Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.77 Durban 2023
http://dx.doi.org/10.17159/0379-4350/2023/v77a07
RESEARCH ARTICLE
Statistical evaluation of the uncertainties in the characterization of South African mine tailings
Kedibone MashaleI, II, *; Bambesiwe MayI; James SehataI; James TshilongoI, II; LukeChimukaII
IAnalytical Chemistry Division, Mintek, Praegville, South Africa
IIMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Braamfontein, South Africa
ABSTRACT
In this study, a South African mine tailing sample was characterized for base metals using X-Ray fluorescence, alkaline fusion, and acid digestion to subsequently evaluate the uncertainties involved. This was based on the importance of characterization data in subsequent methods and obtaining confidence in such methods. It was determined that instrument repeatability and reproducibility contributed most to the overall uncertainty. At a coverage factor of 2 (k=2), the measurement result for iron through alkaline fusion, XRF, and acid digestion was found to be 3.476 ± 0.026%, 3.835 ± 0.023%, and 3.741 ± 0.020%, respectively, and that of arsenic were 88 ± 11 mg kg-1 and 85.5 ± 8.3 mg kg-1 for fusion and acid digestion, respectively. Based on the calculated expanded uncertainties which are at an average of 0.8% of the measurement results, the three methods can be associated with high statistical confidence and therefore eases decision-making regarding subsequent analytical methods.
Keywords: characterization; measurement uncertainty; measurement reliability; measurement confidence; mine tailings
INTRODUCTION
Characterization of geological material is part of subsequent method development and selection. This is based on the knowledge that these materials often contain a variety of minerals and elements which can interfere with analytical procedures. The abundance of the minerals (and elements) is dependent on the matrix, which is further determined by the location and processes it has undergone.1 For example, geological samples from the Witwatersrand Basin are expected to be high in silica whilst samples from the Merensky reef are expected to be high in chromium.2,3 These materials are usually characterized by both qualitative (e.g., mineralogy with X-Ray Diffraction, XRD) and quantitative techniques X-Ray Fluorescence (XRF) for elemental and oxide composition, and ICP-OES/MS which is often coupled with alkaline fusion or acid digestion. For the base metal characterization, acid digestion and alkaline fusion were chosen as they are suitable for the determination of oxides such as CaO, SiO2, Al2O3, Fe2O3, MgO,4 whilst it is also understood that loss of elements such as As, Os, Sb, and Se can occur during the open vessel digestion, making it difficult to compare the methods.5 Due to the processed nature of mine tailings, the concentration of base metals can be expected to be in the lower percentages for elements such as calcium, iron, and magnesium, and higher amounts are expected for silicon/quartz.6 This makes obtaining the quantitative characterization data with the highest accuracy a challenge. However, quantitative characterization has the advantage of allowing for the determination of uncertainties involved in the processes. Despite the existence of several publications on the characterization of tailings, there is usually a lack of the uncertainty component, implying that the confidence and quality of the results cannot be placed. For example, geological material such as tailings from various matrices in South Africa has been successfully characterized which showed the similarities and differences between the matrices.7-11 However, information on the statistical data was not provided.
The common method of assessing the reliability and confidence of the characterization techniques (or any analytical data) is through the evaluation of measurement uncertainty. Quantification of measurement uncertainty in chemical analysis is fast becoming standard procedure as it can highlight the steps that contributed to high errors and uncertainties. In analytical chemistry, it is rather important to evaluate the confidence that can be placed on obtained data, which also represents the quality of the data.12 Therefore it is beneficial for the analytical data to be presented with its uncertainty value. In the ISO/IEC 17025:2017, it is stated that for method validation, uncertainty evaluations must be completed,13 however, this can also be applied to the day-to-day analysis. This includes identifying the sources of uncertainty and formulating the model equation, followed by the combination of the individual uncertainties into the overall uncertainty using the law of propagation.14 In previous studies, the uncertainty of arsenic was evaluated and it was discovered that sample weighing contributed more to the uncertainty.15 Whilst in the uncertainty evaluation of rare earth elements in geological reference materials, the most contribution emanated from analytical instruments16, indicating that such evaluations are rarely predictable. Furthermore, the importance of evaluating the measurement uncertainty was illustrated during a method validation process, thus adding more relevance to the analytical results.17 It is known that most analytical testing laboratories operate using the calibration measurement capability (CMC) that indicates the level of uncertainty that will be acceptable,18 for this reason, evaluating uncertainty during the characterization stages of geological samples, can assist in ensuring that the methods selected leads to accurate results, with a small uncertainty concerning the measured value (measurand). This, therefore, allows for the identification of problematic parameters, which can be optimized and improved, early in the analysis.
