Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.78 Durban 2024
http://dx.doi.org/10.17159/0379-4350/2024/v78a04
SHORT COMMUNICATION
Tetrabutylammonium Bromide (TBAB)-Catalyzed Rapid Conversion of β-ketoesters into β-Enaminoesters under Solvent-free Conditions
Abhijit BachuteI, ; Bhagwansing S. DobhalII
IDepartment of Chemistry, Sambhajirao Kendre College, Jalkot, Dist-Latur, Maharashtra, India
IIDepartment of Chemistry, B. R. Barwale College, Jalna, Maharashtra, India
ABSTRACT
When used with a mortar and pestle at 25 oC in a solvent-free environment, tetrabutylammonium bromide (TBAB) is characterized as an effective catalytic system for the synthesis of β-enaminoesters from β-ketoesters. This method has a number of benefits, including shorter reaction times, use of inexpensive and readily available catalyst, compatible reaction conditions, and high product yields.
Keywords: amines, β-enaminoesters, β-ketoesters, tetrabutylammonium bromide and catalysis
INTRODUCTION
β-Enamino esters are versatile intermediates for the synthesis of nitrogen containing compounds.1-4 Also, they are important subunits present in some biologically important natural products as well as therapeutic agents.5;6 Due to the importance of β-enamino ester derivatives as bioactive leads and versatile building blocks, their synthesis and applications have long been an active topic in organic synthesis.
The synthesis of β-enaminoesters has been described using a variety of methodologies, including tosyl imines, imidoyl halides, addition of enamines or ketimines to activated carboxylic acid derivatives, addition of an ester or amide enolate to a nitrile, and others.7-22 These compounds can also be successfully produced by direct condensation of β-ketoesters with amines.23;24;25 The majority of the protocols are well documented, although they have limitations in chemical yields, harsh reaction conditions, and a lack of widespread applicability. Thus, there is a great need to design a better catalyst for the synthesis of β-enaminoesters.
Synthetic chemists continue to research novel synthetic methods employing new reagents and catalysts to perform chemical transformations. Due to their low production costs and simplicity of usage, solvent-free based synthetic methodologies have found increasing use in the manufacture of pharmacologically relevant heterocyclic compounds.26-30 On the other hand, the mechanochemical method has been increasingly popular due to its affordability and low impact on the environment. Mechanical grinding is one of the typical methods used in solvent-free reactions. Because of its ease of use, clean reaction profile, and potential for larger-scale application with the right experimental set-up, mechanical grinding has emerged as a powerful tool in the paradigm of synthetic organic chemistry.31;32
Tetrabutylammonium bromide (TBAB) is inexpensive, but it's also more selective, non-corrosive, easy to recycle, and is operationally simple. It has been found to be efficient in a number of organic transformations.33,34,35 Only a small amount of TBAB is required to catalyze several reactions such as esterification, oxidation, reduction, and alkylation. Furthermore, it functions as an effective co-catalyst for a range of coupling reactions. It has also been effectively used in a range of molten organic processes as a zwitterionic solvent.
In this article, we present a mild and useful protocol for the synthesis of β-enaminoesters from β-ketoesters from the reaction of amine, β-ketoester in the presence of tetrabutylammonium bromide (10 mol%) in solvent-free conditions at ambient temperature.
EXPERIMENTAL
In CDCl3 at 300 MHz, the 1H NMR spectra were captured using TMS as the internal standard. For solid materials, KBr pellets were used to record IR spectra, and neat was used for liquid samples. Using silica gel, column chromatography was carried out (100-200 mesh). In relation to internal TMS, chemical changes are expressed in ppm, while J values are reported in Hz.
GENERAL PROCEDURE
A mortar and pestle was used to grind the amine (1.5 mmol), β-ketoester (1 mmol), and a tetrabutylammonium bromide (TBAB) (10 mol%) for the allotted amount of time. Water was added once the reaction was completed (TLC), and diethyl ether was used to extract the result. Under low pressure, the organic layer was dried (with Na2SO4) and concentrated. By using column chromatography (silica gel 100-120 mesh, petroleum ether: ethyl acetate = 9:1), the crude product was refined to afford the corresponding β-enaminoester (all known compounds).
