Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.77 Durban 2023
http://dx.doi.org/10.17159/0379-4350/2023/v77a24
RESEARCH ARTICLE
How spiral is the South African Physical Science curriculum? A case study of Electrolytic cells in Grades 10-12
Brighton Mudadigwa
Science Mathematics Technology Education, University of Pretoria, Pretoria, South Africa
ABSTRACT
The South African Physical Science curriculum is regarded as spiral in nature due to its progression of concepts across grades, and its coherence of ideas within grades. A spiral curriculum supports coherence and boosts learner achievement in science. This inquiry examines the fundamental principles of electrolytic cells in the knowledge area of chemical change at the secondary level (Grades 10 to 12). Electrolytic cells' spiral nature was determined by analysing the Physical Science Curriculum (PSC) and 2019 Physical Science Work Schedule (PSWS), using pedagogical link-making for continuity. The PSC passed the spiral test due to the presence of increasingly difficult concepts across grades. Due to the absence of fundamental concepts like oxidation numbers and electrolytes in Grades 10 and 11, the PSC failed the conceptual progression test. Furthermore, the spiral characteristics of the PSC were compromised in the 2019 PSWS document. The sequencing of concepts in the 2019 PSWS does not encourage linking on the meso scale, where the interlinking of ideas is vital for learner comprehension and conceptual understanding. Curriculum designers should introduce oxidation numbers in Grade 10 and electrolytic cells in Grade 11. Furthermore, it is recommended that curriculum designers consider separating chemistry and physics knowledge areas.
Keywords: electrolytic cells; spiral curriculum; pedagogical link-making; promoting continuity, scientific story
INTRODUCTION
The sequencing of Physical Science topics in the South African Physical Science Curriculum (PSC) has been in question since its inception in 2011. In this article, the Physical Science Curriculum Assessment Policy Statement (CAPS) is referred to as the Physical Science Curriculum (PSC). The PSC categorises its topics in terms of knowledge area, and each topic under the knowledge area is assigned the number of hours during which it should be covered in class. The 2019 Physical Science Work Schedule (PSWS) is a working document that teachers are supposed to use in their daily lesson planning. It dictates the topics and concepts to be taught in a term, week, or for a certain duration. The 2019 PSWS further sequences the concepts according to how they should be taught. This study examined the two common documents, the 2019 Gauteng PSWC and the PSC.
The Department of Basic Education (DBE) claims that the curriculum is structured in a spiral manner. The concepts are progressive across grades and coherent within grades.1 An analysis done by Umalusi suggests that the curriculum is largely progressive across the grades, but not coherent within grades.2 In contrast, Sibanda and Hobden3 find no progression within topics across the grade. It should be noted that the report by Umalusi compared the structure and coherence of the PSC against the curriculum in the previous National Curriculum Statement (NCS) across all topics within the science content document.2
Sibanda and Hebden have found significant differences between teachers' sequencing of chemistry topics and the PSC's prescribed approach in Grades 10 to 12.3 While the PSC suggests that the sequencing of topics in chemical bonding start with concepts at the macroscopic level, some teachers prefer to order their lessons starting with concepts at the sub-microscopic level.3 Furthermore, teachers feel overly restricted in implementing the work schedule in its prescribed format.4
For learners to have sound conceptual knowledge of the curriculum and do well in assessments, the order of topics within the curriculum and the conceptual coherence of topics are critical. Students can learn science meaningfully if the science curriculum is sequenced according to a spiral structure.5 It is further suggested that an integrated spiral curriculum accomplishes the primary purpose of giving pupils high-quality learning experiences through consolidating information, fostering a thorough understanding of concepts, and solidifying knowledge over time rather than just at the time of assessment.6 Hypothetically, if Umalusi is correct in saying that the PCS is progressive, this article poses the question: why is electrolysis one of the subjects in which students have consistently fared poorly in their final exams? It has been shown that an adequately executed spiral curriculum frequently leads to improved student performance.7
This study aimed to examine the degree to which the topic ofelectrolytic cells and its underlying concepts conform to a spiral curriculum within and across grades at the secondary school level (Grades 10 to 12). This was done utilising a framework that measures the spiral nature of the curriculum, and tests the connections' meaningfulness. The following questions guided the direction of the investigation:
1. To what extent does the PSC show a progression from Grade 10 to Grade 12 at secondary school level?
2. Does the sequencing of electrolytic cells and its underlying concepts follow a coherent structure that fosters conceptual understanding in each grade?
BACKGROUND
The knowledge area of chemical change in chemistry is a challenging knowledge area in South Africa. In a study of the performance of first-year chemistry students at the University of Cape Town, it was found that students did poorly in reactions and stoichiometry.9 It was shown that these students' comprehension of fundamental scientific concepts, such as atoms and ions, the mole concept, chemical reactions, acids and bases, chemical solutions, and stoichiometry, had declined significantly from what they were taught in Grade 12 the previous year.8;9 Further research highlights that Grade 12 learners may have several alternative conceptions of electrochemistry; however, it has been shown that learners do not comprehend the concepts around electrolysis compared to how they understand galvanic cells.10;11 These topics fall under chemical change in the PSC from Grades 10 to 12.
