanism, however, could not be explained. A similar relationship between FAM and FCM concentrations could not be confirmed in our study.
Despite the limitations of this study, which include low sample size, some inconsistencies in the vaccination protocol and difficulties surrounding behavioural observations of 2 bulls, the results were encouraging. Most studies with GnRH vaccine in other species failed to produce an adequate response in 100 % of treated animals. It seems that a certain number of non-responders can be expected23,32,49,51,52. The reasons for individual variations are not completely understood. Age has clearly been shown to influence the results with older animals showing greater individual variation, less marked responses and shorter duration of effects9,32,49,51,52.In horses, 2 vaccinations with the modified GnRH-tandem-dimer vaccine-Covaccine adjuvant-combination were sufficient to suppress testosterone secretion in young sexually mature pony stallions but further boosters were generally needed in older stallions49. In blackbuck (Antilope cervicapra) and springbok (Antidorcas marsupialis), testosterone concentrations were reduced in young but not in adult rams51. The authors of a recent study23 were able to suppress testosterone concentrations in 4 of 5 stallions treated with a GnRHprotein conjugate (EquityTM) for at least 6 months. The stallion that did not respond also showed the lowest antibody titres. The same stallion, however, showed a marked decrease in libido while 1 of the good responders with low testosterone concentrations demonstrated good libido. The behavioural response (libido suppression) was better than that reported by other authors7,52. These findings show that the control of sexual behaviour is complex and not only testosterone dependent. It is also affected by factors such as age and previous sexual experience in the horse49. Rather than having non-responders in our trial the results in some of the bulls were inconclusive. Two aggressive non-musth young bulls responded whereas in the non-aggressive 3 young bulls a response was difficult to assess. One of these bulls did, however, show a reduction in androgen production. The results for the sexually mature adult bull in the trial were inconclusive as he was probably going out of musth around the time of the primary vaccination.
CONCLUSIONS
The most significant finding of the current study was the effect of the GnRH vaccination on behaviour. Vaccination per se did not cause aggression or disturbance in any of the bulls and no other side effects were observed. At least 2 bulls and possibly a 3rd, showing aggressive behaviour prior to treatment, demonstrated substantial improvement. TGS also ceased. Aggressive behaviour did not recur within the 4-month observation period after the final booster. As expected, the non-aggressive bulls showed no changes in behaviour.
ACKNOWLEDGEMENTS
We thank Johannesburg Zoo, Imire Game Park, Tshukudu Game reserve, Shambala Private Reserve and Elephants for Africa for Ever for allowing us to use their elephant bulls in the trial and for their assistance in the collection of samples and the University of Pretoria and the South African Veterinary Foundation for financial support. We also thank Mrs Monika Höring for the FCM analysis.
REFERENCES
1. Almond G W, Esbenshade K L, Smith C A, Richards G 1992 Effects of chronic gonadotrophin-releasing hormone agonist treatment on serum luteinizing hormone and testosterone concentrations in boars. American Journal of Veterinary Research 53:22-25 [ Links ]
2. Bertschinger H J, Trigg T E, Jöchle W, Human A 2002 Induction of contraception in some African wild carnivores by down-regulation of LH and FSH secretion using the GnRH analogue deslorelin. Reproduction Supplement 60:41-52 [ Links ]
3. Bertschinger H., Delsink A., van Altena J J, Kirkpatrick J., Killian H., Ganswindt A., Slotow R., Castley G 2008 Reproductive control of elephants. In Scholes R J, Mennel K G (eds) Elephant management: a scientific assessment for South Africa. Wits University Press, Johannesburg: 257-328 [ Links ]
4. Blank M S, Fabbri A, Catt K J, Dufau M L 1986 Inhibition of luteinizing hormone release by morphine and endogenous opiates in cultured pituitary cells. Endocrinology 118:2097-2101 [ Links ]
5. Brannian J D, Griffin F, Terranova P F 1989 Urinary androstenedione and luteinizing hormone concentrations during musth in a mature African elephant. Zoo Biology 8:165-170 [ Links ]
6. Brown J L, Bush M, Wildt D E, Raath J R, de Vos V, Howard J G 1993 Effects of GnRH analogues on pituitary-testicular function in free-ranging African elephants (Loxodonta africana). Journal of Reproduction and Fertility 99:627-634 [ Links ]