Therefore, this paper aimed at addressing the statistical uncertainties involved in the initial steps involved when working with geological materials, which is characterization. The study looked at evaluating the individual contributions from XRF, alkaline fusion, and acid digestion coupled with ICP-OES and thereafter, the calculation of the combined uncertainty using the top-down approach for the quantification of iron and arsenic in the tailings. This will highlight the performance of the methods which will serve as a guideline for the selection of methods, common uncertainty contributors, and an approach to processing such data.
EXPERIMENTAL
Materials and Methods
The reagents and chemicals used in this study were of analytical grade and were used as received. The following chemicals and reagents were obtained from Associated Chemical Enterprises Pty Ltd (Johannesburg, South Africa): anhydrous sodium carbonate (Na2CO3), sodium peroxide (Na2O2), hydrogen peroxide (H2O2) and perchloric acid (HClO4, 70%). Hydrochloric acid (HCl, 37%) and nitric acid (HNO3, 65%) were purchased from Sigma Aldrich (Missouri, United States of America). The flux for XRF, lithium tetraborate:lithium metaborate (50:50), was purchased from XRF Scientific (Western Australia, Australia). Calibration standards (10 000 mg L-1) for the base metals were purchased from LGC Standards (Middlesex, United Kingdom). The certified reference materials (CRM), AMIS 610 and AMIS 646 were obtained from African Mineral Standards (Modderfontein, South Africa). All solutions were prepared with deionised water purified using a Milli-Q system from Sigma Aldrich (Missouri, United States of America). The mine tailing sample of 25 kg was sourced from the Elikhulu Tailings Retreatment Plant, which processes tailings from the Ventersdorp Contact Reef (VCR), Witwatersrand Basin. The certified reference materials (AMIS 610 and AMIS 646) were selected based on the matrix.
Particle size distribution, homogeneity and, purity analysis
The finely milled sample was split into smaller fractions using a rotary splitter with 24 fractions obtained with an average mass of 1 kg. Particle size distribution (PSD) was determined using a Malvern hydroEV instrument with the Mastersizer 3000 software (Malvern Pan Analytical Ltd, Worcestershire, United Kingdom). To assess the homogeneity of the fractions, random fractions were selected for tests, also for characterization purposes. The purity analysis of reagents used in the methods was performed to determine if the constituents of the chemicals would be at a significant level to contribute to the uncertainties. Such reagents included the flux used for alkaline fusion and XRF. The reagent blanks of the methods used were therefore analyzed for impurities.
XRD analysis
The samples used for XRD analysis were selected at random across the fractions. After pulverizing in a swing mill to -50 μηι, the material was prepared using a back loading preparation method at XRD Analytical and Consulting cc. The diffractogram was obtained using a Malvern Panalytical Aeris diffractometer (Malvern Pan Analytical Ltd, Worcestershire, United Kingdom) with PIXcel detector and fixed slits with Fe filtered Co-Κα radiation having a scanning range of 5-80° 2θ with a step scan of 0.022° 2θ and measuring time of 48 s. The phases were identified using X'Pert Highscore plus software and PAN-ICSD. The relative phase amounts (wt%) were estimated using the Rietveld method with the method detection limit quantified as 0.5-3 wt%.
