Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.77 Durban 2023
http://dx.doi.org/10.17159/0379-4350/2023/v77a05
RESEARCH ARTICLE
Effects of calcination temperature of eggshell-derived CaO as a catalyst for biodiesel production from waste cooking oil
Raiedhah A. AlsaiariI, *; Esraa M. MusaI; Moustafa A. RizkI, II
IEmpty Quarter Research Unit, Department of Chemistry, College of Science and Art in Sharurah, Najran University, Saudi Arabia
IIDepartment of Chemistry, Faculty of Science, Suez Canal University, Ismailia, Egypt
ABSTRACT
Biodiesel is considered to be more friendly to the environment than petroleum-based fuels, cheaper and capable for producing greener energy which contributed positively in boosting bio-economy. In this work, waste cooking oil (WCO) is converted into biodiesel utilizing a waste eggshell (CaO) nano-catalyst in an effort to discover environmentally beneficial and economically viable processes for social and economic development. The eggshell-based CaO catalyst developed for the production of ecologically friendly biodiesel at a reduced price is calcined at temperatures between 600 and 1100 °C. The synthesized catalysts were assessed in terms of their physical and chemical qualities via BET, TGA and XRD analysis. This revealed that, besides displaying exceptional transesterification activity, the catalyst synthesised at 950 °C also offered the greatest biodiesel yield. Transesterification, used in biodiesel generation, was used to evaluate the catalytic performance of manufactured catalysts under several reaction circumstances. Under prime reaction conditions i.e., a reaction time of 3 hours, an ethanol-oil molar ratio of 9:1, and a catalyst amount of 4 wt.%, it was ascertained that a catalyst which had calcined at 950 °C demonstrated excellent transesterification activity and delivered a ceiling yield of 88% fatty acid ethyl esters. The production of FAME was confirmed by using gas chromatography-mass spectroscopy (GC-MS). Fuel properties of fatty acid ethyl ester complied with ASTM D 6751 which indicated that it would be an appropriate alternative form of fuel.
Keywords: biodiesel, calcination, eggshell, environment-friendly, waste cooking oil
INTRODUCTION
Energy is an important foundation for each country's economy and society. Presently, over 78% of the world's energy consumption is met by non-renewable sources. As a result of their rapid consumption, their supply is dwindling in the modern world.1 Anthropogenic activities produce vast quantities of carbon dioxide (CO2) and greenhouse gases (GHGs), which in turn cause pollution, degradation, and global warming.2,3 Furthermore, climate change is caused by these trace gases. Therefore, it is essential to reduce these emissions; this may be accomplished by concentrating on mitigation techniques, such as the use of renewable energy sources. A renewable, maintainable, economically viable, and environmentally friendly alternative to a fossil fuel-based energy system must be conceived. One potential product is biodiesel: a liquid biofuel currently under consideration as a carbon-efficient alleviation solution. Replacing traditional fossil fuels with a product such as biodiesel is a credible means of reducing greenhouse gas (GHG) emissions and the build-up of CO2. This is of particular significance to the transportation sector, which is viewed as playing a significant role in the reduction of global warming and climate change.4
Biodiesel production costs and the cost of raw materials used in the manufacturing of biodiesel are the key obstacles to commercialization and market competitiveness.2,5 For biodiesel to compete with gasoline, it's essential to find inexpensive feedstocks, alcohols, and catalysts that are both easily available and inexpensive. There is a viable option for the manufacturing of low-cost and ecologically sustainable goods such as biodiesel from waste cooking oil (WCO).6,7
The production of biodiesel requires various procedures which include micro-emulsion,8 blending, pyrolysis, and transesterification (defined as the reaction between a fat/oil triglyceride and alcohol, to produce esters and glycerol). Transesterification is a rescindable procedure and one which requires a surplus of alcohol to impel the equilibrium to the product side. Theoretically, the stoichiometry for this process requires a ratio of 3:1 alcohol to oil but, empirically, this ratio has been seen to fluctuate. When it comes to biodiesel manufacturing, transesterification is the most generally utilized technology due to its low price point, efficiency, and compatibility with diesel oil in any ratio.9 The manufacture of biodiesel from vegetable oils frequently makes use of homogeneous catalysis. Soap formation, excessive wastewater generation, reactor corrosion, and other process problems can occur with homogeneous catalysts, despite the fact that they provide high yields in a short period of time.10 Biodiesel technology is urged to use heterogeneous catalysts to overcome the challenges of homogeneous catalysis and lower the cost of biodiesel production. Among the many advantages of heterogeneous catalysts are environmental friendliness, ease of recyclability, minimal reactor corrosion, ease of separation, and increased purity in the ester and glycerol products.11 The use of low-cost and environmentally favorable solid waste sources for sustainable biodiesel production has recently gained promising scope.