Literature relevant to this field reveals that students in some regions of the world have trouble comprehending a few of the sub-topics in Physical Science. For example, in a study of 100 high school chemistry students in the United States of America, the students considered the issue of chemical change to be significantly more complex than most teachers and textbook authors acknowledge.12 Similar findings confirm that students lack comprehension of solution chemistry,13 and many find it challenging to use their understanding of chemistry in unfamiliar situations. Furthermore, it was found that there were persistent fundamental conceptual errors in the electrochemistry curricula used in high schools and universities. This research focused on electrolysis and its basic concepts under chemical change.14
According to an examination of student responses to problems in the Grade 12 Physical Science paper in South Africa, electrolysis and its basic ideas in chemical change are difficult for high school students to understand. Figure 1 below illustrates a performance analysis conducted question by question over eight years since 2014, the year the first cohort sat for the PSC examinations.
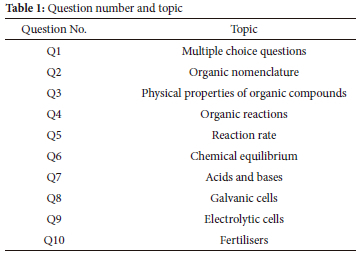
The learners' performance in the chemistry topics evaluated at the Grade 12 final examination is shown in Figure 1. Question 1 was excluded from the illustration as it consisted of multiple-choice questions and tested all topics. Table 1 below lists the topics per question.
It can be seen that learners did not do well on the topic of electrolytic cells (Q9) across the years under examination, indicating an important issue that needs to be addressed. It is the only topic consistently remaining below the 45% threshold over time. Compared to the concept of the electrolytic cell, learners performed well on the concept of acids and bases. The electrolytic cell topic showed the poorest performance over the years, despite a reduction in the breadth of examinable information in 2021. It is significant to note that learners' performance on this topic worsened in the COVID-19 era. For example, the diagnostic report for the 2022 Grade 12 paper showed that electrolytic cells had the lowest percentage score among the three learning areas at 30%, compared to 45% for acids and bases and 49% for organic chemistry.16 According to the report, learners struggle with questions that seek scientific explanations and where the responses require knowledge of fundamental concepts and conceptual interpretation.
The concerns above show that learners struggle with the topic of electrolytic cells, which fall under chemical change. One possible reason for learners' inability to grasp the network of ideas surrounding the electrolytic cell may be the lack of conceptual coherence in the curriculum.17;18 Therefore, this study sought to answer the following question: to what extent does the PSC support the progression and coherence of electrolytic cells and its fundamental concepts at the secondary school level (Grades 10 to 12)?
LITERATURE REVIEW
A curriculum with an incoherent structure of concepts hampers the construction of ideas, and obstructs conceptual understanding.19 Alternatively, a spiral curriculum demonstrates a coherent, logical sequencing of concepts, which enhances meaning-making in the science classroom.20 The PSC advocates for a progression of coherent science concepts. This paper presents an evaluation of the progression of electrolytic cell concepts in the PSC using the yardsticks of the spiral curriculum, pedagogical link-making to promote continuity, and the development of a scientific story.
Spiral Curriculum
A spiral curriculum covers the fundamental information of a subject at various comprehension levels.19 The starting point is where several fundamental concepts are taught and comprehended at lower grades and then revisited in-depth in higher grades for conceptual understanding.1;20 The focus is on a wide base with a number of related concepts connected to more difficult and intricate higher-order ideas.21 This is a process that enhances the comprehension of the inter-relationship and structure of a large body of knowledge.22 The curriculum should permit students to revisit a topic, theme, or subject more than once. Each lesson should bring a greater level of complexity to the concept so that new information is connected to what they have already learned.23;24 Expanding students' competencies is anticipated if there is an increasing level of complexity, where new knowledge is connected to prior understanding.25
At Miami University, a spiral technique was implemented to reduce student attrition in an organic chemistry course.19 The spiral curriculum helped students to have a better conceptual understanding of organic chemistry ideas, which resulted in a reduction in student attrition from between 30% and 50% to 13.1%. On the other end of the spectrum, if the curriculum is not well organised, learners' performance is hindered.25 If science content were organised coherently in the curriculum, students would learn science in a more organised and holistic way.5
If a spiral curriculum is effectively implemented, it has several advantages, including that it improves knowledge retention and increases the level of complexity from one level to the next, enabling students to move beyond memorisation to the application of knowledge.31 Moreover, the concept of revisiting a topic in a spiral curriculum is designed to assist with content mastery. A study was conducted to investigate the mathematics performance of Grade 10 pupils when a spiral progression approach was implemented in teaching mathematics.25 It was found that progressing from one topic to the next in a 'Broken Spiral' is impossible because there is no building on students' existing knowledge.25
The DBE's main goal is for students to learn in a meaningful way, and they recognise that this requires teaching subjects hierarchically. In light of this, the science policy statement claims that the "material and context of each grade reflects the evolution from simple to complex".1
Pedagogical Link-Making to Promote Continuity
The Pedagogical Link-Making (PLM) approach promotes the connection of ideas in a continuous progression of meaning-making and knowledge-building during the teaching and learning process. "Pedagogical link-making is concerned with how teachers and students make connections between ideas in the ongoing meaning-making interactions of classroom teaching and learning".26 PLM is a method of teaching and learning in which instructors work with students to continuously deepen their understanding of subjects through classroom interactions.27 There are three main forms of PLM, namely, pedagogical link-making to support knowledge building, to promote continuity, and to encourage emotional engagement. The spiral structure of the scientific curriculum was examined in this study by focusing on the second form of pedagogical link-making, which encourages continuity.
