Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Chemistry
versión On-line ISSN 1996-840X
versión impresa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.77 Durban 2023
http://dx.doi.org/10.17159/0379-4350/2023/v77a15
RESEARCH ARTICLE
An investigation into arsenic speciation in a wetland impacted by acid mine drainage
Shaeen ChettyI; Marc S. HumphriesII; Katharina BlümleinIII; Letitia PillayI, *
IMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Johannesburg, South Africa
IISchool of Chemistry, University of the Witwatersrand, Johannesburg, South Africa
IIIFraunhofer Institute for Toxicology and Experimental Medicine, Hannover, Germany
ABSTRACT
The formation of acid mine drainage (AMD) and release of toxic contaminants, such as arsenic (As), is a serious environmental problem encountered worldwide. In this study, we investigate the crucial role the Klip River wetland system plays in attenuating As arising from gold mining activities within the Witwatersrand Basin in Johannesburg, South Africa. Mining operations in the region commenced over 130 years ago and have been associated with the widespread pollution of water resources by AMD. We investigated As concentrations, bioavailability and speciation in a peat core from the Klip River wetland as well as in samples from the main tributaries and tailing storage facilities (TSFs) in the upper catchment. Total As concentrations in tributary and TSFs samples ranged between 10.1-89.9 mgkg-1 and 77.4-106 mg kg-1, respectively, with concentrations in the wetland varying between 1.91-73.8 mg kg-1. In general, As bioavailability was low in both catchment (19%) and wetland (4%) samples, with elemental associations suggesting the majority is bound in an immobile form to organic matter and sulfide. As(v) was the predominant species detected in all samples (0.0901-16.6 mg kg-1), with As(m), MMA and DMA present in lower concentrations. Strong correlations between As and S suggest that speciation and methylation are dependent on both chemical and microbial activity. The study highlights the vital role that wetlands can play in sequestering As in the environment.
Keywords: microbial biotransformation, speciation, wetland, Witwatersrand
INTRODUCTION
Arsenic (As) is one of the most significant contributors to environmental pollution worldwide.1-3 A common by-product of many industrial processes, the entry of As into the environment often has severe consequences on both ecosystem and human health due to its extreme toxicity. Arsenic is also commonly associated with gold-bearing ores and co-exists with heavy metals because of its affinity for sulfides and iron hydroxides.4 Mining activities accelerate the natural weathering process of these minerals and can lead to the formation of acid mine drainage (AMD), which releases As bound to sulfide and oxide bearing minerals.5 The mobility of As is controlled by a variety of processes, including redox, adsorption and desorption, ion exchange, precipitation and dissolution, and biological activity.6 These processes are often inter-linked and several may occur simultaneously dictating the speciation and/or dissolution of As minerals.7,8
The behaviour of As can be particularly complicated in dynamic ecosystems, such as wetlands, where there may be rapid changes in sediment and water chemistry, resulting from seasonal variations and the interaction between different biological communities.6,7 Such factors influence the migration and attenuation of As in soil and groundwater. In natural wetland systems, As is often found adsorbed to Fe-Mn oxy-hydroxides,9,10 where speciation is microbially driven.11,12 The speciation of As has a significant influence on fate and toxicity in the environment. There are a range of oxidation states in which As exists, with arsenite (+3) and arsenate (+5) being the predominant forms.6 The trivalent oxidation state is generally considered to be more toxic than the pentavalent, and inorganic forms significantly more toxic than the organic forms (e.g., monomethyl arsenic (MMA), dimethyl arsenic (DMA).5,13 In wetlands affected by anthropogenic activities, pH and organic matter can play a more significant role in As behaviour. Wetlands impacted by upstream mining activities are often subjected to increased acidic conditions,14 which promotes the dissolution of As and other metals from minerals.15,16 The dissolution of As increases its concentration in waterways, the consequent change in water chemistry affecting speciation dynamics. Initially, As(in), the more reactive, toxic species would be more mobile under these conditions and reacts to form organoarsenicals. Over time conversion to As(v) occurs forming more stable, less mobile complexes.17
In wetlands, As(v) tends to be the predominant species, co-precipitating to ferric hydroxides or undergoing biotransformation to render it immobile. However, atypical behaviour is commonly observed for As speciation and mobility in wetlands contaminated by AMD. The objective of this study was to better understand the behaviour, fate and mobility of As in a wetland heavily impacted by gold-mining activities in the Witwatersrand Basin of South Africa. We examine the influx, bioavailability and transformation of As in a wetland system that has been influenced by intensive gold mining activities over the last 100 years. The wetland occupies a strategic position in the landscape and plays a vital role in attenuating pollutants14,18-22 and maintaining downstream water quality in one of Africa's most industrialised cities.