RESULTS AND DISCUSSION
A systematic study was first conducted to assess the effectiveness of tetrabutylammonium bromide as a catalyst for the reaction of aniline and ethylacetoacetate under diverse circumstances (Scheme 1).
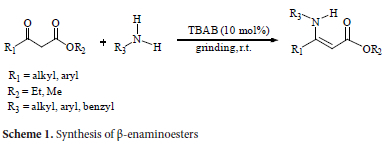
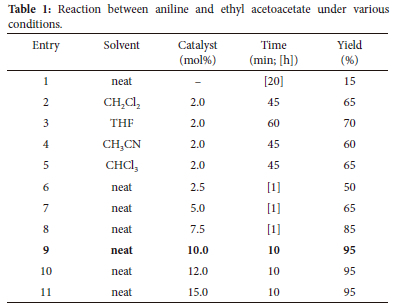
After 20 hours, the reaction between ethyl acetoacetate and aniline produced a meagre amount of product in the absence of a catalyst (entry 1, Table 1), and the outcomes were poor in the presence of solvents (entries 2-5, Table 1). The reaction was completed in 10 minutes with only 10 mol% of tetrabutylammonium bromide, and a nearly quantitative yield of the corresponding β-enaminoester (95%) was obtained. We then optimized the quantity of TBAB for this reaction at ambient temperature and without a solvent (entries 6-11, Table 1). It's important to notice that increasing the amount of TBAB had no effect on yield or response time (entries 10-11). The reaction was not completed with less than 10 mol% tetrabutylammonium bromide, even after milling the reaction mixture for an hour. This resulted in a low yield of the product (50-85%).
The results displayed in Table 2 proved that the approach worked for primary, benzylic, and aromatic amines. For instance, the conversion of methyl and ethyl acetoacetates into the corresponding β-enaminoesters was simple. Excellent yields and short reaction times are just two of the many noteworthy advantages the current approach has over those that have been described. A study claims that this reaction finds steric hindrance when β-ketoesters contain a bulky amine or have a substituent other than hydrogen in the a-position, requiring lengthy reaction times and severe reaction conditions. High product yields were reached even with cyclic and aromatic β-ketoesters (entries, j, k, and l, Table 2).
CONCLUSION
As a result, we have developed a novel methodology for the synthesis of β-enaminoesters in conditions devoid of solvent. The key appealing aspects of this method include the use of a cheap and widely accessible catalyst (TBAB), experimental simplicity, a straightforward work-up procedure, and a quick response.
SUPPLEMENTARY MATERIAL
Supporting information is available for this article online.
ORCID IDS
Abhijit Bachute: https://orcid.org/0009-0003-8784-9284
Bhagwansing S. Dobhal: https://orcid.org/0000-0002-9469-3869
REFERENCES
1. Han Y, Sun J, Sun Y, Gao H, Yan C. Development of domino reactions with β-enamino esters as key intermediates. Youji Huaxue. 2012;32(9):1577-1586. https://doi.org/10.6023/cjoc201206008. [ Links ]
2. Pirnat K, Simunek P, Ursic U, Bezensek J, Groselj U, Golobic A, Meden A, Svete J, Stanovnik B. Enamino esters in the synthesis of heterocyclic systems. Transformation of diethyl acetone-1,3-dicarboxylate into polysub-stituted 1,2,7,8-tetrahydro-2,7-naphthyridine-4-carboxylates. ARKIVOC. 2011;(vi):120-129. [ Links ]
3. Salaheldin AM, Abdullah Al-Sheikh M. β-Enamino esters in heterocyclic synthesis: synthesis of pyrazolone and pyridinone derivatives. Molecules. 2010;15(6):4359-4368. https://doi.org/10.3390/molecules15064359. [ Links ]
4. Govindh B, Diwakar BS, Murthy YLN. A brief review on synthesis & applications of β-enamino carbonyl compounds. Org. Commun. 2012;5(3):105-119. [ Links ]