The use of PLM to promote continuity includes establishing links between teaching and learning activities that are separated according to time scale. There are three categories:
• Macro: continuity links made on an extended time scale (typically months/ years). This involves making references to teaching/ learning in different parts of the science curriculum.
• Meso: continuity links made on an intermediate time scale (typically of days/weeks), which involve making references to different points within a lesson sequence.
• Micro: continuity links made on a short time scale (typically of minutes), which involve making references to different points within a lesson.
The curriculum should be structured so that ideas are connected to similar concepts that have already been studied (in previous grades) or will be studied in the future (grades). In establishing connections between similar ideas, the scientific narrative of a particular concept must be preserved, and the episodes need to accumulate the linked concepts.27
Accordingly, teaching and acquiring scientific conceptual knowledge includes figuring out how scientific concepts fit together in an interconnected system. This paper focuses on link-making to improve continuity in the topic of electrolytic cells and its essential ideas. The PSC promotes a spiral approach in which students gradually develop a grasp of the topics through time. The introduction of an idea and its gradual development in subsequent grades occur from lower grades.1 Science concepts are thus connected across grade levels and topics. This is referred to as conceptual growth and coherence, respectively, by the DBE.1,28 Conceptual development and coherence are equally important to pedagogical link-making to promote continuity, which "involves making connections between teaching and learning events separated in time".27 Therefore, well-organised and hierarchical concepts (spiral) built on pre-existing knowledge enhance and support conditions that are conducive to meaningful learning.29
The interrelationships between concepts across multiple levels should be tracked in the curriculum, focusing on conceptual coherence. In McPhail's view, the creation of curricula should centre on the conceptual linkages made using well-chosen academic material.30 According to Ireland and Mouthaan, students who are taught science using a spiral curriculum develop good theoretical knowledge and foundational abilities, whereas students who are taught using an interdisciplinary curriculum approach develop good factual memory.31 The purpose of this investigation was to determine whether the PSC passes the test of being a spiral curriculum. There are two approaches to promoting link-making: developing a scientific story, and managing or organising time. In this article, the development of the story is emphasised.27
Developing a scientific story
The approach of developing a scientific story in a curriculum focuses on the coherence and progression of hierarchical, substantive lesson content. Mortimer and Scott define the progression of the scientific story as a lens that reflects the development of a scientific narrative. This aids teachers in understanding the evolution of scientific concepts and how they fit into the broader science curriculum.32 McPhail argues that scientific concepts have significance in relation to other concepts;30 therefore, the curriculum must show how scientific concepts are interrelated to form a coherent whole. The hierarchical knowledge structure of science should match the spiral model31 more organically for the meaningful comprehension of scientific concepts to take place. The building of a scientific story depends on the capacity of the teacher to link concepts in current lessons with future lessons using learners' previous knowledge. The links can be made during the lesson through recaps and questioning.27 However, these links should be explicitly vivid in the curriculum document for easy implementation in the science classroom.
A spiral curriculum that seeks to develop a scientific story in a particular science domain should follow the pattern of macro, meso and micro scales.27 In addition to ensuring that knowledge goals are prepared in advance, the spiral's systematic approach to the scope and sequencing of learning objectives also makes it possible for vertical integration within the curriculum as topics are reviewed. The spiral, which is characterised by repeated returns to concepts at escalating levels of complexity, emphasises the necessity for students' understanding of fundamental concepts since mastery is attained via the development of ideas.31
RESEARCH METHODOLOGY
This study used an explanatory, interpretative, and qualitative research paradigm in the form of a case study. A case study methodology was chosen because it allows researchers to conduct in-depth research on a single subject, group, or phenomenon.36 It also provides a comprehensive description and analysis of a single unit.36-38 The unit being analysed in this study was the conceptual structure of the PSC and the 2019 Physical Science Work Schedule (Gauteng province) on the topic of electrolysis and its underlying concepts. Other documents that support the implementation of the curriculum, such as textbooks and lesson plans, were not analysed as participants used different textbooks because there is no single prescribed textbook for the PSC. Participants were not consistent in using lesson plans, some participants had no lesson plans at all.
The coherence and level of progression of these two documents were examined to see if they met the conditions for the spiral curriculum structure claimed by the PSC developers. The underlying electrolysis topic concepts that were scrutinised were reactions in aqueous solutions (Grade 10), redox reactions (Grade 11), and electrolytic cells (Grade 12). In the PSC, there are five knowledge areas: chemical change; chemical systems; matter and materials; mechanics; and waves, sound, and light. The PSC is structured according to knowledge areas per grade, and is suitable for investigating how concepts are hierarchically structured at the secondary school level (Grades 10-12).