The use of CaO as a catalyst in the transesterification process has been well documented by many previous publications. Originating from naturally occurring shells, an exceptionally untainted version of CaO (obtained from the shells of mussels under specific experimental conditions) created biodiesel from soybean oil, with a yield of 94.1%, as reported by Rezaei et al.12 Other methods, such as those employed by Chen et al., manufactured CaO from ostrich eggshells, by implementing a technique involving ultrasound, which yielded 93% biodiesel from palm oil. Another research team, Gupta et al., obtained a 96% biodiesel output from soybean oil, using CaO catalysts based on snail shells. One team demonstrated the feasibility of using waste chicken eggshells and soybean oil to generate high yield and high-quality biodiesel synthesis of over 97%.13
In previous research, it was demonstrated that the transesterification of high-grade vegetable oil is possible when combined with the use of a naturally occurring CaO catalyst. This use of vegetable oil is, however, not viewed as economically viable and, consequently, the use of this process to enter the biodiesel market is particularly challenging. To overcome this issue, there is a great deal of current research exploring alternate, renewable, feedstock that is enduring, economically feasible and environmentally benevolent. This research involves an investigation into the use of waste cooking oil and inedible plant oils, such as jatropha, castor, tobacco and linseed which are essential feedstocks, crucial to transesterification. When considering the production of biodiesel, the use of surplus cooking oil and eggshells has two advantages: a reduction in pollution (caused by the disposal of these two products) and a reduction in production expenditure.
To obtain improved biodiesel yields, CaO's catalytic activity can be boosted through increased basicity and surface area, fabrication of a nanoparticle can greatly enhance the catalytic performance of a heterogenous catalyst via a reduction in crystal size. Biodiesel made from used cooking oil has been extensively studied, although there are still drawbacks such as high levels of free fatty acids and contaminants. For it to be deemed a potential contender for traditional homogenously catalyzed biodiesel production, these constraints must be addressed or reduced.14 A number of research papers on the subject found that biodiesel produced from waste cooking oil using eggshell catalysts yielded between 91% and 100% depending on the reaction conditions. Due to differences in reaction time, heterogeneously catalyzed transesterification processes were slower than those mediated by homogeneous catalysts. In the three-phase systems (oil, alcohol, and catalyst), the mixing difficulty caused this.15 CaO's lack of basicity and surface area may restrict its ability to catalyze. Transesterification can be complicated by the need for more catalysts and a longer reaction time, both of which increase manufacturing costs. To obtain improved biodiesel yields, CaO's catalytic activity can be boosted through increased basicity and surface area.
Currently, no work has been reported studying different calcination temperature and application of green nano particles of CaO derived from waste eggshells as efficient catalyst for synthesizing biodiesel via waste cooking oil that doesn't lead to food competition. This is innovative research, the catalysts engineered were investigated using X-ray diffraction (XRD), TGA and Brunauer-Emmett-Teller (BET) techniques. The ASTM6751 reference standards were used to judge the suitability of the biodiesel product for use as a fuel. Biodiesel production from date seed oil is cost-effective and has no negative impact on food security. It contributes to GHG reduction while also providing a cost-effective alternative to fossil fuels. As a result, our work herein aims to identify the economic benefits associated with conversion of waste material into a value-added product.
EXPERIMENTAL
Preparation of the waste cooking oil sample (WCO)
This WCO was originally gathered from surrounding eateries that used sunflower oil in their cooking. After being heated at 110 °C for roughly half an hour in an oven to eliminate the water contained in the WCO, the oil was filtered to get rid of any remaining food particles. Density, acid value and free fatty acid (FFA) value, were all calculated for WCO in accordance with industry standards.16
Preparation of CaO nanocatalyst from eggshell
Eggshell wastes were gathered from surrounding restaurants and treated in boiling water to harden the gelatinous components sticking to the eggshell's inner wall, allowing for simple removal. It was then oven-dried at 120 °C for 16 hours after being cleaned many times with tap water and rinsing with distilled water to eliminate contaminants. It was ground after drying. The powdered eggshell is placed in a crucible and calcined in a muffle furnace for 3 hours at temperatures of 600, 700, 800, 900, 1000 and 1100 °C.