5. Karthikeyan G, Perumal PT. Ionic liquid promoted simple and efficient synthesis of β-enamino esters and β-enaminones from 1,3-dicarbonyl compounds-One-pot, three-component reaction for the synthesis of substituted pyridines. Can J Chem. 2005;83(10):1746-1751. https://doi.org/10.1139/v05-186. [ Links ]
6. Kim S-H, Bae S, Ko EB, Park GY, Lee E, Hwang HJ, Im CY, Song M. Silica gel-mediated synthesis of β-enamino esters and its application for the synthesis of Indeno 4-hydroxypyridin-2(1H)-ones. Bull Korean Chem Soc. 2019;40(3):262-269. https://doi.org/10.1002/bkcs.11676. [ Links ]
7. Calvet S, David O, Vanucci-Bacque C, Fargeau-Bellassoued M-C, Lhommet G. Chiral heterocyclic β-enamino esters: convenient synthesis and diastereoselective reduction. Tetrahedron. 2003;59(33):6333-6339. https://doi.org/10.1016/S0040-4020(03)00980-3. [ Links ]
8. Waghmare NH Sr. 4Cl Catalyzed synthesis of β-amino Esters. Results Chem. 2020;2:100036. https://doi.org/10.1016/j.rechem.2020.100036. [ Links ]
9. Xin D, Burgess K. A chemoselective route to p-rnamino rsters and thioesters. Org Lett. 2014;16(8):2108-2110. https://doi.org/10.1021/ol5005643. [ Links ]
10. Feng CL, Chu NN, Zhang SG, Cai J, Chen J-Q, Hu H-Y, Ji M. Solvent-free synthesis of β-enamino ketones and esters catalysed by recyclable iron(III) triflate. Chem Pap. 2014;68(8):1097-1103. https://doi.org/10.2478/s11696-014-0544-8. [ Links ]
11. Brandt CA, da Silva ACMP, Pancote CG, Brito CL, da Silveira MAB. Efficient synthetic method for β-enamino esters using ultrasound. Synthesis (Stuttg). 2004;10(10):1557-1559. https://doi.org/10.1055/s-2004-829102. [ Links ]
12. Zhang Z-H, Hu J-Y. Cobalt(II) chloride-mediated synthesis of beta-enamino compounds under solvent-free conditions. J Braz Chem Soc. 2006;17(7):1447-1451. https://doi.org/10.1590/S0103-50532006000700038. [ Links ]
13. Lin J, Zhang L-F. ZrCl4-Catalyzed efficient synthesis of enaminones and enamino esters under solvent-free conditions. Monatsh Chem. 2007;138(1):77-81. https://doi.org/10.1007/s00706-006-0565-2. [ Links ]
14. Zhang Z-H, Yin L, Wang Y-M. A general and efficient method for the preparation of β-enamino ketones and esters catalyzed by indium tribromide. Adv Synth Catal. 2006;348(1-2):184-190. https://doi.org/10.1002/adsc.200505268. [ Links ]
15. Hasaninejad A, Zare A, Mohammadizadeh MR, Shekouhy M, Moosavi-Zare AR. A green solventless protocol for the synthesis of β-enaminones and β-enamino esters using silica sulfuric acid as a highly efficient, heterogeneous and reusable catalyst. E-J Chem. 2010;7(4):1546-1554. https://doi.org/10.1155/2010/829547. [ Links ]
16. Anouar Harrad M, Boualy B, El Firdoussi L, Ait Ali M. Aluminum phosphate catalyzed free solvent preparation of β-enamino esters. Am J Chem. 2012;2(5):271-276. https://doi.org/10.5923/j.chemistry.20120205.05. [ Links ]
17. Chen XM, Li XS, Chan ASC. Highly efficient synthesis of β-amino esters via Mannich-type reaction under solvent-free conditions using ZnCl2 catalyst. Chin Chem Lett. 2009;20(4):407-410. https://doi.org/10.1016/j.cclet.2008.12.030. [ Links ]
18. Shekhar A, Pathak DD. Zeolite (ZSM-5) as a highly efficient and heterogeneous catalyst for the synthesis of β-enaminones and β-enamino esters. E-J Chem. 2011;8(4):1632-1637. https://doi.org/10.1155/2011/176829. [ Links ]
19. Xu F, Lv H-X, Wang J-P, Tian Y-P, Wang J-J. A mild method for the synthesis of β-enaminones and β-enamino esters using KH2PO4 as catalyst. J Chem Res. 2008;2008(12):707-710. https://doi.org/10.3184/030823408X382117. [ Links ]
