Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.78 Durban 2024
http://dx.doi.org/10.17159/0379-4350/2024/v78a09
RESEARCH ARTICLE
Oxidation of 1-hexene using supported ruthenium catalysts under solvent-free conditions
Raiedhah Abdullah Alsaiari
Empty Quarter Research Unit, Department of Chemistry, College of Science and Art in Sharurah, Najran University, Sharurah, Saudi Arabia
ABSTRACT
The study evaluated the solvent-free oxidation of 1-hexene utilizing supported ruthenium catalysts and a small amount of terbutyl hydroperoxide (TBHP) as a radical initiator. The characteristics of the supported catalysts were characterized using transmission electron microscopy (TEM) and N2 adsorption-desorption isotherms. The results of the study revealed that 1% Ru/TiO2 had favorable catalytic performance when subjected to environmentally friendly reaction conditions. This behavior was thought to be a result of Ru spreading and loading on the TiO2. To obtain optimal reaction parameters, the catalyst manufacturing process, temperature, reaction time, type of support, and activating amount were tuned. Compared to catalysts generated using wet-impregnation and precipitation techniques, those produced using sol-immobilization have greater activity.
Keywords: 1-hexene, oxidation, epoxide, ruthenium catalyst, sol-immobilization
INTRODUCTION
The subject of green chemistry has significant importance in contemporary chemical synthesis, including both academic and industrial domains.1,2 Oxidation processes play a crucial role in the production of fine compounds. Selective catalytic C-H oxidation is a very significant process, with considerable importance in both industrial and research contexts. According to the fundamentals of green chemistry, catalytic processes that make use of oxygen derived from the atmosphere are to be favored. Oxygen is a diradical in its ground state, which makes it easy to activate in a variety of radical reactions. This makes oxygen helpful for a variety of low-temperature oxidations since it can be activated quickly and easily in these processes. However, many molecules and catalysts are inactive when exposed to molecular oxygen. As a result, more reactive forms of terminal oxidant must be used. Frequently, this requires the use of a stoichiometric oxygen source that is not environmentally friendly. The oxidation of alkenes has emerged as a captivating subject in the field of heterogeneous catalysis, prompting several investigations on this particular chemical transformation. The allylic oxidation of alkenes, which yields epoxides, allylic alcohols, and a,b-unsaturated ketones, has considerable significance within the field of natural product synthesis.3
There exists a limited number of studies that assess the efficacy of ruthenium (Ru) as a heterogeneous catalyst in the context of epoxidation processes. In the year 1998, researchers used mesoporous MCM41 sieves for their experimental purpose.3-5 [RuII(TDCPP)(CO) (EtOH)] is a Ru compound used for immobilizing meso-tetrakis, also known as (2,6-dichlorophenyl) porphyrin. The supported Ru catalyst impacted heterogeneous alkene epoxidations, with 2,6-dichloropyridine N-oxide and CH2Cl2 being the best terminal oxidants. Subsequent investigations revealed that polyethylene glycol had the capability to establish a covalent etheric linkage with Ru porphyrin. The catalysts include notable characteristics such as high reactivity and the ability to selectively catalyze alkene epoxidation using 2,6-dichloropyridine N-oxide as the terminal oxidant.6,7
Further investigation was conducted to examine the effectiveness of catalysts consisting of Ru-loaded H-montmorillonite (H-Mont) and Ti-pillared clay (PILC) for the oxidation of cyclohexene. tert-butyl hydroperoxide (TBHP) was used as the source of oxygen for this study.
Ru-Ti-PILC's catalytic performance was superior to that of Ru/H-Mont. The latter had a 59% effect on cyclohexene at a concentration of 5%, while the former was responsible for 87% differentiation of 2-cyclohexane-1-one and 13% differentiation of 2-cyclohexane-1-ol. After 6 hours of reaction at 70 oC, no epoxide was produced.8 Prior studies have used supported Ru catalysts (1% Ru/TiO2) for solvent-free 1-decene epoxidation. The 1% Ru/TiO2 catalyst was created using the sol-immobilization procedure, which led to increased activity for the epoxidation of 1-decene.9 Our prior research shown that oxygen as an oxidant and TBHP as a radical initiator may efficiently oxidize a variety of alkenes on supported ruthenium nanoparticles.