The GC-MS for produced biodiesel and eggshell-derived catalysts were subjected to an analysis, including BET, in order to find one that would work well in the manufacture of biodiesel.
Transesterification of waste cooking oil (WCO)
A 100 mL round-bottom flask was used for the transesterification procedure, the -bottom flask sitting in a dish containing an oil bath to control the reaction temperature on the hot plate. The catalyst was weighed and dissolved in the requisite amount of alcohol before being added to the round-bottom flask containing the measured and heated waste cooking oil for the reaction. By adjusting the catalyst loading, alcohol/oil molar ratio, reaction temperature, and reaction duration, a high biodiesel yield was achieved by the transesterification process. The following equation was used to calculate an expected yield of biodiesel:

Different experimental runs were performed to examine and optimize the impact of catalyst loading, alcohol to oil molar ratio, reaction temperature, and reaction time. The effect of catalyst loading was studied by varying the loading amount from 2 to 6 wt% in each run (the mass of catalyst was calculated based on the weight of waste cooking oil). A molar ratio of 1:9 was chosen for the oil to ethanol, and 75 °C and 3 hours reaction time were applied.
The next step of the experiment was to maintain a steady catalyst loading. A study was conducted to assess the impact of factors such as reaction temperature, reaction duration, and catalyst loading. A study was conducted to assess the impact of oil to ethanol molar ratio. Variations in the oil-to-ethanol molar ratio (1:3, 1:6, 1:9, and 1:12) were tested, and the value with the greatest biodiesel output was chosen. To determine the best temperature range for the reaction, temperatures of 50-80 °C were tested. As the optimal value, the reaction temperature with the maximum biodiesel yield was selected.
Lastly, to study the effect of reaction time, the other values for catalyst loading, oil to alcohol molar ratio, and reaction temperature were held constant while reaction times of 30, 60, 90, 120, and 180 minutes were tested to see which one resulted in the maximum biodiesel production. The solution was then put into a separate funnel when the reaction was completed. The catalyst, Glycerol and the ethyl ester all formed distinct layers. Overnight, the product was left standing to separate effectively. As can be seen in Figure 1, after an overnight stand, the three phases are clearly separated. After that, the biodiesel layer was mixed with 15 mL of distilled water and then shaken on a hotplate for 15 minutes, and then transferred to a separating funnel and left for 24 hours to end up with two clear layers. The lower layer was discarded, and the biodiesel layer was mixed with 0.05 g of sodium sulfate and shaken for 10 min, then separated to measure the weight of the biodiesel layer.

The calcined samples underwent X-ray Diffraction analysis (XRD) using a Brucker AXS-D8 Advance diffractometer (Germany). This diffractometer is outfitted with a copper anode that produces Ni-filtered CuKa radiation (k = 1.5406) from a generator running at 40 kV and 40 mA, in the 2 range between 20 and 80. The instrument is maintained by interfaces of DIFFRAC plus SEARCH and DIFFRAC plus EVA to enable an automatic search and match of the crystalline phases for identification. For BET, 1 g of catalyst samples were degassed for 50 minutes at 120 °C in a sample tube to eliminate moisture and other surface contaminants. The tube was allowed to cool to ambient temperature before being connected to a gas intake (liquid N2 at -196 °C) that was running parallel to an empty reference tube. Both tubes were immersed in liquid nitrogen in a Dewar. For TEM, Sample preparation was as follows: the catalyst powder was dispersed in high purity ethanol, then a drop of the suspension was allowed to evaporate on a holey carbon film supported on a TEM grid. Images were acquired in transmission mode and particle size distributions were calculated using Image J software.
RESULTS AND DISCUSION
Catalyst preparation and characterizations
TGA analysis
TGA studies were conducted to determine the best calcination temperature for eggshells CaO derived catalyst in order to build an effective catalyst for biodiesel synthesis from date seed oil. The findings of a thermal experiment on uncalcined eggshell are shown in Figure 2. The results revealed a modest loss of mass, which was most likely caused by water evaporation and the burning of some organic elements. The temperature range of 600-1000 °C, on the other hand, was linked to considerable weight loss. This weight loss at high temperatures might be attributed to CaCO3 breakdown into CaO. Catalysts were made from wasted eggshell powder in a muffle furnace at three different temperatures, 800 °C, 950 °C, and 1000 °C, as measured by thermogravimetric analysis.