The 2019 PSWS documents were structured as teaching units within a grade thus it was important to examine this document since it was the designated teachers' instrument for implementing the PSC. The PSWS documents are structured as teaching units within a grade which teachers should then follow when planning their lessons. Furthermore, the 2019 PSWS provides the sequencing of topics that have to be taught within each grade. Teachers in government schools were to follow the sequencing prescribed in the 2019 PSWS document religiously to ensure that learning of the topic takes place.
In this study, chemical change was singled out for investigation due to students' poor performance in the topic, particularly in electrolytic cells. Since the curriculum is an acknowledged spiral curriculum, the topic of electrolytic cells was investigated to ascertain how coherent, progressive, and hierarchical concepts are from Grades 10 to 12. The PSC and the 2019 PSWS were mined for information on the topic of electrolytic cells and its underlying principles as covered in these grades. Only the topics of reactions in aqueous solution (Grade 10), types of reactions (Grade 11), and electrolytic cells (Grade 12) were subjected to the analysis; each of these topics contributes to the conceptual understanding of the idea of the electrical cell. The topics were examined for consistency between and within each grade. Using the macro, meso and micro scales from the pedagogical link-making approach to promote continuity,27 the PSC and 2019 PSWS were analysed to determine how the concepts within the topic, electrolytic cell, fit into a spiral curriculum structure. Two research questions were developed to address the spiral nature of the PSC and the 2019 PSWS documents, as indicated in the last paragraph of the introduction.
ANALYSIS OF RESULTS
The presence of pedagogical link-making to promote continuity, specifically the approach of developing a scientific story,27 was used to analyse whether the PSC and 2019 PSWS do indeed follow a spiral approach. The concept of developing a scientific story is divided into three categories: macro scale (concept progression across grades), meso scale (topic arrangements within a grade), and microscale (concepts linked within a lesson). 'Macro' refers to long-term continuity links (months and years) that involve teaching from various sections of the science curriculum. 'Meso' refers to continuity links made on an intermediate time scale (days and weeks), which involves the referencing of concepts within a lesson sequence. 'Micro' entails continuity links made on an immediate time scale (minutes or within the lesson), involving the referencing of concepts or events within a lesson.
FINDINGS
The two sub-research questions were used to categorise the study's findings. The first sub-research question addressed the progression and hierarchical structure of electrolytic cells across Grades 10 to 12. To analyse the interrelationships of concepts, the macro scale of constructing a scientific story was adopted in light of pedagogical link-making for continuity. The second research question addressed the coherence of electrolytic cell concepts within each grade. The meso and micro scales were used to investigate the sequential coherence of the electrolytic cell and its underlying concepts from Grades 10 to 12.
Conceptual Progression Across Grades
The extract (Table 2) shows how concepts on the topic of chemical change are structured in the PSC across Grades 10 to 12.
Table 2 shows concepts that are covered in Grade 10 under reactions in aqueous solutions, such as ions in aqueous solutions, ion interaction, electrolytes, conductivity, and precipitation reactions. Acid-based processes, redox reactions, and oxidation numbers are among the higher-level topics covered in Grade 11. By the end of Grade 12, students are required to grasp electrochemical processes and possess more in-depth knowledge of acids and bases.
Basically, these topics, which are covered from Grades 10 to 12, show the progression of both content coverage and level of abstraction. In Grade 10, teachers are expected to teach two types of reactions: ion exchange (precipitation, gas forming, and acid-base reactions) and redox reactions. The PSC instructs that for redox reactions, students should be taught the folowing1:
Redox reactions are electron transfer reactions. (Use the charge of the atom as an indication of electron transfer, no redox reaction terminology is required here). Use the charge of the atom to demonstrate how losing or gaining electrons affects the overall charge of an atom.
The instructions to teachers in the content document of the PSC explain why the various types of reactions are being covered in this grade. This is based on the rationale that it helps students balance equations; as a result, more emphasis is placed on charges than on oxidation numbers. Students might be more ready for the more complex ideas of redox processes studied in Grade 11 if the basic ideas of oxidation numbers were introduced earlier in this grade. The importance of oxidation numbers is recognised by the PSC when it uses oxidation numbers rather than the charge on an atom to distinguish between redox processes and ion exchange reactions.1
Displacement reactions in ion-exchange reactions and displacement in redox reactions differ due to no change in oxidation numbers of elements (in ion-exchange reactions) and change in oxidation numbers of elements (in displacement reactions in redox reactions).
The scientific phrase 'redox reactions' should be used by both students and teachers. Curriculum developers accurately distinguish between displacement in ion exchange and redox reactions, employing oxidation numbers. Nonetheless, teachers in Grade 10 do not teach oxidation numbers. Changes in the charges of species must be used to balance chemical equations. However, allowing pupils to use technical language without comprehending what it means encourages rote learning because oxidation numbers are essential in understanding how various species have gained their distinctive charges through electron loss or gain. The comprehension of redox reactions and balancing chemical equations would be improved by linking oxidation numbers with charges. If curriculum designers decide against using oxidation numbers at this stage, it will make it more difficult for students in Grade 10 to balance equations. Charges and inspection are used in the balancing of equations,33 however, this is a hurried approach that is insufficient for balancing redox processes.