This study aims to expand upon our prior research on the oxidation of different alkenes using catalysts composed of supported ruthenium nanoparticles. The objective is to explore the possibility of oxidizing straight chain alkenes by a comparable mechanism. This study provides more evidence supporting the selective oxidation of 1-hexene under ecologically sustainable circumstances, even in solvent-free conditions of moderate severity. This study showcases the potential for improving the conversion of 1-hexene while simultaneously increasing selectivity towards 1-hexene-3-ol and 1-hexene-3-one by the optimization of reaction conditions, specifically focusing on the support and catalyst production process.
MATERIALS AND METHODS
The chemicals used in this investigation were obtained from commercial entities, namely Sigma Aldrich, and employed in their original form without any alterations. In order to facilitate a comparative analysis, the catalyst was synthesized using three established methodologies: sol-immobilization, deposition precipitation, and wet-impregnation techniques. The quantity of catalyst metal deposited onto the support was expressed as a weight percentage. RuCl3-xH2O, 1-hexene, terbutyl hydroperoxide (TBHP) (radical intitators), polyvinyl alcohol (PVA, Sigma-Aldrich, 80% hydrolysed); sodium borohydride (NaBH4, Sigma-Aldrich, 99.99%).
Catalyst preparation - sol immobilization
Some 0.5 g of 1% Ru/support catalysts were produced using the following method: in a 600 mL glass container, aqueous precursor solution (RuCl3.xH2O) and PVA (10 mg mL-1, PVA mass: metal mass = 1: 1) were added to vigorously agitated DI water (140 mL). Following a 15-minute period of mixing, a newly made solution of NaBH4 (0.15 M, with a molar ratio of 4:1 for metal to mole) was promptly introduced, and the resultant solution was agitated for an additional 30 minutes. The support material was introduced into the colloidal solution and subjected to agitation for an additional duration of 30 minutes, facilitating the process of immobilization. The catalyst obtained was subjected to filtration, followed by washing with deionized water at a ratio of 1 L per gram, in order to eliminate any inorganic ions. Subsequently, the catalyst was dried under static air conditions at a temperature of 110 °C for a duration of 16 hours.10,11
In order to obtain 0.5 g of supported metal catalyst using wet-impregnation method, the calculated amount of RuCl3.xH2O was dissolved in suitable amount of distilled water, a specific amount of support was added, and the mixture was evaporated while being stirred continuously at 80 oC. After drying the paste at 110 °C for 16 hours, it was ground into a fine powder and calcined at 300 °C for 3 hours, with a calcination heating rate of 20 oC per minute.
Deposition precipitation
A catalyst consisting of 1% Ru/TiO2 was made using the following procedure: 0.99 g of support was combined with 150 mL of distilled water and subjected to stirring at a temperature of 60 °C. In this experimental procedure, a necessary solution of RuCl3-xH2O was introduced, followed by the gradual addition of a NaOH solution drop by drop to the combination in order to maintain a consistent pH level of 9. After a duration of 1.5 hours, the solution underwent filtration, and subsequently, the solid was rinsed with a volume of 1 liter of distilled water. The catalyst underwent a drying process at a temperature of 110 °C for a duration of 16 hours, followed by calcination in a static air environment at a temperature of 300 °C for a period of 3 hours.
Catalyst testing and characterization
The oxidation of 1-hexene was conducted using air in a glass reactor, which included a round-bottomed flask with a volume of 50 mL and was equipped with a reflux condenser. The experiment included the suspension of ruthenium catalyst (0.1 g) in 1-hexene (10 mL) at a specific temperature. Subsequently, a very small quantity of the radical initiator TBHP was added. The resultant mixture was agitated at a temperature of 45oC for a duration of 24 hours. The reaction mixture was heated to the necessary reaction temperature using a hotplate. Following the designated period of reaction time, the amalgamation of the resultant reaction products and unreacted 1-hexene was subjected to cooling until it reached ambient temperature. Subsequently, the mixture was subjected to filtration and subsequently analyzed using a Varian star 3400 CX GP system equipped with a CP wax 52 capillary column (25 m, 0.35 mm ID, 0.2 μm) in conjunction with a Flame Ionization Detector (FID).10,11
The XRD analysis was performed with a PANalytical X'pert pro diffractormeter equipped with a CuK X-ray source. The scans began from 10 to 80° 2 at 40 kV. Surface area analysis was performed using a Micromeritics Gemini 2360 Analyzer. In the presence of He, samples were degassed for around 50 minutes at 120 °C in order to measure the surface area. Once the sample was in a sample vessel that was attached to a gas intake (liquid N2 at -196 °C), it was ready for use. Using inductively coupled plasma mass spectrometry, the leaching of the active catalyst was verified (ICP-MS). It was carried out using an Agilent 7900 ICP-MS that has a MicroMist nebulizer attached to it. The quantification of the ruthenium under analysis was done by comparing the results to a calibration curve. For TEM investigation, a JEOL 2000FX TEM running at 200 kV was used.