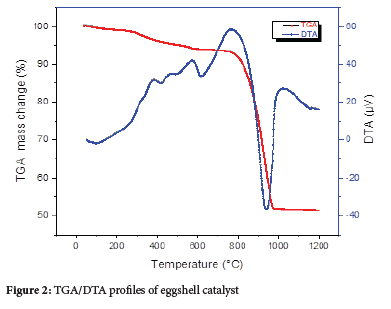
BET analysis
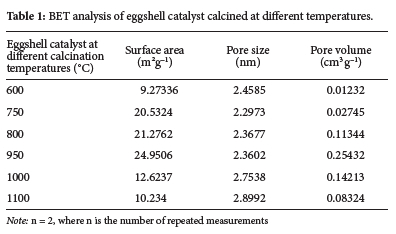
C950's BET surface area and pore volume were determined to be much larger (Table 1). Because of the increased surface area and pore volume, the catalyst displayed enhanced catalytic activity in the transesterification of date seed oil for biodiesel production. As a result, the C950 catalyst was chosen to optimize biodiesel properties.
Characteristics of waste cooking oil
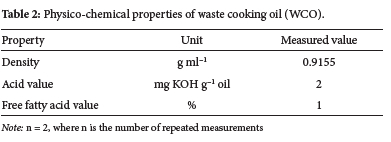
The density, acid value and free fatty acid value of waste cooking oil were evaluated after filtering and separation of the biodiesel layer; the findings are shown in Table 2.
Influence of reaction conditions on biodiesel production
Effects of calcination temperature on the catalyst activity
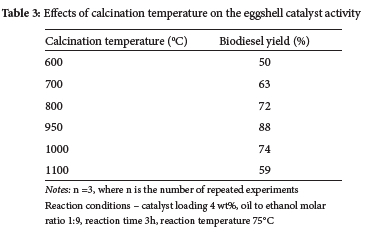
For selected feedstocks, the influence of activation temperature on transesterification activity was studied in the range of (600-1100 °C) as shown in Table 3. The results revealed that as the activation temperature for eggshell-derived material was raised, the biodiesel conversion rose linearly. In comparison to other catalysts, the 950 °C calcined catalyst had the highest catalytic activity. Catalysts calcined at 950 °C (C950) showed strong catalytic activity which may be due to the catalyst's surface containing an optimal number of active sites. In comparison to catalysts activated at different temperature, the BET surface area and pore volume of C950 were found to be considerably greater (Table 1). As a result of the increased surface area and pore volume, the catalyst demonstrated greater catalytic activity in the transesterification process of waste cooking oil (WCO) for the manufacture of biodiesel. Because of this, the C950 catalyst was selected for optimizing biodiesel characteristics.
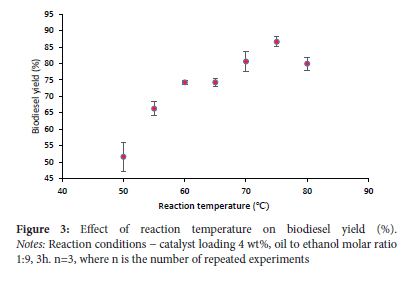
Effect of reaction temperature
Biodiesel production is very temperature dependent. Figure 3 demonstrates that the highest biodiesel yield was achieved at 75 °C. Because ethanol continuously vaporizes at temperatures above 75 °C, the concentration of ethanol in the reaction medium decreases because of the redux, resulting in a lower biodiesel yield.3 At an ideal temperature of 75 °C for producing biodiesel, a maximum yield of 88% was achieved.