METHODS
Study Area
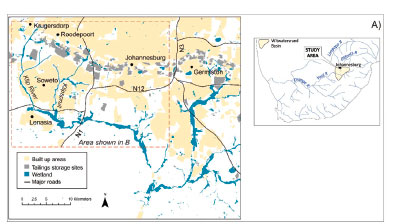
The Witwatersrand Basin, located in central South Africa, is the largest gold reserve in the world with an estimated reserve of 30 000 tonnes.23 Gold mining has had a significant impact on water resources and wetlands in the region, largely as a result of AMD.24 The Klip River catchment is a sub-basin of the Vaal-Orange River system and represents the most significant drainage system for the southern Witwatersrand region (Figure 1A).21
The Klip River and Klipspruit are the primary tributaries draining the Central Witwatersrand mining-industrial complex and discharge into the extensive wetland area downstream. Mining and industrial activities, located predominantly to the north of the wetland, are in close proximity to river tributaries.20 In the Klip River wetland, AMD arising from tailings storage facilities (TSFs) result in leaching of metals into both surface and groundwater. The primary mechanism of entry in this system is via groundwater discharge.18,19 The wetland also receives runoff from several large informal settlements and industrial regions, as well as discharge from three sewerage treatment plants. Elevated concentrations of metals (Cu, Co, Ni, Pb, U and Zn) and organic pesticides (polyaromatic hydrocarbons and polychlorinated biphenyls) have been reported.22
Sampling
Surface sediment samples (n = 7) were collected from the two main tributaries, the Klip River (KR1, KR2, KR3, KR4) and Klipspruit (KS1, KS2, KS3), as well as from three TSFs (T1, T2, T3) located in the upper catchment of the Klip River wetland (Figure 1B). A 3.5 m sediment core (C1; 26.309608° S, 27.859830° E) was collected in the upper section of the Klip River wetland (Figure 1B) using a Russian peat corer. Sub-samples for analysis were taken at 10 cm intervals and subsequently stored in bags at 4 °C. Field work took place at the end of the rainy season in March 2017 and the wetland inundated at the time of sampling. The pH and redox potential of sediment samples were measured using a portable combination meter (Thermo Scientific Orion Star) calibrated against appropriate standard solutions.
Chemical analysis
Total concentrations
Sediment samples (n = 42) collected from the wetland and its tributaries were air-dried, milled to a fine powder and combusted in a furnace at 500 °C for 4 hours to measure loss on ignition (LOI). Combusted sample powders (~25 mg, n = 3) were microwave digested in 9 mL HF:HNO3:HCl (6:3:1) using an Anton Paar Multiwave Go system.25 Solutions were evaporated to dryness following the addition of perchloric acid (0.5 mL), before finally being diluted with 1% HNO3 and spiked with Rh as internal standard for final analysis.
Total As concentrations in sediment was measured against external matrix-matched standards with an Agilent 7700x ICP-MS at the University of the Witwatersrand. Procedural blanks (n = 12) were analysed with each digestion batch and used to correct final sample concentrations. The sediment reference material CGL-111 (Central Geological Laboratory) was used to assess the accuracy and reproducibility of the method, with recoveries of As ranging between 84% and 102% (n = 42). Internal precision was typically <3% for all As analyses. Major elements (Fe2O3 and MnO) were analysed by powder X-ray fluorescence (XRF) using a Bruker Ranger S2, following calibration against a range of local and USGS rock standards. Sulfur was measured using a Thermo Scientific Flash 2000 Organic Elemental CHNS Analyser.
Arsenic speciation
Sediment samples (n = 34) for speciation analysis were selected based on measured total As concentrations. Air-dried sediment (100 ± 50 mg, n = 3) was extracted using a mixture of 5 mL 50 mM KH2PO4 and 5 mL 50 mM K2HPO4. The phosphate-based extraction solution was prepared by dissolving appropriate amounts of K2HPO4 and KH2PO4 in distilled water resulting in a solution of 50 mM K2HPO4 and 50 mM KH2PO4. Samples were vortexed before being placed in an ice-cooled ultra-sonic bath for 30 minutes. Samples were then centrifuged at 1500 rpm and the supernatant extracted and stored at 4 °C before analysis. Prior to analysis, sample extracts were diluted (1:5) with 0.1 M HCl26,27 and quantified by HPLC-ICP-MS. The bioavailable fraction was considered to be the sum of the extracted species.
Chromatographic separation was performed by elution with a 0.5 mL min-1 flow rate through an anion-exchange Dionex Ion Pac AS7 analytical column (2 x 250 mm) and a ThermoScientific Dionex ICS-5000+ IC system with a ThermoFischer iCAP TQ detector. A gradient elution consisting of 200 mM (NH4)2CO3 (eluent A) and 20 mM NH4HCO3 (eluent B) was used with the following conditions: 0-2 min 100% eluent B, 2-10 min 50% eluent B, and 10 min 100% eluent B. Reagent blanks (n = 3) were included in sample sequences and quantification was done against the As(v) species. To exclude possible interference from 40Ar35Cl+, the determination of 75As (dwell time = 100 ms) was carried out in the SQ-KED mode using He as the collision gas.
Standard solutions of As species (As(in), As(v), MMA and DMA) were made using sodium salts (>98%, Sigma-Aldrich). Appropriate dilutions of stock solutions were made from working standards to prepare calibration standards. Calibration of each As species was performed on six points over a range of 0.05 to 5.0 |ig L-1.
RESULTS
Chromatographic separation
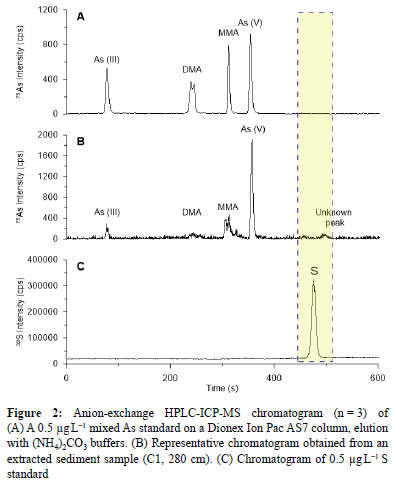
Ion-exchange chromatography hyphenated to ICP-MS was used to separate and identify the four different As species in a 0.5 |ig L-1 combined species standard (Figure 2A). All targeted As species were adequately baseline separated. The formation of a shoulder was evident at low concentrations for DMA but did not affect the analysis and subsequent quantification. Calibration curves with good correlation coefficients (R2 > 0.999) were obtained. The QC standards analysed during the course of each analysis batch indicated stability (80-90% recovery) for DMA, MMA and As(v), while the phosphate buffer mobile phase/standard matrix introduced some variability in the As(iii) quantification. A correction factor (1.3-1.4) was applied to the data accordingly. Limits of detection (0.04 ng L-1) and quantification (0.12 ng L-1) were calculated based on repeated measures of blank phosphate buffer injections.