The following equation (1) was used to calculate the conversion factor as a percentage:
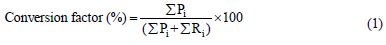
where, Pi is the concentration of the product i and Ri is the concentration of the reactant i.
The selectivity for each product was calculated as a percentage using equation (2):
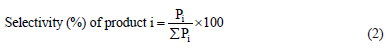
RESULTS AND DISCUSSION
Catalyst characterization
The incorporation of Ru into the support pores may be seen by the comparison of surface areas between the 1% Ru/TiO2 catalyst and the undoped supports, as shown in Table 1. Similarly, the 1%Ru/TiO2 catalyst that was reused had a little decrease in surface area, going from 46 to 40 m2 g-1. This reduction might perhaps be attributed to the adsorption of specific products and residual substrate on the catalyst's surface. The study was conducted on two separate times, ensuring that the margin of error did not exceed 0.7 m2 g-1.

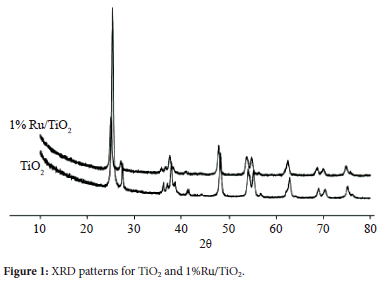
No observation was made of the typical XRD reflections of the ruthenium (Figure 1). This suggests that Ru was dispersed uniformly in the TiO2 support.
Influence of radical initiators on 1-hexene oxidation (blank reaction)
When utilizing oxygen as a terminal oxidant, it is important to determine the degree to which the reaction can take place without the catalyst present. Due to the fact that the ground state of molecular oxygen is diradical, it is capable of taking part in radical reactions even in the absence of a catalyst and especially when radical initiators are present. Therefore, it is important to proceed the reaction in the presence of TBHP only without the catalyst. There was no conversion seen when TBHP and catalyst were not present. Table 2 indicates that after the addition of TBHP to the process, a trace of conversion to the epoxide was seen at 40 oC and 45 oC; additional products were also discovered, although their yields were insignificant. In order to assess the impact of radical initiator concentration on the oxidation of 1-hexene, a range of concentrations of tert-butyl hydroperoxide (TBHP) were used (0.005-0.09 mmol) at varying reaction temperatures. It is reasonable to see that the oxidation of 1-hexene is enhanced with an increase in the quantity of TBHP within the reaction medium. No observable reaction occurred at higher reaction temperatures, even when the concentration of TBHP was low. Hence, a concentration of 0.07 mmol of TBHP was chosen as the reference concentration for further investigations.
When compared to various reaction temperatures in the absence of the ruthenium catalyst, it was shown that TBHP exhibited the lowest amount of activity at 40 °C.
Ruthenium-catalyzed reactions
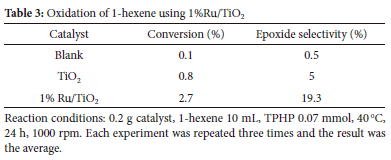
The investigation focused on the oxidation of 1-hexene under solvent-free conditions, using 1% Ru/TiO2. The catalyst was synthesized using the sol-immobilization method. The experimentation started by conducting tests on the TiO2 support. The results indicated that the support exhibited a 0.8% conversion rate of 1-hexene and a 5% selectivity towards epoxide. The addition of ruthenium supported on TiO2 has been shown to result in an enhanced conversion of 1-hexene. Specifically, the conversion rate was seen to reach 2.7%, while the selectivity rose to 19.3% with the addition of a 1% Ru/TiO2 catalyst, as illustrated in Table 3.