Alkaline fusion analysis
Alkaline fusion was performed as stated in the literature.5 Using a sensitive mass balance, 0.2 g of the sample was weighed into a zirconium crucible and mixed with 0.7 g of Na2CO3 and 1.5 g of Na2O2, which acts as the flux and mixed until homogenous. The mixture was fused using an open-flame Bunsen burner (>950 °C) until a clear red melt was obtained. After cooling, the crucibles were placed in a 250 mL beaker with 100 mL of distilled water and 40 mL HCl was added to leach out the metals. This solution was transferred into a 200 mL volumetric flask containing 10 μg mL-1 scandium as an internal standard and analyzed on an ICP-OES for base metals.
Acid digestion method
For further elemental characterization and tracking of methods using the reference materials (AMIS 610 and AMIS 646), multi-acid digestion was carried out (in triplicate). For this method,5 0.5 g of sample was weighed into a Teflon beaker, wet with water, and put on the hotplate (110 °C) for 1.5 hours with 15 mL HNO3 and 5 mL HClO4. After the time lapsed, the solutions were allowed to cool before 15mL HNO3, 5 mL HClO4, and 10 mL HF were added and heated until dry. The dry residue was wet with water and dissolved using 10 mL HCl and a few drops of H2O2. The solution was then transferred into a 50 mL volumetric flask containing 10 μg mL-1 scandium as the internal standard.
XRF analysis
A small amount (2 g) of the sample was weighed and mixed with 5 g lithium tetraborate: lithium metaborate (50:50) flux and mixed until homogenous. The mixture was transferred into a platinum crucible and fused at a temperature of 850 °C for 30 minutes to obtain a melt. The melt was then poured into a platinum lid and allowed to cool to form a bead which was analyzed on the Rigaku Primus IV WDXRF (Rigaku Corporation, Tokyo, Japan). The instrument was calibrated using fused beads containing the elements of interest in a range of 10-1000 mg kg-1 for lower elements and 0.050-50% for higher elements. The method detection limits were 0.0011% for iron and 18 mg kg-1 for arsenic.
ICP-OES analysis
The solutions with an ICP-OES finish were analyzed on the Agilent 5900 ICP-OES (Agilent Technologies Inc, California, United States of America) equipped with the ICP-Expert software and a charged-coupled device (CCD) detector. The operating conditions employed were 1.25 kW RF power, 12 L min-1 plasma flow, 1.3 L min-1 auxiliary flow and a sample uptake of 0.3 mL min-1. The analysis was carried out in radial mode with high purity Argon used to sustain the plasma and as a carrier gas. Calibration standards in the range of 0.050-50% including the calibration blank, were prepared from single-element stock solutions. A concentration factor of 1000 was incorporated into the preparation of the standards to enable the analysis to cover a concentration range of 0.05-50%.The calibration was checked using quality control standards of 5% and 25%, with reference materials incorporated in the analysis. The method detection and quantification limits were calculated as 5.5 mg kg-1 and 18 mg kg-1 for arsenic and 0.00024% and 0.0079% for iron.
STATISTICAL ANALYSIS
Homogeneity analysis
Homogeneity analysis was carried out by calculating the RSD of the multiple measurements and relating it to the mass used. This calculation is based on the modification of the Ingamells' sampling constant19 as in Equation 1, where Ks is the sampling constant and m is the mean sample mass used.

The homogeneity of the split fractions was assessed using a statistical homogeneity test,19

where HE is the relative homogeneity, which was calculated based on the mean sample mass and the number of observations. The objective is to obtain a low homogeneity factor (< 10%), that will eventually lead to the requirement of a small sample mass that will give an uncertainty of <5% at a 95% confidence interval, according to Equation 3,20 where UNC is the required uncertainty at a specific confidence interval.