Physical characteristics and total As concentrations
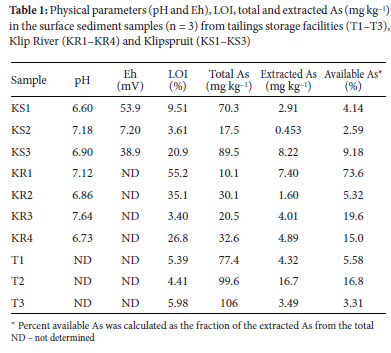
The pH of surface sediment from the tributaries varied within a narrow range of 5.79-7.86 and were predominantly anoxic (7.20-53.9 mV). Surface samples collected from TSFs and the two tributaries, Klip River (KR) and Klipspruit (KS), show highly variable As concentrations (Table 1). Material from TSFs (77.4-106 mg kg-1) was characterised by considerably higher As concentrations compared to sediments from both the Klip River (10.01-32.6 mg kg-1) and Klipspruit (17.4-9.9 mg kg-1). Arsenic was relatively bioavailable in the Klip River (28.4 ± 30.7%), with KR1 having 73.6% of total As in bioavailable form. Sites closer to the wetland (KS3 and KR4) tended to have higher total and bioavailable As (32.6-89.5 mg kg-1 and 9.18-15.0%, respectively).
For the sediment core, pH ranged between 6.54 and 7.41, while Eh varied between -317 and 124 mV and showed no obvious downcore trend (Figure 3). The upper part of the sediment profile was characterised by high %LOI (65-90%), but values decreased sharply at ~250 cm with material from the bottom of the core containing little organic matter (LOI = 5%). Concentrations of S (0.05-6%) showed a gradual decrease downcore and have some similarity to LOI. Arsenic concentrations varied between 15 and 75 mg kg-1 with the highest concentration recorded at a depth of 220 cm. Arsenic is relatively unavailable, ranging between 1.13-10.0%, limiting its mobility in the wetland.
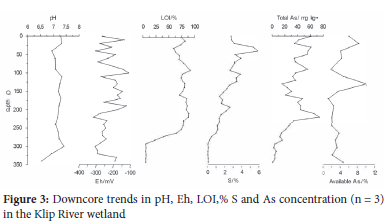
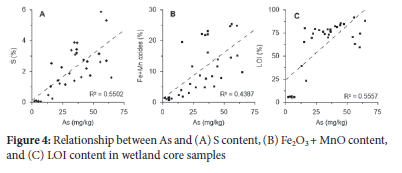
Possible As-complexes were investigated using correlation plots (Figure 4). Strong positive correlations between total As and S (R2 = 0.55), Fe+Mn oxides (R2 = 0.44) and LOI (R2 = 0.56) suggest that these complexes are likely to have the strongest influence on As mobility and speciation.
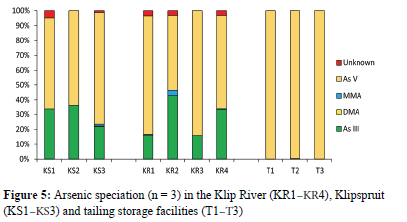
Arsenic speciation
The predominant species detected in extracted sediment samples was As(v), followed by As(in), MMA and an unidentified As species (Table S1, Figure 5). Material from TSFs was characterised by a very different speciation distribution pattern compared to the river and wetland samples, with As(v) the only species present in concentrations ranging between 3.49 and 16.5 mg kg-1 (Figure 5). River tributaries had low levels of MMA (<0.11 mg kg-1) and As(iii) contributing ~30% of the total As present, with As(v) the predominant species present (0.288-6.17 mg kg-1).
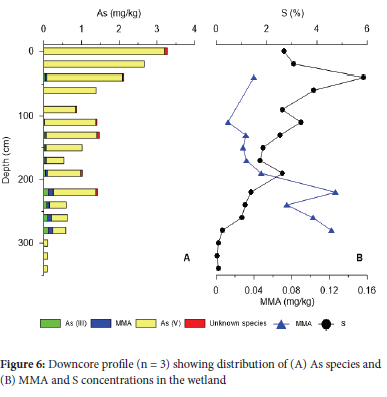
Arsenic concentrations in the sediment core reveal a high degree of variability in speciation (Figure 6A,Table S2). In the upper 100 cm As(v) concentrations were elevated (0.0789-0.333 mg kg-1 and 0.831-3.19 mg kg-1, respectively), followed by a general decline in concentration with depth. Conversely, As(iii) increases below 100 cm (0.0343-0.141 mg kg-1). MMA and DMA were more prevalent in the wetland core compared to the surface river samples. Concentrations of MMA generally increased with depth (0.0123-0.126 mg kg-1). There appears to be an inverse relationship between MMA and S with depth (Figure 6B).
Most wetland and tributary samples were characterised by an unknown As peak (Figure 2B) at approximately 500 s. The concentration of this unknown As species ranged between 0.0296 and 0.253 mg kg-1.