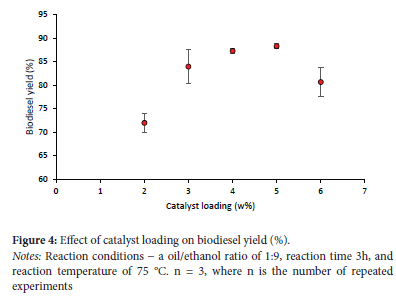
Effect of different catalyst loading
One of the most important aspects of a successful transesterification reaction is the amount of catalyst used. In Figure 4, we see how changing the catalyst loading affects the biodiesel yield. Increasing the catalyst loading from 1% to 4% wt resulted in a greater biodiesel production. This study's findings suggest that increasing catalyst loading can increase the biodiesel yield by increasing the active surface area of the catalyst involved in the transesterification process. This process has, however, negatively impacted the biodiesel yield, which declined by 4 to 6 wt.%. This may be due to the transesterification reaction product becoming stickier, due to the larger catalyst quantities, which inhibits the mass transfer process taking place in the liquid (oil/ alcohol/catalyst) structure, resulting in a reduced biodiesel yield once the optimal amount is attained. The decrease in biodiesel production with low catalyst loading is likely attributable to the insufficiency of the catalyst for complete conversion and the creation of methyl ester. This research found that a catalyst loading of 4% wt resulted in an 88% biodiesel yield.
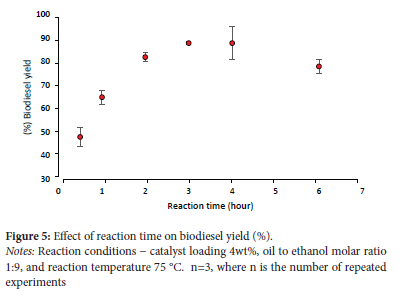
Effect of reaction time
Figure 5 depicts the influence of reaction time on the transesterification process. The highest biodiesel production was achieved after three hours of reaction. After the optimal reaction time, the biodiesel yield declines significantly due to the transesterification process's reversible nature, which results in product loss with longer reaction times. Longer reaction times may also cause the hydrolysis of esters and the production of more fatty acids, which lowers the amount of biodiesel that can be made.17
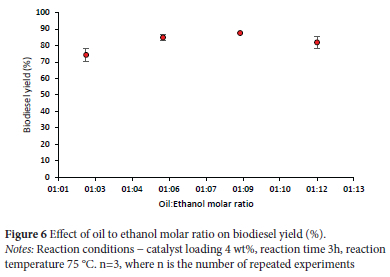
Effect of oil to ethanol molar ratio
Another factor that has a significant influence on biodiesel yield, is the oil-to-ethanol ratio. As observed in Figure 6, an increase in the molar ratio of oil to ethanol from 1:3 to 1:12 creates a comparative growth in biodiesel production. When the ratio exceeds 1:12, biodiesel production decreases which can be ascribed to the high molar ratio of oil to ethanol impeding the separation of glycerin, as the solubility of glycerol, in surplus ethanol, escalates. It is necessary to keep the glycerin in solution, to reduce the production of esters and assist the formation of mono-, di-, and triglycerides. In this investigation, the optimal molar ratio of oil to ethanol for a maximum biodiesel yield of 89% was determined to be 1:12.
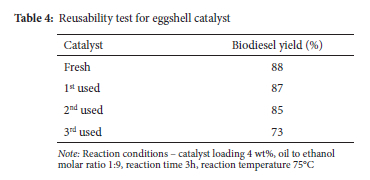
Reusability test
An extremely useful method of determining a catalyst's long-term viability from an economic standpoint is to examine its reusability in the biodiesel synthesis process. Experiments on the CaO-1000 catalyst's reusability were conducted using fresh reactants and improved reaction conditions as shown in Table 4. Used catalyst was centrifuged out of the reaction mixture, washed in n-hexane to remove adsorbed materials, and then dried in an oven at 110 °C for 16 hours after each run. Biodiesel yields of over 80% were recorded for as many as two separate cycles. Once the catalyst was reused more than three times, however, the biodiesel yield began to drop. The accumulation of organic contaminants on the surface of the catalyst may account for its loss of transesterification activity.
Comparison with previous studies
A comparison has been made between previous studies and what has been studied in this study as shown in Table 5. In this work the focus was on the effect of calcination temperature of the catalyst whereas in previous studies one calcination temperature was selected. Also, in this study, this catalyst was compared with pure calcium oxide under the same reaction conditions to be close to the same effectiveness as the eggshell catalyst, while this comparison was not made in previous studies.