Analysis of variance (ANOVA)
The results from the analysis were compared using Single Factor ANOVA using Microsoft Excel (Microsoft Office Professional Plus 2016) to assess the significance of the differences in the variances of the method. The decision-making was based on the following criteria:21 H0: variances of the methods are not significantly different, H1: variance of the methods are significantly different Rule: Reject the null hypothesis if Fstatistic>Fcritical
Uncertainty evaluations
After the sample preparation consisting of alkaline fusion, XRF, and acid digestion, the resulting samples were quantified on the respective instruments. The analysis aimed to quantify the elements in terms of concentration; therefore, the operational model equation is:15

whereby C is the concentration of the element in the sample, C0 is the element concentration in the sampling concentration, V is the volume of the sampling solution and W is the mass of the sample.
Based on Equation 4, the factors contributing to the uncertainty in the analysis are those arising from; the calibration of volumetric glassware used, the effect of temperature during preparation, weighing of the solid samples, and instrumental analysis which consists of preparation of calibration standards, instrument drift and stability, method repeatability and linear regression of the calibration curve.
For repetitive measurements, the standard uncertainty was calculated using standard deviation for Type A and certificate data for Type B uncertainty, in which Equation 5 13 was used:

RESULTS AND DISCUSSION
This section will present the results starting with particle size distribution, homogeneity, and purity analysis based on their importance, followed by the interpretation of the characterization data and the statistical analysis encompassing ANOVA and uncertainty evaluation.
Purity analysis
The purity analysis was performed in comparison to the specifications of the chemicals and the amount of specific analytes detected in the sample. The combination of the Na2CO3 and Na2O2 in the alkaline fusion showed impurities of 0.083% for aluminum, 0.017% for chromium, 0.04% for iron, and no impurities as a result of silicon are present. The majority of the base metals impurities in the flux are insignificant when compared to the amounts detected in the samples, with the exception being calcium at 0.33% impurity and 1.11% in the real sample, thus contributing 0.21% to the uncertainty. This effect on calcium is also observed in the result by alkaline fusion reading 1.1%, while the acid digestion and XRF read 0.87 and 0.77%, respectively. The impurities (except for calcium) are regarded as insignificant contributors to uncertainty as they contribute less than 10%.13 Similar observations were made for the purity analysis of the acids and the flux for XRF sample preparation. Therefore, the contribution of the purity of reagents to the overall uncertainty for acid digestion and XRF is regarded as insignificant.
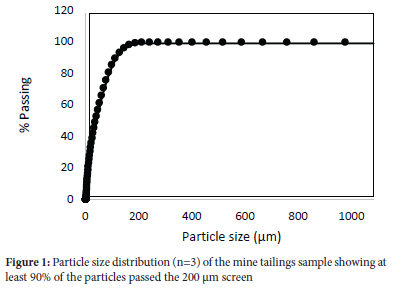
Particle size distribution and homogeneity analysis
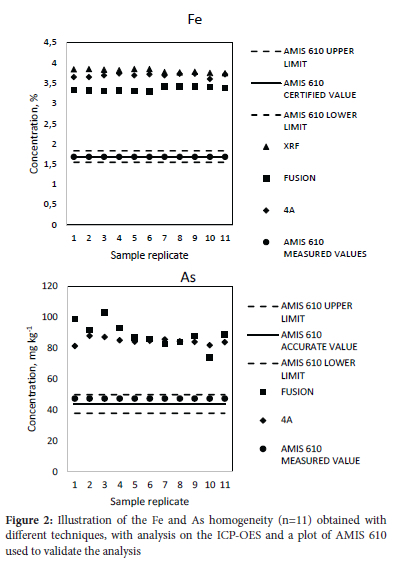
The majority of the particles were in the 80-150 μm range, with 90% of the particles passing the 150 μηι screen (Figure 1), implying there should be satisfactory homogeneity. The homogeneity factor was calculated as 0.63% for iron with alkaline fusion, acid digestion, and XRF and 4.1% for arsenic with the same methods. These were satisfactory compared to the homogeneity factors of 42.3% and 20.8% which were obtained for arsenic and iron in the analysis of a geological certified reference material.16 Majority of the base metals evaluated for homogeneity reported a homogeneity factor of <10%, which indicates high homogeneity of the sample (Figure 2). The high homogeneity of the sample is expected as the VCR is one of the reefs that are homogenous and base metals are typically homogenous in geological material due to their high content22. Based on this assumption, homogeneity calculations are rarely done.