DISCUSSION
Transport and accumulation of As in the wetland
Arsenic concentrations in the Klip River wetland system reflect the long-term impacts associated with mining activities and industrial development around Johannesburg over the last century. It is likely that As entry into the wetland follows the pattern observed for other metals associated with mining waste in this system18,19 i.e. via groundwater and to a lesser extent surface inflow. The two likely sources of As are from the leaching of residual waste stored in TSFs and seepage from flooded mine voids, which contain highly acidic and sulfidic water, with high concentrations of dissolved metals including As. Dry TSFs, the most common type of facility in South Africa, are typically characterised by an outer oxidation zone and an inner reducing region.28 In the outer oxidation zone, As is likely to be stable and precipitated to Fe, Al or Mn oxides. However, As is likely to be mobile in the inner reducing region of TSFs, providing a source of As contamination into the tributaries and wetland. The leaching of As from TSFs may also be promoted through the microbial oxidation of sulphur-containing ores.29,30 Flooded voids associated with abandoned mines in the Witwatersrand Basin are characterised by highly acidic (pH < 3) water containing elevated concentrations of sulfur (>3000 mg L-1) and various heavy metals. In the Central Basin, mine water is characterised by As concentrations ranging between 22 and 116 |ig L-1.31
Arsenic associations in the sediment influence its availability and movement. In wetlands affected by mining, the presence of iron-based sulfur minerals, such as FeS2, results in As either incorporating into the structure of Fe-pyrites or adsorbing to or co-precipitating with other minerals (including sulfur) rendering it unavailable.8,32,33 Correlation plots (Figure 4) indicate that the most likely As-associations; Fe-Mn oxides and organic matter (e.g., peat) and S. The TSFs are characterised by high concentrations of Fe-Mn oxides and As and low concentrations of organic material. Bioavailability data (Table 1) indicates that the majority of As is not mobile with only 8.54% of the total As measured bioavailable.
The wetland core samples indicate stronger associations with organic and sulfur content, with a lower bioavailability of 3.54%. Given the nature of As speciation in wetlands affected by AMD, and significant correlation between As and S (Figure 4), it is plausible that the unidentified species (Figure 2) is an As-sulfur species. Degradation or reaction of the sulfur species may have resulted in the slight shift in the retention times of samples containing the unknown peak.34,35 The physico-chemical parameters (particularly Eh) of the wetland suggest that the As may be complexed to a sulfate or sulfide species.36 This postulated As-S species was only present in samples where elemental S concentrations were >0.07% (Figure 6) and it is therefore likely that As and S are related. Arsenic adsorbed to both organic and sulfur based substrates are generally considered not bioavailable.37,38
Speciation
Arsenate is the predominant species in both the tributaries (surface runoff) and the wetland system. Sediment composition plays an important role in the speciation and sequestration of arsenic in wetlands. Inorganic As tends to adsorb to metal oxides in sediments removing As from solution.6 Iron and Mn oxides/hydroxides may facilitate the oxidation of As(iii) and adsorption onto mineral surfaces. Under highly anoxic conditions in wetlands, As tends to become more mobile due to the reductive dissolution of Fe-Mn oxyhydroxides.39 Additionally, arsenate tends to reduce to the more mobile and toxic As(iii). The trend noted in the Klip River wetland appears to be typical for wetlands impacted by AMD40-44 and in systems with high S and organic matter.41 Although wetland sediments are anoxic, arsenate remains the predominant species over arsenite, which may be due to various attenuation processes occurring within the wetland. In this instance, it is likely primarily oxidation (through chemical or microbial changes) followed by surface sorption organic matter or other sulfide bearing minerals.39 Such a mechanism is supported by the observed relationships between As and S, and organic matter (Figure 2, 3, and 4).
Additionally, high concentrations of organic matter and sulfur often provide a rich environment for microbial activity45 which may result in the biotransformation of As species.11 Bacteria which thrive in acid-rich environments may reduce or oxidise Fe, S, and As changing the speciation and in some instances producing volatile methylated compounds such as DMA(v), MMA, and volatile TMAO, TMA(iii).12,46-48 More specifically, Fe- or S-reducing bacteria (e.g., Desulfurella and Desulfuromonas), and arsenate respiring bacteria (e.g., Chrysiogenes arsenatis) result in arsenate dissociation from mineral phases.49,50 Conversely, the microbial oxidation of minerals containing As leads to attenuation of the by-products (e.g., As-Schwertmannite).51
Bacteria from the species Aeromonas hydrophila Bacillus sp., Pseudomonas sp., Bacillus licheniformis and genera Pseudomonas, Aeromonas sp., and Arthrobacter have previously been identified in the Klip River.52 These species are typically As resistant and act as oxidants.53-55 Arsenic biotransformation may result in a change of oxidation state As(iii) to As(v),11,30 indirectly by bacterial oxidation of Fe(ii), increasing adsorption sites for As,56 facilitating precipitation. Further, sulfur is often utilised as the electron donor in the methylation processes of As within bacteria.57,58 The decrease of S and increase in MMA content (Figure 6B) infers a methylation mechanism, most likely through microbes. Monomethyl arsenate may be further transformed by the presence of sulfur to thiolated As species, sulfide containing As(v) metabolites e.g., monomethyl monothioarsonic acid and dimethyl monothioarsinic acid which are then sequestered by wetland plants.59 Furthermore, microbial reduction of sulfate to form sulfides may also have contributed to the formation ofthiolated species with As.36 Alternatively, MMA may be retained in the wetland through adsorption to organic matter or may undergo further methylation forming DMA and TMA, all of which are volatile and bioavailable leaving a limited signature in the wetland.60,61
The Klip River wetland is an effective trap for As, sequestering it in relatively immobile forms. However, the dynamic nature of a wetland, due to the constant changing of processes (flooding, sorption, climatic), and the on-going anthropogenic activity in this area puts the As(v) stability at risk. Disrupting the microbial balance and sequestration mechanisms currently utilised to oxidize As, could result in dissolution of Fe-oxyhydroxide minerals releasing As(v) into the environment. One of the major influences on the biogeochemistry of the wetland and As speciation, is the sustained and continuous mining of peat for use in horticulture.62 Severe reduction in metal attenuation capacity has been with reported as early as 2007 with parts of the wetland in a state of collapse.20 The removal of peat adversely impacts wetland function and would result in the release of sequestered As. The release of As species, particularly the inorganic forms, into the environment poses a serious and significant threat to both ecosystem and human health. Contaminated drinking water is the most common pathway of exposure for humans. Long-term exposure to elevated concentrations of As may lead to a range of health issues including dermal, gastrointestinal, renal, neurological and carcinogenic effects.63-65 The implications of As present in drinking water as a result of the collapse of the Klip River wetland are significant considering that the wetland feeds into the Vaal dam, which supplies approximately 23% of the South African population with potable drinking water.66 In addition to the impact on water quality and the associated health effects, the economic impact from the cost of improving water purification systems would be considerable.