It must be acknowledged that the sequencing of topics from Grades 10 to 12 is hierarchical and the curriculum is designed to build concepts sequentially. The terminology and ideas of redox reactions and oxidation numbers need to be introduced, along with links to prior knowledge. This should be based on the types of reactions to which Grade 10 and 11 students are exposed.
The Grade 12 concept of electrolytic cells and its applications marks the end of the topic reactions in an aqueous solution, a scientific story that began in Grade 10. The order in which the topics are covered across the grades is shown in Figure 2.
Other significant ideas left out in Grade 11 include electrolytes or simple electrolysis in addition to the aforementioned omission of oxidation numbers in Grade 10. During the entire year of chemistry in Grade 11, students are typically not exposed to any concepts that include electrolysis. As a result, there is a conceptual gap, and students have to depend entirely on their fundamental knowledge of conductivity and electrolytes from Grade 10 when they study the notion of electrolytic cells in Grade 12. As can be seen, the conceptual advancement and meaningful learning of students are hindered by concept gaps within the spiral curriculum.
In Grade 11, students should be able to study strong and weak electrolytes by decomposing conducting materials with electricity, which will help them better understand conductivity and electrolytes. This would fill the conceptual gap by building on the concepts of nonconducting solutions (non-electrolytes) and conducting solutions (electrolytes) covered in Grade 10. Due to poor exposure to concepts in Grade 11 related to the topic of electrolytic cells, the progression of ideas across grades fails to meet the requirement of a spiral curriculum.
In this section, I described how the PSC addresses the transition between grades, which are separated by years (macro scale). The weaknesses and strengths of the hierarchical arrangements of topics in the curriculum were further highlighted. The next section examines the conceptual coherence between grade-level topics, focusing on links intended to encourage continuity at the macro scale (separated by months and weeks) and the meso scale (separated by days and lessons).
Arrangements of Topics between Grades
The topics in the two documents, the PSC and the 2019 PSWS, are arranged according to terms. The content document outlines the topics that should be covered per term, as well as the work schedule. It has detailed time frames related to terms, months, weeks and the number of hours that should be spent teaching a particular topic or concept. Teachers are supposed to follow the time frame to the letter, and there is a monitoring tool called the Curriculum Management Model (CMM) in place to check the syllabus coverage for each teacher. There is no leeway to change how the topics are ordered in the work schedule, as shown in Table 3.
In general, all physics topics are taught sequentially and logically throughout all grades. Since there is minimal correlation between, for example, waves and electric circuits at this level, physics is easier to sequence because the topics are distinct. So, separating the two topics with another, different, topic would not considerably affect the coherence of concepts. The placement of unrelated physics topics between chemistry topics, however, must be done with caution because chemistry topics are interconnected.
Consider the topics covered in the first and second terms of Grade 10, as taken from Table 2:
Atomic Structure -> Chemical Bonding -> Waves -> Atoms, Compounds & Molecules -> Chemical Change (Matter and Energy & Balancing Equations) -> Magnetism -> Electrostatics -> Electric Circuits -> Chemical Change (Reactions in Aqueous Solutions) - Mole Concept.
Atoms, compounds, and molecules are linked concepts with interlinking ideas that pertain to chemical bonding. However, there is a three-week break between the first and second term, and the chemistry topics are separated by the topics of waves, which lasts for four weeks. When discussing atoms, compounds, and molecules again in the second term, teachers and students cannot make references to meso-scale concepts. The same applies to chemical change, and it is even worse in this case. The teaching of magnetism, electrostatics, and electricity lasts for three weeks, and a two-week break divides the theme of chemical change into two parts. Like atoms, molecules, and compounds, hardly any meso-scale interlinking occurs during the teaching of reactions in aqueous solutions. The 2019 work schedules for Grades 11 and 12 followed the same pattern.
The same topic of electrostatics, electricity, magnetism, and energy and power is covered in Grade 11 for five weeks before a three-week break, dividing the theme of chemical change into two halves again. The focus of this study, redox reactions, is presented sequentially with oxidation numbers, acids, and bases as the third topic after electromagnetism and electricity. Therefore, teachers and learners in Grade 11 can create links at the meso scale. Contrary to this view, concept sequencing in Grade 12 is just as poor as in Grade 10. After covering electromagnetic and electricity, the topic of electrochemical cells is addressed. There is a six-week break between this topic and the rates of reactions, and acids and bases. As a result, the meso scale is not emphasised throughout the teaching of the electrochemical cell, particularly during the first lesson.
These conceptual gaps encourage students to understand isolated pockets of ideas, which promote rote learning which does not promote the coherence and flow of concepts from one notion to the next. How can the 2019 work schedules be implemented if there are coherence anomalies given that teachers are required to use them in their current form?