20. Zhang M, Abdukader A, Fu Y, Zhu C. Efficient synthesis of β-enaminones and β-enaminoesters catalyzed by gold (I)/silver (I) under solvent-free conditions. Molecules. 2012;17(3):2812-2822. https://doi.org/10.3390/molecules17032812. [ Links ]
21. Guizzetti S, Benaglia M, Bonsignore M, Raimondi L. Triclorosilane-mediated stereoselective synthesis of β-amino esters and their conversion to highly enantiomerically enriched p-lactams. Org Biomol Chem. 2011;9(3):739-743. https://doi.org/10.1039/C0OB00570C. [ Links ]
22. Chen H-Y, Patkar LN, Ueng S-H, Lin C-C, Lee S-Y. Synthesis of β-amino esters by regioselective amination of allyl bromides with aryl and alkyl amines synthesis of β-amino esters. Synlett. 2005;13:2035-2038. [ Links ]
23. Kamble VT, Joshi NS, Atkore ST. Cyanuric chloride catalyzed rapid conversion of β-ketoesters into β-enaminoesters under mild and solvent-free conditions. J. Iranian Chem. Soc. 2011;8(3):616-621. https://doi.org/10.1007/BF03245892. [ Links ]
24. Bartoli G, Sambri L, Bosco M, Locatelli M, Marcantoni E, Melchiorre P. Zn(ClO4)2-6H2O as a powerful catalyst for the conversion of β-ketoesters into enamino esters. Synlett. 2004;2(2):239-242. https://doi.org/10.1055/s-2003-44974. [ Links ]
25. Varala R, Nuvula S, Adapa SR. Efficient synthetic method for β-enamino esters catalyzed by Yb(OTf)3 under solvent-free conditions. Aust J Chem. 2006;59(12):921-924. https://doi.org/10.1071/CH06239. [ Links ]
26. Tanaka K, Toda F. Solvent-free organic synthesis. Chem Rev. 2000;100(3):1025-1074. https://doi.org/10.1021/cr940089p. [ Links ]
27. Zangade S, Patil P. A review on solvent-free methods in organic synthesis. Curr Org Chem. 2020;23(21):2295-2318. https://doi.org/10.2174/1385272823666191016165532. [ Links ]
28. Cave GWV, Raston CL, Scott JL. Recent advances in solventless organic reactions: towards benign synthesis with remarkable versatility. Chem Commun (Camb). 2001;(21):2159-2169. https://doi.org/10.1039/b106677n. [ Links ]
29. Lei L, Zhou Y. Solvent-Free or Less-Solvent Solid State Reactions. Huaxue Jinzhan. 2020;32(8):1158-1171. [ Links ]
30. Hernandez JG, Friscic T. Metal-catalyzed organic reactions using mechanochemistry. Tetrahedron Lett. 2015;56(29):4253-4265. https://doi.org/10.1016/j.tetlet.2015.03.135. [ Links ]
31. Wang G-W. Mechanochemical organic synthesis. Chem Soc Rev. 2013;42(18):7668-7700. https://doi.org/10.1039/c3cs35526h. [ Links ]
32. Banik BK, Banerjee B, Kaur G, Saroch S, Kumar R. Tetrabutylammonium bromide (TBAB) catalyzed synthesis of bioactive heterocycles. Molecules. 2020;25(24):5918. https://doi.org/10.3390/molecules25245918. [ Links ]
33. Khan MU, Khan SU, Kiriratnikom J, Zareen S, Zhang X. CoCo-PBA/ tetrabutylammonium bromide as highly efficient catalyst for CO2 and epoxides coupling reaction under mild conditions [J]. Chin Chem Lett. 2022;33(2):1081-1086. https://doi.org/10.1016/j.cclet.2021.06.002. [ Links ]
34. Navath S. Tetrabutylammonium bromide (TBAB): A facile phase transfer catalyzed direct synthesis of a,a-dihalo methyl sulfones. Int J Org Chem (Irvine). 2021;1:19-23. [ Links ]
Received 27 July 2023
Revised 30 October 2023
Accepted 8 December 2023
* To whom correspondence should be addressed. Email: abhijitbachute7783@gmail.com




![C-H activation: a Critical Evaluation of a Published Method and its Application Towards Inherently Chiral Calix[4]arenes](/img/pt/prev.gif)