CONCLUSION
Total metal analysis indicates that AMD is likely responsible for the release and transport of As from TSFs and mine voids via groundwater plumes to the Klip River wetland. Attenuation of As occurs primarily through two mechanisms; oxidation of As(in) to As(v) with subsequent surface sorption to organic matter and/or sulfide minerals, and microbial biotransformation of As(iii) to As(v) or methylated arsenic species. These processes result in As being sequestered in a relatively immobile form within the wetland. However, deterioration in wetland function could result in large volumes of previously sequestered pollutants releasing into the environment severely affecting water quality and the surrounding environment. Preserving wetland integrity is thus critical for water resource management and human health.
ACKNOWLEDGEMENTS
This research was supported by the University of the Witwatersrand and the National Research Foundation of South Africa (NRF) (Grant: 102390). The authors would like to thank Fraunhofer Institute for Toxicology and Experimental Medicine (ITEM) for their assistance with the As speciation analysis.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPLEMENTARY INFORMATION
Supplementary information for this article is provided in the online supplement
ORCID IDs
Shaeen Chetty: https://orcid.org/0000-0001-6363-4113
Marc Steven Humphries: https://orcid.org/0000-0002-4047-1451
Katharina Blümlein: https://orcid.org/0000-0002-1556-1172
Letitia Pillay: https://orcid.org/0000-0002-6707-9222
REFERENCES
1. Chung JY, Yu SD, Hong YS. Environmental Source of Arsenic Exposure. J. Prev. Med. Pub. Health 2014;47(5):253-257. https://doi.org/10.3961/jpmph.14.036 [ Links ]
2. Fatoki JO, Badmus JA. Arsenic as an Environmental and Human Health Antagonist: A Review of Its Toxicity and Disease Initiation. J. Hazard. Mater. Adv. 2022;5:100052. https://doi.org/10.1016/j.hazadv.2022.100052 [ Links ]
3. Raju NJ. Arsenic in the Geo-Environment: A Review of Sources, Geochemical Processes, Toxicity and Removal Technologies. Environ. Res. 2022;203:111782. https://doi.org/10.1016/j.envres.2021.111782 [ Links ]
4. Sami K, Druzynski AL. Trace Constituents; WRC Report No. 1236/1/03; Water Research Commission: South Africa. 2003:89. [ Links ]
5. Paikaray S. Arsenic Geochemistry of Acid Mine Drainage. Mine Water Environ. 2014;34:181-196. https://doi.org/10.1007/s10230-014-0286-4 [ Links ]
6. Smedley P, Kinniburgh D. Source and Behaviour of Arsenic in Natural Waters; United Nations Synthesis report; 2001. https://www.semanticscholar.org/paper/Source-and-behaviour-of-arsenic-in-natural-waters-Smedley-Kinniburgh/5ad9cda11550955b7c83ce538e6daeeb9fdea999 (accessed 2023-01-18). [ Links ]
7. Barral-Fraga L, Barral, MT, MacNeill, KL, Martiná-Prieto D, Morin S, Rodríguez-Castro MC, Tuulaikhuu, BA, Guasch H. Biotic and Abiotic Factors Influencing Arsenic Biogeochemistry and Toxicity in Fluvial Ecosystems: A Review. Int. J. Environ. Res. Public. Health 2020;17(7): 2331. https://doi.org/10.3390/ijerph17072331 [ Links ]
8. Pillay L, Kindness A. A Preliminary Investigation into the Stability of Inorganic Arsenic Species in Laboratory Solutions Simulating Sediment Pore Water. South Afr. J. Chem. 2016;69: 9-14. https://doi.org/10.17159/0379-4350/2015/v69a2 [ Links ]
9. Fendorf S, Nico PS, Kocar BD, Masue Y, Tufano, KJ. Chapter 12 - Arsenic Chemistry in Soils and Sediments. In Developments in Soil Science; Singh B, Grafe M. Eds.; Synchrotron-Based Techniques in Soils and Sediments; Elsevier, 2010; 34:357-378. https://doi.org/10.1016/S0166-2481(10)34012-8 [ Links ]
10. Neil CW, Yang YJ, Schupp D, Jun YS. Water Chemistry Impacts on Arsenic Mobilization from Arsenopyrite Dissolution and Secondary Mineral Precipitation: Implications for Managed Aquifer Recharge. Environ. Sci. Technol. 2014;48(8):4395-4405. https://doi.org/10.1021/es405119q [ Links ]
11. Zhang J, Zhou W, Liu B, He J, Shen Q, Zhao FJ. Anaerobic Arsenite Oxidation by an Autotrophic Arsenite-Oxidizing Bacterium from an Arsenic-Contaminated Paddy Soil. Environ. Sci. Technol. 2015;49(10):5956-5964. https://doi.org/10.1021/es506097c [ Links ]
12. Zhang SY, Williams PN, Luo J, Zhu YG. Microbial Mediated Arsenic Biotransformation in Wetlands. Front. Environ. Sci. Eng. 2016;11(1): 1. https://doi.org/10.1007/s11783-017-0893-y [ Links ]