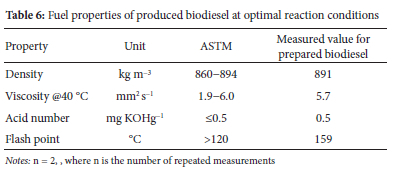
Characteristics of synthesized biodiesel
CaO nano-catalyst was synthesized by calcination under optimum reaction circumstances, and the fuel qualities of the resulting biodiesel were analyzed using the American Society for Testing and Materials' technique (ASTM). Table 6 shows that the produced biodiesel had high-quality fuel attributes that were within the range of the biodiesel standard and were generally consistent with earlier results.22
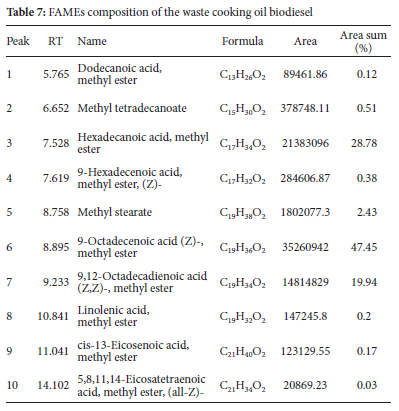
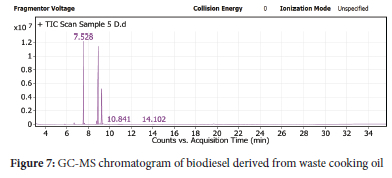
Fatty acid ethyl esters (FAMEs) in biodiesel were identified using GC-MS analysis. Table 7 described the fatty acid ethyl esters and other products in more details. All the fatty acid ethyl esters expected to appear and responsible for biodiesel appeared in the analysis (Figure 7).
CONCLUSION
Biodiesel has been successfully manufactured via a combination of low-cost, waste cooking oil and a financially viable, high-performance, low-impact CaO nano-catalyst created from the waste eggshell. Under ideal conditions, of 4 wt.% catalyst loading, 9:1 ethanol to oil molar ratio, 75 °C reaction temperature, and 3 h reaction time, the C950 catalyst functioned commendably, yielding a maximum biodiesel yield of 88%. The high BET surface area, and optimal pore volume of this catalyst, created increased transesterification activity in the surplus cooking oil. However, following many reuses, this activity was observed to diminish because of two factors: active sites on the surface of the catalyst becoming covered by glycerol and other organic molecules and agglomeration of the catalyst particles. In conclusion, the use of a catalyst created from surplus eggshells, combined with low-cost, waste cooking oil, is a commercially viable and ecologically sound alternative to traditional fossil fuels.
ACKNOWLEDGMENTS
The authors are thankful to the Deanship of Scientific Research at Najran University for funding this work under the Research Groups Funding program grant code (NU/RG/SERC/11/2).
ORCID IDs
Raiedhah A. Alsaiari: https://orcid.org/0000-0002-0735-3162
Esraa M. Musa: https://orcid.org/0000-0002-9746-392X
Moustafa A. Rizk: https://orcid.org/0000-0002-4482-2992
REFERENCES
1. Alsaiari R, Musa E, Alqahtani H, Rizk M. Biodiesel production from date seed oil via CaO-derived catalyst from waste eggshell. Biofuels. 2023. https://doi.org/10.1080/17597269.2023.2172769. [ Links ]
2. Bankovic-Ilic IB, Miladinovic MR, Stamenkovic OS, Veljkovic VB. Application of nano CaO-based catalysts in biodiesel synthesis. Renew Sustain Energy Rev. 2017;72:746-760. https://doi.org/10.1016/j.rser.2017.01.076. [ Links ]
3. Yang X, Wang Y-T, Yang Y-T, Feng E-Z, Luo J, Zhang F, Yang W-J, Bao G-R. Catalytic transesterification to biodiesel at room temperature over several solid bases. Energy Convers Manage. 2018;164:112-121. https://doi.org/10.1016/j.enconman.2018.02.085. [ Links ]
4. Osman AI, Hefny M, Abdel Maksoud MIA, Elgarahy AM, Rooney DW. Recent advances in carbon capture storage and utilisation technologies: a review. Environ Chem Lett. 2021;19(2):797-849. https://doi.org/10.1007/s10311-020-01133-3. [ Links ]
5. Laca A, Laca A, Díaz M. Eggshell waste as catalyst: a review. J Environ Manage. 2017;197:351-359. https://doi.org/10.1016/j.jenvman.2017.03.088. [ Links ]
6. Leung D, Wu X, Leung M. A review on biodiesel production using catalyzed transesterification. Appl Energy. 2010;87(4):1083-1095. https://doi.org/10.1016/j.apenergy.2009.10.006. [ Links ]