7. Clement F, Vidament M, Daels P, Van der Meer F, Larry J L, Colenbrander B, Turkstra J 2005 Immunocastration in stallions: effect on spermatogenesis and behaviour. Animal Reproduction Science 89:230-233 [ Links ]
8. Cooper K A, Harder J D, Fredrick D L, Lodge G A, Peachey H C, Spellmire T J, Winstel D P, Clawson D H 1990 Serum testosterone and musth in captive male African and Asian elephants. Zoo Biology 9:297-306 [ Links ]
9. Curtis P D, Pooler R L, Richmond M E, Miller L A, Mattfeld G F, Quimby F W 2002 Comparative effects of GnRH and porcine zona pellucida (PZP) immunocontraceptive vaccines for controlling reproduction in white-tailed deer (Odocoileus virginianus). Reproduction Supplement 60:131-141 [ Links ]
10. de Oliveira C A, West G D, Houck R, Leblanc M 2004 Control of musth in an Asian elephant bull (Elephas maximus) using leuprolide acetate. Zoo and Wildlife Medicine 35:70-76 [ Links ]
11. Dickerman R D, Zachariah N Y, Fouraker M, McConathy W J 1997 Neuroendocrine-associated behavioral patterns in the male Asian elephant (Elephas maximus). Physiology and Behavior 61:771-773 [ Links ]
12. Eisenberg J F, McKay G M, Jainudeen M R 1971 Reproductive behavior of the Asiatic elephant (Elephas maximus maximus). Behaviour 38:193-225 [ Links ]
13. Foerner J J, Houck R I, Copeland J F, Schmidt M J, Byron H T, Olsen J H 1994 Surgical castration of the elephant (Elephas maximus and Loxodonta africana). Journal of Zoo and Wildlife Medicine 25:355-359 [ Links ]
14. Fowler M E, Hart R 1973 Castration of an Asian elephant, using etorphine anesthesia. Journal of the American Veterinary Medical Association 163:539-543 [ Links ]
15. Ganswindt A, Heistermann M, Borragan S, Hodges J K 2002 Assessment of testicular endocrine function in captive African elephants by measurement of urinary and faecal androgens. Zoo Biology 21:27-36 [ Links ]
16. Ganswindt A, Palme R, Heistermann M, Borragan S, Hodges J K 2003 Non-invasive assessment of adrenocortical function in the male African elephant (Loxodonta africana) and its relation to musth. General and Comparative Endocrinology 134:156-166 [ Links ]
17. Ganswindt A, Heistermann M, Hodges J K 2005 Physical, physiological and behavioural correlates of musth in captive African elephants (Loxodonta africana). Physiological and Biochemical Zoology 78:505-514 [ Links ]
18. Ganswindt A, Rasmussen H B, Heistermann M, Hodges J K 2005 The sexually active states of free-ranging male African elephants (Loxodonta africana): Defining musth and non-musth using endocrinology, physical signals, and behaviour. Hormones and Behavior 47(1):83-91 [ Links ]
19. Garai M E 1997 The development of social behaviour in translocated juvenile African elephants Loxodonta africana (Blumenbach). PhD thesis, University of Pretoria [ Links ]
20. Hall-Martin A J, Van Der Walt L A 1984 Plasma testosterone levels in relation to musth in the male African elephant. Koedoe 27:147-149 [ Links ]
21. Jainudeen M R, Katongole C B, Short R V 1972 Plasma testosterone levels in relation to musth and sexual activity in the male Asiatic elephant, Elephas maximus. Journal of Reproduction and Fertility 29:99-103 [ Links ]
22. Jainudeen M R, Mc-Kay G M, Eisenberg J F 1972 Observations on musth in the domesticated Asiatic elephant (Elephas maximus). Mammalia 36:247-261 [ Links ]
23. Janett F, Stump R, Burger D, Thun R 2009 Suppression of testicular function and sexual behaviour by vaccination with GnRH (EquityTM) in the adult stallion. Animal Reproduction Science 115:88-102 [ Links ]
24. Katsiia G V, Todua T N, Gorlushkin V M, Chirkov, A M, Goncharov N P 1989 [Effect of immobilization stress on the gonadotropic function of the hypophysis in male hamadryas baboons (Papio hamadryas)]. Biulleten' Eksperimental'Noi Biologii i Meditsiny 107:231-234 [ Links ]
25. Kock N, Kock M 1984 Management of two Indian elephants (Elephas maximus indicus) in a Middle Eastern zoo. American Association of Zoo Veterinarians Annual Proceedings 1984:75-81 [ Links ]
26. Kumar N, Savage T, DeJesus W, Tsong Y Y, Didolkar A, Sundaram K 2000 Chronic toxicity and reversibility of antifertility effect of immunization against gonadotropin-releasing hormone in male rats and rabbits. Toxicological Sciences 53:92-99 [ Links ]
27. Ladd A 1993 Progress in the development of anti-LHRH vaccine. American Journal of Reproductive Immunology 29:189-194 [ Links ]
28. Ladd A, Tsong Y Y, Walfield A M, Thau R 1994 Development of an antifertility vaccine for pets based on active immunization against luteinizing hormone-releasing hormone. Biology of Reproduction 51:1076-1083 [ Links ]