Mineralogy and base metal characterization
The dominant minerals are shown in Figure 3, with quartz (SiO2) being the most dominant at approximately 80 wt% and calcite as a minor phase at 0.2 wt%, which is expected for a VCR matrix and similar to previous studies focusing on tailings.23,24 The presence of quartz at that percentage indicates the possible acidity, which can interfere in analytical techniques such as fire assay by hindering the chemical reaction that takes place,25 whilst the presence of pyrite (FeS2) can be an interferent during fire assay and instrumental analysis. In Table 1, the most abundant elements by alkaline fusion, XRF, and acid digestion are silicon, iron, calcium, and aluminum which are characteristic of the minerals observed in the XRD analysis, shown in Figure 3. Furthermore, repeatability and reproducibility calculations showed the methods to give satisfactory precision with the limit of repeatability and reproducibility being below the set targets. The described latter methods have limitations regarding the elements, these can be compared to each other for the quantification of iron and arsenic based on the recoveries that were obtained for the reference materials on the methods. In the previous characterization of tailings in two studies, the major minerals that were detected compare with those detected in this study were quartz (58-82%), mica (3-10%), chlorite (3-10%) for Witwatersrand Basin11 and quartz, magnetite and magnesioferrite for the East Rand Basin,9 which were both quantified by XRD.
Statistical evaluations
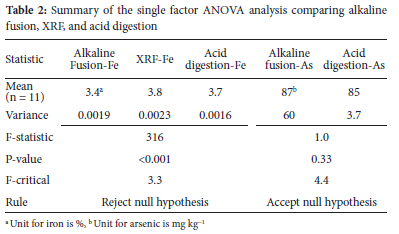
For base metal quantification, XRF, alkaline fusion, and acid digestion were performed repeatedly and a statistical comparison was performed. According to the single factor ANOVA (Table 2), the three methods showed significant differences (Fstatistic>Fcritical) for elements such as iron and calcium, whereas for arsenic the difference was insignificant (Fstatistic<Fcritical)). However, the difference being significant for iron is not an implication that the individual methods cannot be trusted or are incapable of producing accurate quantification. The methods are allowed to differ to a certain extent and the use of a reference material further supports this, provided that the quantification of the reference material falls within the certified limits. This was observed in the analysis of AMIS 610 (iron) by the three methods with the certified range; alkaline fusion and acid digestion (1.6-1.8%) and XRF (1.6-1.9%) and recovered concentrations reported as 1.7% for alkaline fusion, 1.7% for acid digestion and 1.9% for XRF.
Uncertainty evaluations
Volumetric glassware calibration
For a 200 mL volumetric flask, the capacity allowance is 0.02 mL at 20 °C, with a triangular distribution (k= ), therefore;

The temperature at which the glassware was used differed from the calibration temperature, further uncertainty was calculated based on the expansion of water at that particular temperature (23 °C), resulting in a temperature difference of 3 °C and the expansion coefficient was at 2.1 χ 10-4 °C-1. The uncertainty due to the temperature was then calculated to be:
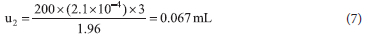
Therefore, the uncertainty that affects the measurement result from the use of the volumetric glassware is:
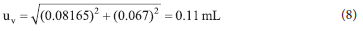
with the calculated relative uncertainty being 0.000547 mL. The uncertainties for the other factors were also calculated in this manner to populate Table 3.