Effect of catalyst mass
In order to investigate the impact of catalyst mass on the oxidation of 1-hexene, the quantity of catalyst used was modified within the range of 0.05-0.25 g per 10 g of substrate. The data shown in Table 4 demonstrates a positive correlation between catalyst mass and the conversion of 1-hexene. Similarly, an increase in catalyst mass is seen to enhance the selectivity towards 1, 2-epoxyhexane. On the other hand, the catalytic selectivity towards 2-hexenal exhibits a decline as the mass of the catalyst increases.
Effect of stirring rate
The mass transport constraint is a crucial element in the process of catalysis. In order to investigate whether the reaction was conducted under circumstances of mass limitation, the stirring speed of the reaction was examined. The outcomes obtained at a temperature of 40 °C are shown in Figure 2. It is evident that the increase of the stirring speed results in an increase in the conversion rate of 1-hexene. Nevertheless, it can be seen that the catalyst's reactivity reaches a stable state at rotational speeds of 1200 rpm and higher, suggesting that the reaction is mostly governed by kinetic factors. Consequently, a speed of rotation of 1000 rpm was chosen for following investigations.
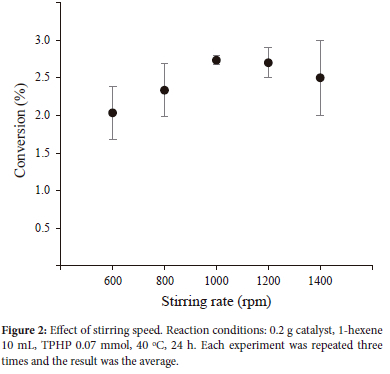
Effect of different supports
Previous studies have revealed that the qualities of the support material have a significant influence on the performance of a catalyst. This influence may be seen in terms of reaction conversion rates and selectivity, particularly in the context of oxidation reactions.12 Initial experimentations was performed on the pure supports, as shown in Table 5. However, it was noted that the enhancement of conversion was not significant when compared to the blank test. The addition of ruthenium to the support resulted in increased conversion of 1-hexene in all cases, as seen in Table 5. However, when combined with a Ru catalyst, TiO2 demonstrated the greatest overall activity, with a conversion rate of 2.7% and an epoxide selectivity of 19.3% at a temperature of 40 oC. TiO2 and other reducible supports have the potential to diffuse onto Ru, which has the potential to have a significant effect in terms of the catalytic activity. This phenomenon is described by the acronym SMSI, which stands for strong metal support interaction.13 In addition, the literature has established that TiO2 is a suitable material for oxidation reactions and interacts favorably with the metal when used as a support. Consequently, TiO2 was included in subsequent investigations.
Effect of the preparation method
The preparation method is a significant aspect that may influence the activity of a catalyst. Three distinct procedures were used to produce 1% Ru/TiO2, as shown in Table 6. The catalysts prepared by wet-impregnation and deposition precipitation methods exhibit comparable levels of activity. However, as shown before, a sol-immobilization approach produced catalysts with increased activity, presumably as a result of a larger dispersion of ruthenium with a considerably smaller nanoparticle size. According to these findings, sol-immobilization is the best way to produce catalysts of this type for oxidation of 1-hexene. The 1% Ru/TiO2 catalyst synthesized via sol-immobilization was the most active of the bunch.
The particle size distribution (PSD) and the results of the transmission electron microscopy (TEM) study for the 1% Ru/TiO2 catalyst synthesized via sol-immobilization are shown in Figure 3. Based on the above-mentioned findings, it can be seen that the 1% Ru/TiO2 sample mostly comprised of nanoparticles, with the majority measuring no larger than 3-4 nm. Additionally, the size distribution range of these particles was rather narrow, ranging from 1 to 7 nm, as shown in Figure 3. The greater activity of this catalyst may be attributed to the better dispersion of Ru, which may be attributed to the smaller size of the particles.