13. Nguyen MH, Pham TD, Nguyen TL, Vu HA, Ta TT, Tu MB, Nguyen THY, Chu DB. Speciation Analysis of Arsenic Compounds by HPLC-ICP-MS: Application for Human Serum and Urine. J. Anal. Methods Chem. 2018; 2018:e9462019. https://doi.org/10.1155/2018/9462019 [ Links ]
14. McCarthy TS, Humphries MS. Contamination of the Water Supply to the Town of Carolina, Mpumalanga, January 2012. South Afr. J. Sci. 2013;109(9/10):10. https://doi.org/10.1590/sajs.2013/20120112 [ Links ]
15. Fischel MHH. The Biogeochemical Cycling of Arsenic in a Changing Climate. PhD Thesis, University of Delaware, USA, 2019. http://udspace.udel.edu/handle/19716/24243 (accessed 2023-01-18). [ Links ]
16. Hussain MM, Wang J, Bibi I, Shahid M, Niazi NK, Iqbal J, Mian IA, Shaheen SM, Bashir S, Shah NS, Hina K, Rinklebe J. Arsenic Speciation and Biotransformation Pathways in the Aquatic Ecosystem: The Significance of Algae. J. Hazard. Mater. 2021;403:124027. https://doi.org/10.1016/j.jhazmat.2020.124027 [ Links ]
17. Lee K, Shim H, Lee D, Chung D. The Fate and Factors Determining Arsenic Mobility of Arsenic in Soil-A Review. Korean J. Soil Sci. Fertil. 2015; 48:7380. https://doi.org/10.7745/KJSSF.2015.48.2.073 [ Links ]
18. Chetty S, Pillay L, Humphries MS. Gold Mining's Toxic Legacy: Pollutant Transport and Accumulation in the Klip River Catchment, Johannesburg. South Afr. J. Sci. 2021;117. https://doi.org/10.17159/sajs.2021/8668 [ Links ]
19. Humphries MS, McCarthy TS, Pillay L. Attenuation of Pollution Arising from Acid Mine Drainage by a Natural Wetland on the Witwatersrand. South Afr. J. Sci. 2017;113(1-2):1-9. https://doi.org/10.17159/sajs.2017/20160237 [ Links ]
20. McCarthy TS, Arnold V, Venter J, Ellery WN. The Collapse of Johannesburg's Klip River Wetland. South Afr. J. Sci. 2007;103(9-10):391-397. [ Links ]
21. Vermaak VA. Geomorphological Investigation of the Klip River Wetland, South of Johannesburg. MSc Thesis, University of the Witwatersrand, South Africa, 2009. [ Links ]
22. Olasupo A. Assessment of Persistent Organic Pollutant Accumulation in Sediments of the Klip River. MSc Thesis, University of the Witwatersrand, South Africa, 2018. https://doi.org/10.13140/RG.2.2.30447.38566 [ Links ]
23. Frimmel HE. The Witwatersrand Basin and Its Gold Deposits. In The Archaean Geology of the Kaapvaal Craton, Southern Africa; Kroner, A., Hofmann, A., Eds.; Regional Geology Reviews; Springer International Publishing: Cham, 2019; pp 255-275. https://doi.org/10.1007/978-3-319-78652-0_10 [ Links ]
24. McCarthy TS, Venter JS. Increasing Pollution Levels on the Witwatersrand Recorded in the Peat Deposits of the Klip River Wetland : Research Article. South Afr. J. Sci. 2006;102(1):27-34. https://doi.org/10.10520/EJC96496 [ Links ]
25. Balaram V, Rao, T. Rapid Determination of REEs and Other Trace Elements in Geological Samples by Microwave Acid Digestion and ICP-MS. At. Spectrosc. 2003;24:206-212. [ Links ]
26. Georgiadis M, Cai Y, Solo-Gabriele HM. Extraction of Arsenate and Arsenite Species from Soils and Sediments. Environ. Pollut. 2006;141(1):22-29. https://doi.org/10.1016/j.envpol.2005.08.028 [ Links ]
27. Yuan CG, He B, Gao EL, Lü JX, Jiang GB. Evaluation of Extraction Methods for Arsenic Speciation in Polluted Soil and Rotten Ore by HPLC-HG-AFS Analysis. Microchim. Acta. 2007;159(1):175-182. https://doi.org/10.1007/s00604-006-0709-4 [ Links ]
28. Hansen RN. Contaminant Leaching from Gold Mining Tailings Dams in the Witwatersrand Basin, South Africa: A New Geochemical Modelling Approach. Appl. Geochem. 2015;61:217-223. https://doi.org/10.1016/j.apgeochem.2015.06.001 [ Links ]
29. Manchisi J, Hansford GS, GaylardI P, Simukanga S, Nyirenda RL, Sichalwe A. Potential for Bioleaching Copper Sulphide Rougher Concentrates of Nchanga Mine, Chingola, Zambia. J. South. Afr. Inst. Min. Metall. 2012;112(12):1051-1058. [ Links ]
30. Muravyov M, Panyushkina A. Distinct Roles of Acidophiles in Complete Oxidation of High-Sulfur Ferric Leach Product of Zinc Sulfide Concentrate. Microorganisms 2020; 8(3):386. https://doi.org/10.3390/microorganisms8030386 [ Links ]
31. Department of Water Affairs, D. Feasibility study long-term solution to acid mine drainage (AMD) - East, Central and West Rand underground mining basins in the Gauteng province. https://www.environment.co.za/acid-mine-drainage-amd/feasibility-study-long-term-solution-to-acid-mine-drainage-amd-east-central-and-west-rand-underground-mining-basins-in-the-gauteng-province.html (accessed 2023-01-18). [ Links ]