OVERVIEW OF THE 2023 ANNUAL TEACHING PLANS
Since the COVID-19 era work schedules have been replaced by Annual Teaching Plans (ATPs). The 2023 ATPs from Grades 10 to 12 are currently authored nationally, unlike the 2019 work schedules and prior years that were produced provincially. The Grade 2023 10 ATP is coherently structured, and chemistry topics are no longer separated by physics topics. However, this is not the case with the 2023 ATPs for Grades 11 and 12.39
Table 4 shows how Grades 11, and 12 topics are sequenced over three terms. The matter and material knowledge area are separated by 8 weeks of chemical change topics, and the knowledge area of chemical change is separated by 2 weeks of matter and material topics.40 In Grade 12, the knowledge area of chemical change is separated by 4 weeks of teaching about electricity & magnetism and matter & material.41 The 2023 ATPs address the coherent sequencing of topics to a certain extent. However, they do not address the broken spiral episodes as the CAPS curriculum has not changed.
DISCUSSION
The study revealed that the Grade 12 topic of the electrolytic cell and its essential concepts of redox processes in Grade 11, and reactions in aqueous solutions in Grade 10, are organised progressively over the grades. The topics adhere to the standards of a spiral approach for coherence, the hierarchy of concepts, and organisation.22 The basic concepts in Grade 10 are revisited at each grade to Grade 12 with increasing levels of complexity.31 However, basic ideas were noticeably left out of Grades 10 and 11 on the topic of chemical change.
Despite being crucial to the conceptual understanding of redox reactions and balancing chemical equations, the idea of oxidation numbers is not covered in Grade 10. To comprehend the transfer of charge between any two species, students must use the change in oxidation values to calculate the quantity of charge transferred.31 Omitting oxidation numbers leaves a conceptual gap and falls short of spiral curricular requirements. When students are introduced to more complex phenomena in higher grades without grasping them in the lower grades, this creates a broken spiral in the curriculum.25
In a broken spiral, students are not able to fully understand the topic, which will hinder their understanding of upcoming and more difficult concepts in higher grades.
Electrolytes are not taught in Grade 11, according to this study. Grade 10 students learn about the topic by examining the conductivity of solutions. The idea of electrolytes is covered in detail in Grade 12 when students explore the concept of electrolysis and its applications, despite the topic not being further developed in Grade 11. Thus there is a conceptual gap in Grade 11, which cannot be filled by introducing the ideas of weak and strong electrolytes. The conceptual gap in Grade 11 is another instance of a broken spiral that impacts the macro-scale interconnection of ideas between grades. According to McPhail, a concept inside a topic receives its meaning from other concepts within the phase, hence the curriculum must show how they are connected.30
Additionally, it was discovered that the 2019 work schedule had infrequent chemistry topics across grades. Large conceptual gaps caused by physics concepts that separate the chemistry topics compromise learners' conceptual coherence, and restrict meaningful learning. Finally, there is no meso-scale linking of ideas separated by days and weeks in the teaching and learning processes. Staarman and Ametller argue that pedagogical linkages are understood as a means of making apparent the connections between various knowledge parts that must be connected to develop scientific knowledge.26 By connecting scientific concepts, a scientific story is created and the degree to which these connections are made affects students' conceptual understanding of the subject matter.35 The lack of vivid interlinking of science concepts in the curriculum uncovered in this study will negatively impact science learning and the comprehension of scientific ideas in the classroom.
CONCLUSION
In conclusion, failure to include the topic of oxidation numbers in the Grade 10 curriculum affects how well Grade 11 students learn about and understand redox reactions. Similarly, omitting electrolytes in Grade 11 impairs students' conceptualisation of electrolytic cells in Grade 12. These significant gaps undermine the spiral nature of the topic of chemical change, leading to content overload at the Grade 12 level. Furthermore, the lack of proper sequencing of concepts in each grade across the phase negatively affects the development of a scientific story, and impacts the understanding of the subject matter. Correct sequencing of the curriculum and how it is implemented affects how students grasp the content and build knowledge.
Curriculum developers are, therefore, advised to revise chemical change content to include oxidation numbers in Grade 10, and electrolytic cells in Grade 11. Including essential electrolysis content in Grade 11 will also reduce the load that has to be covered in Grade 12. The sequencing of scientific concepts is of great importance in the quest for meaningful teaching and learning. Therefore, it is recommended that curriculum developers revisit how the curriculum is sequenced in all grades. The DBE should consider covering each topic in a sequence of lessons. That is, chemical change should be taught without concepts being dispersed between other chemistry or physics topics. The best way to rectify this problem is to broaden the curriculum by offering physics and chemistry as separate subjects.
This paper only investigated the concept of electrolytic cells. However, there are other topics in this knowledge area, such as acids and bases, galvanic cells, and reaction rates, which need further investigation regarding whether they have broken spiral episodes. To determine whether the PSC conforms to a spiral curriculum in the South African setting, more research on the full science curriculum is advised. It is consequently recommended that as a starting point, curriculum planners investigate the possibility of oxidation numbers being incorporated into a broken spiral curriculum at the Grade 10 level.