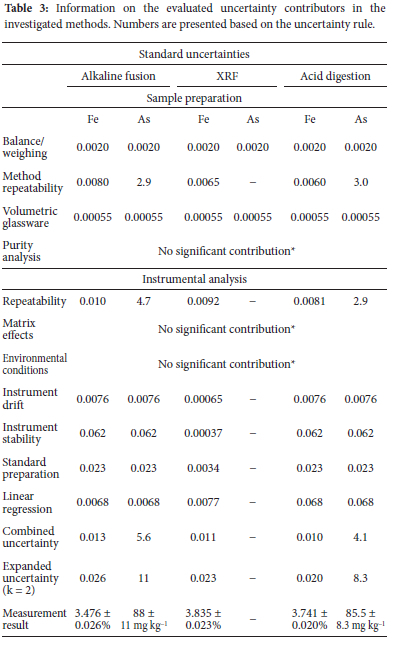
The parameters with the most contribution to the uncertainty for iron are the method and instrument repeatability which is quantified at a standard uncertainty of 0.010%, contributing 79% to the overall uncertainty, making it a significant contributor. At a coverage factor of 2 (k=2) and 95% confidence interval, the measurement result for iron is expressed as 3.476 ± 0.026%, 3.835 ± 0.023% and 3.741 ± 0.020% for the three methods (Table 3). The expanded uncertainties affect the measurement result by at most 0.8%, rendering them tolerable, considering that the best estimate of the measurand is high. With a tolerance of 5%, the uncertainty of alkaline fusion for iron is 0.026% with the benchmark being 0.17%, indicating the satisfactory accuracy of the results. The same trend was observed in the uncertainties of arsenic by alkaline fusion and acid digestion. In this case, either one of the methods can be used to quantify iron, based on the low uncertainty it reported. Given that quantifying uncertainty places confidence on an analytical result, it helps in choosing methods to use downstream of characterization. In this study, the iron has low uncertainty concerning the analytical result, implying that the result can be trusted and relied on. This observation was also made in a study in which iron was quantified as 0.23%, with an uncertainty value of 0.0153%. This was considered low as it give iron result in the range of 0.2147-0.2453%.26 Since iron is an interferent during fire assay and in the instrumental analysis of gold, a suppressor can be added to counter the effect of the iron as an interferent, based on how much interferent (Fe) is present. In geological samples, it is rather uncommon to statistically quantify and analyze characterization data, moreover uncertainty due to issues such as homogeneity of the material (for most matrices). Both iron and arsenic have been quantified with methods such as XRF and have been evaluated for uncertainty in geological and biological matrices. For example, in the uncertainty quantification of arsenic after ICP-MS analysis,15 sample weighing and sample digestion contributed the most to the overall uncertainty which is not the case with this study.
In another study, global uncertainty was calculated in which accuracy and precision uncertainties were propagated for 27 elements from geological samples by XRF. The study also included assessing factors such as sample matrix and instrumental effects in order to create an uncertainty dataset that can be utilised irregardless of differences in matrices and conditions.27 Although environmental factors are usually different in these studies, these uncertainty evaluations for characterization methods allow for the presence of a footprint, which can always be referred to in other studies. This also shows the importance of evaluating the uncertainty routinely as the contributing factors can alter.
CONCLUSION
In this paper, a synopsis of elemental characterization methods; fusion, acid digestion, and XRF coupled with uncertainty evaluation was given. It discussed the importance of uncertainty as a measure of confidence and the level of accuracy in the methods used. This is mainly due to the significance of characterization in subsequent methods. The study aimed to quantify uncertainty in the various methods which will assist in selecting a method to use. The analysis of the data obtained showed that although the methods yield results that vary (within a tolerable range), the uncertainties obtained are significantly low, which makes decision-making in terms of method selection, easier and more efficient. At a 95% confidence interval, the uncertainties calculated for the methods are significantly low; 0.020-0.026% for iron by XRF, peroxide fusion and acid digestion and 8.3-11 mg kg-1 for arsenic by fusion and acid digestion. This implies that, with statistical backing through uncertainty analysis, it can be inferred that the techniques used were optimum and, either method can be used. Therefore, it is recommended that uncertainty evaluations are integrated into the characterization methods.