32. Saunders JA, Lee MK, Dhakal P, Ghandehari SS, Wilson T, Billor MZ, Uddin A. Bioremediation of Arsenic-Contaminated Groundwater by Sequestration of Arsenic in Biogenic Pyrite. Appl. Geochem. 2018;96:233-243. https://doi.org/10.1016/j.apgeochem.2018.07.007 [ Links ]
33. Wilkin RT, Wallschlâger D, Ford RG. Speciation of Arsenic in Sulfidic Waters. Geochem. Trans. 2003;4(1): 1. https://doi.org/10.1186/1467-4866-4-1 [ Links ]
34. Kalenga PM. Determination and Characterization of Sulphur in South African Coal. MSc Thesis, University of the Witwatersrand, South Africa, 2011. [ Links ]
35. Vu HA, Nguyen MH, Vu-Thi HA, Do-Hong Q, Dang XH, Nguyen TNB, Trinh HQ, Ly Bich T, Nguyen TT, Le-Van D, Tu MB, Chu DB. Speciation Analysis of Arsenic Compounds by High-Performance Liquid Chromatography in Combination with Inductively Coupled Plasma Dynamic Reaction Cell Quadrupole Mass Spectrometry: Application for Vietnamese Rice Samples. J. Anal. Methods Chem. 2019;2019:e5924942. https://doi.org/10.1155/2019/5924942 [ Links ]
36. Couture R.-M, Wallschlâger D, Rose J, Van Cappellen P. Arsenic Binding to Organic and Inorganic Sulfur Species during Microbial Sulfate Reduction: A Sediment Flow-through Reactor Experiment. Environ. Chem. 2013;10. https://doi.org/10.1071/EN13010 [ Links ]
37. Hu J, Zhou S, Wu P, Qu K. Assessment of the Distribution, Bioavailability and Ecological Risks of Heavy Metals in the Lake Water and Surface Sediments of the Caohai Plateau Wetland, China. PLOS ONE 2017;12(12):e0189295. https://doi.org/10.1371/journal.pone.0189295 [ Links ]
38. Wang H, Liu R, Wang Q, Xu F, Men C, Shen Z. Bioavailability and Risk Assessment of Arsenic in Surface Sediments of the Yangtze River Estuary. Mar. Pollut. Bull. 2016;113(1-2): 125-131. https://doi.org/10.1016/j.marpolbul.2016.08.076 [ Links ]
39. Langner P, Mikutta C, Kretzschmar R. Arsenic Sequestration by Organic Sulphur in Peat. Nat. Geosci. 2012;5(1):66-73. https://doi.org/10.1038/ngeo1329 [ Links ]
40. Cassone G, Chillé D, Foti C, Giuffré O, Ponterio RC, Sponer J, Saija F. Stability of Hydrolytic Arsenic Species in Aqueous Solutions: As3+vs. As5+. Phys. Chem. Chem. Phys. 2018;20(36): 23272-23280. https://doi.org/10.1039/C8CP04320E [ Links ]
41. Kocourková-Vísková E, Loun J, Sracek O, Houzar S, Filip J. Secondary Arsenic Minerals and Arsenic Mobility in a Historical Waste Rock Pile at Kank near Kutná Hora, Czech Republic. Mineral. Petrol. 2015;109(1):17-33. https://doi.org/10.1007/s00710-014-0356-0 [ Links ]
42. Meharg AA, Zhao FJ. Biogeochemistry of Arsenic in Paddy Environments. In Arsenic & Rice; Meharg AA, Zhao, FJ., Eds.; Springer Netherlands: Dordrecht, 2012; pp 71-101. https://doi.org/10.1007/978-94-007-2947-6_5 [ Links ]
43. Gnanaprakasam ET, Lloyd JR, Boothman C, Ahmed KM, Choudhury I, Bostick BC, van Geen A, Mailloux BJ. Microbial Community Structure and Arsenic Biogeochemistry in Two Arsenic-Impacted Aquifers in Bangladesh. mBio 2017;8(6): e01326-17. https://doi.org/10.1128/mBio.01326-17 [ Links ]
44. Yu Z, Qiu W, Wang F, Lei M, Wang D, Song Z. Effects of Manganese Oxide-Modified Biochar Composites on Arsenic Speciation and Accumulation in an Indica Rice (Oryza Sativa L.) Cultivar. Chemosphere 2017;168:341-349. https://doi.org/10.1016/jxhemosphere.2016.10.069 [ Links ]
45. Lescure T, Joulian C, Bauda P, Hénault C, Battaglia-Brunet F. Bacterial Oxidation of Arsenic in Polluted Soils: Role of Organic Matters; Conference: Interfaces Against Pollution. 2012:1 [ Links ]
46. Chen J, Sun GX, Wang XX, Lorenzo V, Rosen BP, Zhu YG. Volatilization of Arsenic from Polluted Soil by Pseudomonas Putida Engineered for Expression of the ArsM Arsenic(III) S-Adenosine Methyltransferase Gene. Environ. Sci. Technol. 2014;48(17):10337-10344. https://doi.org/10.1021/es502230b [ Links ]
47. Huang K, Xu Y, Zhang J, Chen C, Gao F, Zhao FJ. Arsenicibacter Rosenii Gen. Nov., Sp. Nov., an Efficient Arsenic Methylating and Volatilizing Bacterium Isolated from an Arsenic-Contaminated Paddy Soil. Int. J. Syst. Evol. Microbiol. 2017;67(9):3186-3191. https://doi.org/10.1099/ijsem.0.002068 [ Links ]