ACKNOWLEDGEMENT
I am deeply grateful for the unwavering support and financial resources from the University of Pretoria's Faculty of Education and the Mathematics Science Technology Education Department, which have been instrumental in ensuring the success of this project. I am thankful to my spouse Sipiwe Mudadigwa, and our two sons Agape Prince Mudadigwa and Elnathan Tonderai Mudadigwa for their emotional support and encouragement throughout the writing of this paper.
ORCID ID
Brighton Mudadigwa: https://orcid.org/0000-0002-5366-6207
REFERENCES
1. Department of Education, DoE. National Curriculum Statement: Curriculum and Assessment Policy Statement, Grades 10-12 Physical Science. Pretoria: Department of Basic Education; 2011. https://www.education.gov.za/Portals/0/CD/National%20Curriculum%20Statements%20and%20Vocational/CAPS%20FET%20%20PHYSICAL%20SCIENCE%20WEB.pdf?ver=2015-01-27-154258-683 [ Links ]
2. Grussendorff S, Booyse C, Burroughs E. What's in the CAPS Package? A Comparative Study of the National Curriculum Statement (NCS) and the Curriculum and Assessment Policy Statement (CAPS): Physical Sciences FET Phase. Umalusi; 2014. https://www.umalusi.org.za/docs/reports/2014/overview_comparitive_analysis.pdf [ Links ]
3. Sibanda D, Hobden P. The sequencing of basic chemistry topics by physical science teachers. Afri. J. of Res. Maths. Sci. Tech. Edu. 2016;20(2):142-153. [ Links ]
4. Ramatlapana K, Makonye J. From too much freedom to too much restriction: The case of teacher autonomy from national curriculum statement (NCS) to curriculum and assessment statement (caps). Afr. Educ. Rev. 2012;9(sup1):S7-S25. [ Links ]
5. Bain K, Siddique MNA. Organization of contents in intended junior secondary science curriculum of Bangladesh: An explorative study. Sci. Educ. Int. 2017;28(2):156-166. https://doi.org/10.33828/sei.v28.i2.9. [ Links ]
6. Coelho C, Moles D. Student perceptions of a spiral curriculum. Eur. J. of Dent. Educ. 2016;20(3):161-166. https://doi.org/10.1111/eje.12156. [ Links ]
7. Dunton JB, Wilhelmina S. Spiral progression approach in teaching science and the performance of learners in district I, capiz. J. Phys.: Conference Series. 2019;1254(1):012045. https://doi.org/10.1088/1742-6596/1254/1/012045. [ Links ]
8. Potgieter M, Davidowitz B. Grade 12 achievement rating scales in the new national senior certificate as indication of preparedness for tertiary chemistry. S. Afr. J. Che. 2010;63:75-82. [ Links ]
9. Davidowitz B, Chittleborough G, Murray E. Student-generated submicro diagrams: A useful tool for teaching and learning chemical equations and stoichiometry. Chem. Edu. Prac. 2010;11(3):154-164. https://doi.org/10.1039/C005464J. [ Links ]
10. Amponsah KD. South African twelfth-grade students' conceptions regarding electrochemistry. J. of Edu. Lrng. (EduLearn). 2020;14(3):362-368. https://doi.org/10.11591/edulearn.v14i3.16273. [ Links ]
11. Amponsah KD, Kotoka JK, Beccles C, Dlamini SN. Effectiveness of collaboration on low and high achieving school students' comprehension of electrochemistry in South Africa. Eur. J. STEM Educ. 2018;3(2):4. https://doi.org/10.20897/ejsteme/2685. [ Links ]
12. Hesse III JJ, Anderson CW. Students' conceptions of chemical change. J. Res. in Sci. Tchng. 1992;29(3):277-299. [ Links ]
13. Devetak I, Vogrinc J, Glazar SA. Assessing 16-year-old students' understanding of aqueous solution at submicroscopic level. Res. Sci. Educ. 2009;39(2):157-179. https://doi.org/10.1007/s11165-007-9077-2. [ Links ]
14. Rahayu S, Treagust DF, Chandrasegaran AL. High school and preservice chemistry teacher education students' understanding of voltaic and electrolytic cell concepts: Evidence of consistent learning difficulties across years. Int. J. Sci. Maths Educ. 2021;1-24. [ Links ]
15. Department of Education, DoE. National senior certificate examination 2017 diagnostic report part 1. Pretoria: Department of Education; 2018. [ Links ]
16. Department of Education, DoE. National senior certificate examination 2021 diagnostic report part 1: Content Subjects. Pretoria: Department of Education; 2022. [ Links ]
17. Mudadigwa B, Msimanga A. Exploring teacher pedagogical practices that help learners make connections during the teaching of reactions in aqueous solutions at senior secondary level. Afr. J. of Res. in Maths. Sci. Tech. Edu. 2019;23(3):332-343. https://doi.org/10.1080/18117295.2019.1688476. [ Links ]
18. Widarti HR, Rokhim DA, Syafruddin AB. 2020. The development of electrolysis cell teaching material based on stem-pjbl approach assisted by learning video: A need analysis. J. Pndkn IPA Indon. 2020;9(3):309-318. [ Links ]