ACKNOWLEDGEMENTS
This research is part of a study funded by the National Research Foundation (South Africa) under grant number (128110) and the Analytical Chemistry Division at Mintek.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
ORCID IDs
Kedibone Mashale: https://orcid.org/0000-0003-2502-5688
Bambesiwe May: https://orcid.org/0000-0001-8842-2459
James Sehata: https://orcid.org/0000-0001-7018-3082
James Tshilongo: https://orcid.org/0000-0001-5661-4907
Luke Chimuka: https://orcid.org/0000-0002-8552-2478
REFERENCES
1. Kossoff D, Dubbin E, Alfredsson M, Edwards SJ, Macklin MG, HudzonEdwards KA. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochemistry. 2014;51:229-245. https://doi.org/10.1016/j.apgeochem.2014.09.010 [ Links ]
2. Hunt EJ, Latypov R, Horváth P. The merensky cyclic unit, bushveld complex, South Africa: Reality or myth? Minerals. 2018;8(4):1-35. https://doi.org/10.3390/min8040144 [ Links ]
3. Agangi A, Hofmann A, Rollion-bard C, Marin-Carbonne J, Cavalazzi B, Large R, Meffre S. Gold accumulation in the Archean Witwatersrand Basin, South Africa-Evidence from concentrically laminated pyrite. Earth-Sci Rev. 2015;140:27-53. https://doi.org/10.1016/j.earscirev.2014.10.009 [ Links ]
4. Guo Z, Bai Y, Cui J, Mei Y, Ma Z. Determination of multi-element contents in gypsum by ICP-AES. Guang Pu Xue Yu Guang Pu Fen Xi. 2014;34(8):2250-2253. [ Links ]
5. Hu Z, Qi L. Chapter 15: Sample digestion methods. In: Holland HD, Turekian KK, editor. Treatise on Geochemistry. 2nd Ed. Amsterdam: Elsevier; 2014, p.87-109. https://doi.org/10.1016/B978-0-08-095975-7.01406-6 [ Links ]
6. Lottermoser BG. Mine waste: Characterization, treatment, environmental impact. 2nd Ed. Hedeilberg: Springer Berlin. 2007. [ Links ]
7. Malatse M, Ndlovu S. The viability of using the Witwatersrand gold mine tailings for brickmaking. J. South. African Inst. Min. Metall. 2015;115(4):321-327. https://doi.org/10.17159/2411-9717/2015/v115n4a8 [ Links ]
8. Singo NK, Kramers JD. Feasibility of tailings retreatment to unlock value and create environmental sustainability of the Louis Moore tailings dump near Giyani, South Africa. J. South. African Inst. Min. Metall. 2021;121(7):361-367. https://doi.org/10.17159/2411-9717/1138/2021 [ Links ]
9. Okereafor U, Makhatha M, Mekuto L, Mavumengwana V. Gold mine tailings: A potential source of silica sand for glass making. Minerals. 2020;10(5):1-12. https://doi.org/10.3390/min10050448 [ Links ]
10. Fashola MO, Ngole-Jeme VM, Babalola OO. Physicochemical properties, heavy metals, and metal-tolerant bacteria profiles of abandoned gold mine tailings in Krugersdorp, South Africa. Can. J. Soil Sci. 2020;100(3):217-233. https://doi.org/101139/cjss-2018-0161 [ Links ]
11. Nengovhela AC, Yibas B, Ogola JS. Characterisation of gold tailings dams of the Witwatersrand Basin with reference to their acid mine drainage potential, Johannesburg, South Africa. Water SA. 2006;32(4):499-506. https://doi.org/10.4314/wsa.v32i4.5290 [ Links ]
12. Kessel W. Measurement uncertainty according to ISO/BIPM-GUM. Thermochim. Acta. 2002;382(1-2);1-16. https://doi.org/10.1016/s0040-6031(01)00729-8 [ Links ]
13. Joint Committee for Guides in Metrology (JCGM) 101:2008. Evaluation of Measurement Data - Supplement 1 to the Guide to the Expression of Uncertainty in Measuremen. JCGM/Working Group 1. 1st Ed. 2008. [ Links ]