Reusability and stability of ruthenium catalysts
In order to determine whether the 1% Ru/TiO2 catalyst synthesized via sol-immobilization could be reused, an excessive quantity of the catalyst was utilized in the above reaction. After the reaction was complete, the catalyst was filtered, washed with acetone, and dried in an oven at 110 °C for 16 hours. Following that, the required quantity of catalyst for a standard reaction was retrieved in order to be reused. The data pertaining to the activity of the catalyst in its first and subsequent states is shown in Table 7. The use of the fresh catalyst facilitated a conversion rate of 1-hexene and a selectivity towards epoxide of 2.7% and 19.3%, respectively. In contrast, the catalyst that was reused exhibited diminished activity and was unable to facilitate effective reutilization when subjected to drying without prior washing. The substandard performance observed might perhaps be attributed to the deactivation of the catalyst by the reaction products that were adsorbed at the time. In contrast to the unwashed catalyst, the reused catalyst that underwent pre-washing in acetone exhibited enhanced activity, as shown by a conversion rate of 2 and an epoxide selectivity of 17%. In contrast to the newly introduced catalyst, the catalyst that underwent a washing process prior to reuse exhibited suboptimal performance. The observed outcome is likely attributable to the phenomenon of active site inhibition caused by carbon.
The leaching of the active component in the solution is a significant challenge that is often seen in heterogeneous catalysts, particularly in the liquid phase. However, in this study, the leaching of ruthenium was not detected by the use of inductively coupled plasma (ICP) analysis. The determination of the features of active species in a reaction may be achieved via the implementation of hot filtration experiments. Table 8 presents a comparative analysis of the oxidation of 1-hexene under two different conditions: ordinary oxidation at 5 and 24 hours, and a hot filtration reaction where the catalyst is filtered after 5 hours of reaction time, followed by an additional 19 hours of reaction. However, there was a significant reduction in both the conversion of 1-hexene and the selectivity of epoxide, dropping from 2.7% to 1.1% and from 19.3% to 11%, respectively. Based on the available evidence, it may be inferred that the major catalytic route exhibits heterogeneity, whereas homogeneous catalysis does not seem to be implicated.
The oxidation reaction of 1-hexene was not seen in the absence of TBHP, even in the presence of 1%Ru/TiO2 catalyst. As a result, a conversion rate of just 0.2% was attained, and there was no synthesis of epoxide. The role of a little amount of TBHP in activating oxygen in the presence of 1% Ru/TiO2. this reaction was investigated using the radical scavenger BHT (2,6-di-tert-butyl-4-methylphenol). As seen in Table 9, this scavenger interacted with radicals and inhibited the radical process from spreading. This suggests that radical chemistry was at work during the process. Therefore, it may be inferred that free-radical species facilitate the process of oxygen activation from atmospheric air. The involvement of oxygen in the process was further elucidated using an additional diagnostic experiment, in which the reaction was conducted under a nitrogen (N2) environment instead of ambient air. Table 8 shows that the absence of epoxide is indicative of atmospheric molecular oxygen's role in the oxidation process.
CONCLUSIONS
The findings of the study revealed that the supported Ru catalyst exhibited activity in the solvent-free oxidation of 1-hexene when oxygen from air was used as the principal oxidant at a temperature of 40 °C. Under the conditions of the experiment, using a catalytic amount of TBHP as a radical initiator along with supported ruthenium catalysts made a big difference in how environmentally friendly this reaction was. The experimental procedure of hot filtration served to emphasize the heterogeneous characteristics of the supported ruthenium catalyst.
ACKNOWLEDGEMENTS
Raiedhah Alsaiari is thankful to the Deanship of Scientific Research at Najran University for funding this work under Research Priorities and Najran Research funding program. (NU/NRP/SERC/12/22).
CONFLICT OF INTEREST
The author declared that there is no conflict of interests.