29. Lincoln G A, Fraser H M, Fletcher T J 1982 Antler growth in male red deer (Cervus elaphus) after active immunization against LH-RH. Journal of Reproduction and Fertility 66:703-708 [ Links ]
30. Lincoln G A, Ratnasooriya W D 1996 Testosterone secretion, musth behaviour and social dominance in captive male Asian elephants living near the equator. Journal of Reproduction and Fertility 108:107-113 [ Links ]
31. López-Calderón A, Ariznavarreta C, González-Quijano M I, Tresguerres, J A, Calderón, M D 1991 Stress induced changes in testis function. Journal of Steroid Biochemistry and Molecular Biology 40:473-479 [ Links ]
32. Malmgren L, Andresen O, Dalin A M 2001 Effect of GnRH immunisation on hormonal levels, sexual behaviour, semen quality and testicular morphology in mature stallions. Equine Veterinary Journal 33:75-83 [ Links ]
33. Merl S, Scherzer S, Palme R, Möstl E 2000 Pain causes increased concentrations of glucocorticoid metabolites in horse feces. Journal of Equine Veterinary Sciences 20:586-590 [ Links ]
34. Miller L A, Johns B E, Killian G J 2000 Immunocontraception of white-tailed deer with GnRH vaccine. American Journal of Reproductive Immunology 44:266-274 [ Links ]
35. Möstl E, Maggs J L, Schrotter G, Besenfelder U, Palme R 2002 Measurement of cortisol metabolites in faeces of ruminants. Veterinary Research Communications 26:127-139 [ Links ]
36. Niemuller C A, Brown J L, Hodges J K 1998 Reproduction in elephants. In Knobil E, Neill J D (eds) Encyclopedia of reproduction. Academic Press, New York: 1018-1029 [ Links ]
37. Norman R L 1993 Effects of corticotrophin-releasing hormone, testosterone and cortisol secretion in intact male rhesus macaques. Biology of Reproduction 40:148-153 [ Links ]
38. Olsen J H, Byron H T 1993 Castration of the elephant. In Fowler M E (eds) Zoo and wild animal medicine: current therapy. Saunders Company, Philadelphia: 441-444 [ Links ]
39. Oonk H B, Turkstra J A, Schaaper W M, Erkens J H, Schuitemaker-de Weerd M H, van Nes A, Verheijden J H, Meloen R H 1998 New GnRH-like peptide construct to optimize efficient immunocastration of male pigs by immunoneutralization of GnRH. Vaccine 16:1074-1082 [ Links ]
40. Palme R, Möstl E 1994 Biotin-streptavidin enzyme immunoassay for determination of oestrogens and androgens in boar faeces. In Görög S (ed.) Advances of steroid analysis '93' Akadémiai Kiadó, Budapest: 111-117 [ Links ]
41. Poole J H, Moss C J 1981 Musth in the African elephant, Loxodonta africana. Nature 292:830-831 [ Links ]
42. Poole J H, Kasman L H, Ramsay E C, Lasley B L 1984 Musth and urinary testosterone concentrations in the African elephant (Loxodonta africana). Journal of Reproduction and Fertility 70:255-260 [ Links ]
43. Poole J H 1987 Rutting behavior in African elephants: the phenomenon of musth. Behaviour 102:283-316 [ Links ]
44. Poole J H 1987b Raging bulls. Animal kingdom 90:18-25 [ Links ]
45. Poole J H, Granli P K Visual and tactile signals of African savannah elephants. Online at: http://www.elephantvoices.org/ (accessed January 2009) [ Links ]
46. Reimers T J, Lamb S V, Bartlett S A, Matamoros R A, Cowan R G, Engle J S 1991 Effects of hemolysis and storage on quantification of hormones in blood samples from dogs, cattle, and horses. American Journal of Veterinary Research 52:1075-1080 [ Links ]
47. Slotow R, van Dyk G, Poole J, Page B, Klocke A 2000 Older bull elephants control young males. Nature 408:425-426 [ Links ]
48. Stojilkovicacute S, Dufau M L, Catt K J 1987 Receptors and secretory actions of sigma/phencyclidine agonists in anterior pituitary cells. Endocrinology 121:2044-2054 [ Links ]
49. Stout T A E, Colenbrander B 2004 Suppressing reproductive activity in horses using GnRH vaccines, antagonists or agonists. Animal Reproduction Science 82-83:633-643 [ Links ]
50. Thakuria D B, Barthakur T 1996 Management of musth in a male African elephant by chemical sedatives in the Assam State Zoo, Guwahati. Indian Veterinary Journal 73:339-340 [ Links ]
51. Turkstra J A, Schaftenaar W, Klaver P, Meloen R H 2001 Immunization against GnRH to control fertility and sexual behaviour in zoo-animals. Proceedings of the 40th International Symposium on Diseases of Zoo and Wildlife Animals, Rotterdam, The Netherlands, 23-26 May 2001: 313 [ Links ]
52. Turkstra J A, Van der Meer F, Knaap J, Rottier P, Teerts K, Colenbrander, Meloen R 2005 Effects of GnRH immunization in sexually mature pony stallions. Animal Reproduction Science 3-4:247-259 [ Links ]
Received: September 2009.
Accepted: January 2010.
* Author for correspondence. E-mail: henkbert@tiscali.co.za