48. Kuramata M, Sakakibara F, Kataoka R, Abe T, Asano M, Baba K, Takagi K, Ishikawa S. Arsenic Biotransformation by Streptomyces Sp. Isolated from Rice Rhizosphere. Environ. Microbiol. 2015;17(6):1897-1909. https://doi.org/10.1111/1462-2920.12572 [ Links ]
49. Ahmann D, Roberts AL, Krumholz LR, Morel FM. Microbe Grows by Reducing Arsenic. Nature. 1994;371(6500):750. https://doi.org/10.1038/371750a0 [ Links ]
50. Ahmann D, Krumholz LR, Hemond HF, Lovley DR, Morel FMM. Microbial Mobilization of Arsenic from Sediments of the Aberjona Watershed. Environ. Sci. Technol. 1997;31(10):2923-2930. https://doi.org/10.1021/es970124k [ Links ]
51. Duquesne K, Lebrun S, Casiot C, Bruneel O, Personné JC, Leblanc M, Elbaz-Poulichet F, Morin G, Bonnefoy V. Immobilization of Arsenite and Ferric Iron by Acidithiobacillus Ferrooxidans and Its Relevance to Acid Mine Drainage. Appl. Environ. Microbiol. 2003;69(10):6165-6173. https://doi.org/10.1128/AEM.69.10.6165-6173.2003 [ Links ]
52. Chihomvu P, Stegmann P, Pillay M. Identification and Characterization of Heavy Metal Resistant Bacteria from the Klip River. Int. J. Biol. Food Vet. Agric. Eng. 2014;8(11):1107-1117. [ Links ]
53. Ike M, Miyazaki T, Yamamoto N, Sei K, Soda S. Removal of Arsenic from Groundwater by Arsenite-Oxidizing Bacteria. Water Sci. Technol. 2008;58(5):1095-1100. https://doi.org/10.2166/wst.2008.462 [ Links ]
54. Shi K, Wang Q, Wang G. Microbial Oxidation of Arsenite: Regulation, Chemotaxis, Phosphate Metabolism and Energy Generation. Front. Microbiol. 2020;11:569282. https://doi.org/10.3389/fmicb.2020.569282 [ Links ]
55. Herrera C, Moraga R, Bustamante B, Vilo C, Aguayo P, Valenzuela C, Smith CT, Yánez J, Guzmán-Fierro V, Roeckel M, Campos V. Characterization of Arsenite-Oxidizing Bacteria Isolated from Arsenic-Rich Sediments, Atacama Desert, Chile. Microorganisms 2021;9(3):483. https://doi.org/10.3390/microorganisms9030483 [ Links ]
56. Hao L, Liu M, Wang N, Li GA. Critical Review on Arsenic Removal from Water Using Iron-Based Adsorbents. RSC Adv. 2018;8(69):39545-39560. https://doi.org/10.1039/C8RA08512A [ Links ]
57. Chen C, Li L, Huang K, Zhang J, Xie WY, Lu Y, Dong X, Zhao FJ. Sulfate-Reducing Bacteria and Methanogens Are Involved in Arsenic Methylation and Demethylation in Paddy Soils. ISME J. 2019;13(10):2523-2535. https://doi.org/10.1038/s41396-019-0451-7 [ Links ]
58. Bentley R, Chasteen TG. Microbial Methylation of Metalloids: Arsenic, Antimony, and Bismuth. Microbiol. Mol. Biol. Rev. 2002;66(2):250-271. https://doi.org/10.1128/MMBR.66.2.250-271.2002 [ Links ]
59. Raab A, Williams PN, Meharg A, Feldmann J. Uptake and Translocation of Inorganic and Methylated Arsenic Species by Plants. Environ. Chem. Lett. 2007;4(3):197-203. https://doi.org/10.1071/EN06079 [ Links ]
60. Rensing C, Rosen BP. Heavy Metals Cycle (Arsenic, Mercury, Selenium, Others); Elsevier, 2009; pp 205-219. https://doi.org/10.1016/B978-012373944-5.00053-5 [ Links ]
61. Kumarathilaka P, Seneweera S, Meharg A, Bundschuh J. Arsenic Accumulation in Rice (Oryza Sativa L.) Is Influenced by Environment and Genetic Factors. Sci. Total Environ. 2018;642:485-496. https://doi.org/10.1016/j.scitotenv.2018.06.030 [ Links ]
62. Kitir N, Yildirim E, Üstün $, Metin T, Melek E, Selda O, Raziye K, Hüsnü Ü, Halime Ü. Peat Use in Horticulture. IntechOpen. https://www.intechopen.com/chapters/62735 (accessed 2023-01-18). [ Links ]
63. Ravenscroft P, Brammer H, Richards K. Health Effects of Arsenic in Drinking Water and Food. In Arsenic Pollution; John Wiley & Sons, Ltd, 2009; pp 157-212. https://doi.org/10.1002/9781444308785.ch5 [ Links ]
64. Hong YS, Song KH, Chung JY. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Pub. Health 2014;47(5):245-252. https://doi.org/10.3961/jpmph.14.035 [ Links ]
65. Sage AP, Minatel BC, Ng KW, Stewart GL, Dummer TJB, Lam WL, Martinez VD. Oncogenomic Disruptions in Arsenic-Induced Carcinogenesis. Oncotarget 2017;8(15):25736-25755. https://doi.org/10.18632/oncotarget.15106 [ Links ]
66. Chinyama A, Ochieng GM, Snyman J, Nhapi I. Occurrence of Cyanobacteria Genera in the Vaal Dam: Implications for Potable Water Production. Water SA 2016;42(3):415-420. https://doi.org/10.4314/wsa.v42i3.06 [ Links ]
Received 23 January 2023
Revised 17 July 2023
Accepted 18 July 2023
* To whom correspondence should be addressed: Email: letitia.pillay@gmail.com