14. Heydorn K, Madsen BS. Verification of uncertainty budgets. Accredit. Qual. Assur. 2005;10(8):403-408. https://10.1007/s00769-005-0015-6 [ Links ]
15. Jia Y, Che M, Hu W, Liu T. Evaluation of the Uncertainty in the Determination of Arsenic in Cosmetics by Inductively Coupled Plasma-mass Spectrometry (ICP-MS). Esse. 2017;539-550. https://doi.org/10.1515/9783110540048-055 [ Links ]
16. Verma SP, Santoyo E, Velasco-Tapia F. Statistical evaluation of analytical methods for the determination of rare-earth elements in geological materials and implications for detection limits. Int. Geol. Rev. 2002;44(4):287-335. https://doi.org/10.2747/0020-6814.44.4.287 [ Links ]
17. Senila M, Drolc A, Pintar A, Senila L, Levei E. Validation and measurement uncertainty evaluation of the ICP-OES method for the multi-elemental determination of essential and nonessential elements from medicinal plants and their aqueous extracts. J. Anal. Sci. Technol. 2014;5(11):1-9. https://doi.org/10.1186/s40543-014-0037-y [ Links ]
18. Beckert SF, Flöres RE. Calibration and measurement capability (CMC) and customer technical qualification. 19th International Congress of Metrology. Paris; September 24-26, 2019. [ Links ]
19. Rossbach M, Grobecker KH. Homogeneity studies of reference materials by solid sampling - AAS and INAA. Accredit. Qual. Assur. 1999;59(2):498-503. https://doi.org/10.1007/s007690050422 [ Links ]
20. Daniel L, Clarke BR, Parsons DF. Performance of Sample Preparation for Determination of Gold in Samples of Geological Origin. Geostand. Geoanalytical Res. 2019;43(3):435-452. https://doi.org/10.1111/ggr.12278 [ Links ]
21. Larson MG. Analysis of variance. Circulation. 2008;117(1):115-121. https://doi.org/10.1161/CIRCULATIONAHA.107.645335 [ Links ]
22. Minnitt RCA. Sampling in the South African minerals industry. A. Sampling in the south african minerals industry. J. South. African Inst. Min. Metall. 2014;114(1):63-81. [ Links ]
23. Tabelin CB. Igarashi T. Mechanisms of arsenic and lead release from hydrothermally altered rock. J. Hazard. Mater. 2009;169:980-990. https://doi.org/10.1016/j.jhazmat.2009.04.049 [ Links ]
24. Tomiyama S, Igarashi T, Tabelin CB, Tangviroon P, Ii H. Acid mine drainage sources and hydrogeochemistry at the Yatani mine, Yamagata, Japan: A geochemical and isotopic study. J. Contam. Hydrol. 2019;225,103502. https://doi.org/j.jconhyd.2019.1 [ Links ]
25. Turan A, Yucel O. The effect of iron and oxidizing flux addition on the fire assay of low grade pyritic refractory gold ores. J. Min. Metall. Sect. B Metall. 2011;4(2):219-227. https://doi.org/10.2298/JMMB110127007T [ Links ]
26. Gazulla Barreda MF, Edo, RM, Cordero MO, Vaquer MJV. Determination of minor and trace elements in geological materials used as raw ceramic materials. Bol. la Soc. Esp. Ceram. y Vidr. 2016;55(5):185-196. https://doi.org/10.1016/j.bsecv.2016.06.003 [ Links ]
27. Wang YY, Zhan XC. The uncertainty evaluation of analytical results for 27 elements in geological samples by x-ray fluorescence spectrometry. Guang Pu Xue Yu Guang Pu Fen Xi. 2014;34(4):1118-1123. [ Links ]
Received 12 September 2022
Revised 24 April 2023
Accepted 28 April 2023
* To whom correspondence should be addressed Email: kedibonema@mintek.co.za