ORCID ID
Raiedhah A. Alsaiari: https://orcid.org/0000-0002-0735-3162
REFERENCES
1. Feng Y, Dechezelles J-F, D'Acremont Q Courtade E, De Waele V, Pera-Titus M, Nardello-Rataj V. D'Acremont, Courtade E, De Waele V, M. Pera-Titus M, Nardello-Rataj V. Light-driven Pickering interfacial catalysis for the oxidation of alkenes at near-room temperature. Green Chem. 2023;25(4):1417-1423. https://doi.org/10.1039/D2GC04591E. [ Links ]
2. Gupta UN, Dummer NF, Pattisson S, Jenkins RL, Knight DW, Bethell D, Hutchings GJ. Solvent-free aerobic epoxidation of dec-1-ene using gold/graphite as a catalyst. Catal Lett. 2015;145(2):689-696. https://doi.org/10.1016/j.cattod.2018.09.005. [ Links ]
3. Liu C-J, Yu W-Y, Li S-G, Che C-M. Ruthenium meso-tetrakis(2,6-dichlorophenyl)porphyrin complex immobilized in mesoporous MCM-41 as a heterogeneous catalyst for selective alkene epoxidations. J Org Chem. 1998;63(21):7364-7369. https://doi.org/10.1021/jo981003l. [ Links ]
4. Dali A, Rekkab-Hammoumraoui I, Choukchou-Braham A, Bachir R. Allylic oxidation of cyclohexene over ruthenium-doped titanium-pillared clay. RSC Adv. 2015;5(37):29167-29178. https://doi.org/10.1039/C5RA90001H. [ Links ]
5. Kasper JB, Saisaha P, de Roo M, Groen MJ, Vicens L, Borrell M, de Boer JW, Hage R, Costas M, Browne WR. A common active intermediate in the oxidation of alkenes, alcohols and alkanes with H2O2 and a Mn(II)/ Pyridin-2-Carboxylato Catalyst. ChemCatChem. 2023;15(1):e202201072. https://doi.org/10.1002/cctc.202201072. [ Links ]
6. Suresh AK, Sharma MM, Sridhar T. Engineering Aspects of Industrial Liquid-Phase Air Oxidation of Hydrocarbons. Ind Eng Chem Res. 2000;39(11):3958-3997. https://doi.org/10.1021/ie0002733. [ Links ]
7. Zheng S-L, Yu W-Y, Che C-M. Ruthenium(II) Porphyrin Catalyzed Formation of (Z)-4-Alkyloxycarbonyl- methylidene-1,3-dioxolanes from y-Alkoxy-a-diazo-p-ketoesters. Org Lett. 2002;4(6):889-892. https://doi.org/10.1021/ol010283f. [ Links ]
8. Dali A, Rekkab-Hammoumraoui I, Choukchou-Braham A, Bachir R. Allylic oxidation of cyclohexene over rutheniumdoped titanium-pillared clay. RSC Adv. 2015;5(37):29167-29178. https://doi.org/10.1039/C4RA17129B. [ Links ]
9. Alsaiari R. Supported Ruthenium and Tetrapropylammonium bromide catalysts for oxidative carboxylation of 1-decene. Asian J Chem. 2020;32(4):771-775. https://doi.org/10.14233/ajchem.2020.22350. [ Links ]
10. Alsaiari R. Supported ruthenium catalyst as an effective catalyst for selective oxidation of toluene. J Indian Chem Soc. 2022;99(8):100593. https://doi.org/10.1016/j.jics.2022.100593. [ Links ]
11. Raiedhah Alsaiari RA, Moustafa A Rizk MAR, Esraa Musa EM, Huda Alqahtani HA, Fatima Alqadri FA, Mervat Mohamed MM, Mabkhoot Alsaiari MA, Ali Alkorbi AA, Iman Shedaiwa IS, Faeza Alkorbi FA. Supported Ruthenium Catalysts for Oxidation of Benzyl Alcohol under Solvent-Free Conditions. J Chem Soc Pak. 2022;44(4):322-329. https://doi.org/10.52568/001069/JCSP/44.04.2022. [ Links ]
12. Engel RV, Alsaiari R, Nowicka E, Pattisson S, Miedziak PJ, Kondrat SA, Morgan DJ, Hutchings GJ. Oxidative Carboxulation of 1-Decene to 1,2-Decylene Carbonate. Top Catal. 2018;61(5-6):509-518. https://doi.org/10.1007/s11244-018-0900-y. [ Links ]
13. Bowker M. The Basic and Application of Heterogeneous Catalysis, Oxford chemistry primers, Oxford, 1998, 29. [ Links ]
Received 16 October 2023
Revised 20 December 2023
Accepted 3 February 2024
* To whom correspondence should be addressed. Email: kaade_77@hotmail.com