19. Grove NP, Hershberger JW, Bretz SL. Impact of a spiral organic curriculum on student attrition and learning. Chem. Educ. Res. Pract. 2008;9(2):157-162. https://doi.org/10.1039/B806232N. [ Links ]
20. Biesta G. Reclaiming teaching for teacher education: Towards a spiral curriculum. Beij. Int. Rev. Educ. 2019;1(2-3):259-272. https://doi.org/10.1163/25902539-00102015. [ Links ]
21. Ozdem-Yilmaz Y, Bilican K. Science Education in Theory and Practice. Cham: Springer; 2020. Disc. lrnng; p. 177-190. [ Links ]
22. Bruner JS. The process of education: A searching discussion of school education opening new paths to learning and teaching. Cambridge: Harvard University Press; 1960. [ Links ]
23. Gibbs B C. Reconfiguring Bruner: Compressing the spiral curriculum. Phi Delta Kappan. 2014;95(7):41-44. https://doi.org/10.1177/003172171409500710. [ Links ]
24. Neumann Y, Neumann E, Lewis S. The robust learning model with a spiral curriculum: Implications for the educational effectiveness of online master degree programs. Cont. Iss. Educ. Res. 2017;10(2):95-108. [ Links ]
25. Orale RL, Uy MEA. When the spiral is broken: Problem analysis in the implementation of spiral progression approach in teaching mathematics. J. of Aca. Res. 2018;3(3):14-24. [ Links ]
26. Staarman JK, Ametller J. The Routledge International Handbook of Research on Dialogic Education. Routledge; 2019. Pedagogical link-making with digital technology in science classrooms: New perspectives on connected learning; p. 497-508. [ Links ]
27. Scott P, Mortimer E, Ametller J. Pedagogical link-making: A fundamental aspect of teaching and learning scientific conceptual knowledge. Stud. in Sci. Educ. 2011;47(1):3-36. https://doi.org/10.1080/03057267.2011.549619. [ Links ]
28. Department of Education. National curriculum statement: Physical science content. Pretoria: Department of Basic Education; 2006. [ Links ]
29. Ausubel DP. The facilitation of meaningful verbal learning in the classroom. Educ. Psych. 1977;12(2):162-178. https://doi.org/10.1080/00461527709529171 [ Links ]
30. McPhail G. The search for deep learning: A curriculum coherence model. J. Curr. Stu. 2021;53(4):420-434. [ Links ]
31. Ireland J, Mouthaan M. Perspectives on curriculum design: Comparing the spiral and the network models. Res. Mat. 2020;30(Autumn):7-12. [ Links ]
32. Mortimer EF, Scott P. Meaning making in secondary science classroom. Philadelphia: Open University Press; 2003. [ Links ]
33. Jensen WB. Balancing redox equations. J. Chem. Educ. 2009;86(6):681. https://doi.org/10.1021/ed086p681. [ Links ]
34. Lehesvuori S, Ametller J. Exploring coherence and authorship in pedagogical link-making in science. Int. J. of Sc. Educ. 2021;43(17):2791-2813. https://doi.org/10.1080/09500693.2021.1991599. [ Links ]
35. Borg WR, Gall MD. Educational research: An introduction (4th ed). New York: Longman;1983. [ Links ]
36. McMillan JH, Schumacher S. Research in education: Evidence-based inquiry. My education lab series; 2010. [ Links ]
37. Merriam SB. Qualitative research and case study applications in education. San Francisco: Jossey-Bass Publishers; 1998. [ Links ]
38. Opie C. Doing educational research. London: SAGE; 2004. Chapter 5, Res. Appr; p. 87-92. [ Links ]
39. Department of Education, DoE. 2023/24 Annual Teaching Plans: Physical Sciences: Grade 10: Pretoria Department of Basic Education; 2022. https://www.education.gov.za/Portals/0/Documents/Recovery%20plan%20page/2023%20ATPs/FET%20Content%20Subjects/Grade%2010/1.520%20ATP%202023-24%20Gr%2010%20Phys%20Sci%20final.pdf?ver=2022-12-22-114024-427 [ Links ]
40. Department of Education, DoE. 2023/24 Annual Teaching Plans: Physical Sciences: Grade 11 : Pretoria. Department of Basic Education; 2022. https://www.education.gov.za/Portals/0/Documents/Recovery%20plan%20page/2023%20ATPs/FET%20Content%20Subjects/Grade%2011/1.530%20ATP%202023-24%20Gr%2011%20Phys%20Sci%20final.pdf?ver=2022-12-22-114024-333 [ Links ]
41. Department of Education, DoE. 2023/24 Annual Teaching Plans: Physical Sciences: Grade 12: Pretoria. Department of Basic Education; 2022. https://www.education.gov.za/Portals/0/Documents/Recovery%20plan%20page/2023%20ATPs/FET%20Content%20Subjects/Grade%2012/1.540%20ATP%202023-24%20Gr%2012%20Phys%20Sci%20final.pdf?ver=2022-12-22-114024-350 [ Links ]
Received 13 February 2023
Revised 8 August 2023
Accepted 26 October 2023
* To whom correspondence should be addressed. Email: bbmudadigwa@gmail.com