7. Mamo T, Mekonnen YS. Microwave-assisted biodiesel production from Microalgae, Scenedesmus species, using goat bone: made nano-catalyst. Appl Biochem Biotechnol. 2020;190(4):1147-1162. https://doi.org/10.1007/s12010-019-03149-0. [ Links ]
8. Rajaeifar A, Akram A, Ghobadian B, Rafiee S, Heijungs R, Tabatabaei M. Environmental impact assessment of olive pomace oil biodiesel production and consumption: A comparative lifecycle assessment. Energy. 2016;106:87-102. https://doi.org/10.1016/j.energy.2016.03.010. [ Links ]
9. Taher H, Al-Zuhair S, Al-Marzouqi H, Haik Y, Farid M. A review of enzymatic transesterification of microalgal oil-based biodiesel using supercritical technology. Enzyme Res. 2011; 468292. https://doi.org/10.4061/2011/468292. [ Links ]
10. Sivasamy A, Cheah KY, Fornasiero P, Kemausuor F, Zinoviev S, Miertus S. Catalytic Applications in the production of biodiesel from vegetable oils. ChemSusChem. 2009;2(4):278-300. https://doi.org/10.1002/cssc.200800253. [ Links ]
11. Zhou Q, Zhang H, Chang F, Li H, Pan H, Xue W, Hu Y, Yang S. Nano La2O3 as a heterogeneous catalyst for biodiesel synthesis by transesterification of Jatropha curcas L. oil. J Ind Eng Chem. 2015;31:385-392. https://doi.org/10.1016/j.jiec.2015.07.013. [ Links ]
12. Rezaei R, Mohadesi M, Moradi R. Optimization of biodiesel production using waste mussel shell catalyst. Fuel. 2013;109:534-541. https://doi.org/10.1016/j.fuel.2013.03.004. [ Links ]
13. Gupta J, Agarwal M. Preparation and characterization of CaO nanoparticle for biodiesel production. AIP Conf Proc. 2016;1724:020066. https://doi.org/10.1063/1.4945186. [ Links ]
14. Zhang Y, Dubé A, McLean D, Kates M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour Technol. 2003;89(1):1-16. https://doi.org/10.1016/S0960-8524(03)00040-3. [ Links ]
15. Alsaiari A. Supported ruthenium catalyst as an effective catalyst for selective oxidation of toluene. J Indian Chem Soc. 2022;99(8):100593. https://doi.org/10.1016/j.jics.2022.100593. [ Links ]
16. Ejim I, Kamen F. Physiochemical characterization of algae oil from microalgae of Nike Lake Enugu. J Eng Appl Sci (Asian Res Publ Netw). 2013;5:181-187. [ Links ]
17. Eevera T, Rajendran K, Saradha S. Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew Energy. 2009;34(3):762-765. https://doi.org/10.1016/j.renene.2008.04.006. [ Links ]
18. Ali N, Khairuddin N, Siddique B. Eggshell waste as a catalyst for biodiesel production: A preliminary study. Mater Sci Eng. 2021;1195:012043. [ Links ]
19. Erchamo YS, Mamo TT, Workneh GA, Mekonnen YS. Improved biodiesel production from waste cooking oil with mixed methanol-ethanol using enhanced eggshell-derived CaO nano-catalyst. Sci Rep. 2021;11(1):6708. https://doi.org/10.1038/s41598-021-86062-z. [ Links ]
20. Ali NA, Khairuddin N, Tengku Azmi TSM, Siddique MBM. The preparation of CaO catalyst from eggshells and its application in biodiesel production from waste cooking oil. Arab J Sci Eng. 2023;48(1):383-388. https://doi.org/10.1007/s13369-022-07125-5. [ Links ]
21. Nadeem F, Bhatti I, Ashar A, Yousaf M, Iqbal M, Mohsin M, Nisar J, Tamam N, Alwadai N. Eco-benign biodiesel production from waste cooking oil using eggshell derived MM-CaO catalyst and condition optimization using RSM approach. Arab J Chem. 2021;14(8):103263. https://doi.org/10.1016/j.arabjc.2021.103263. [ Links ]
22. Yasar F. Biodiesel production via waste eggshell as a low-cost heterogeneous catalyst: its effects on some critical fuel properties and comparison with CaO. Fuel. 2019;255:115828. https://doi.org/10.1016/j.fuel.2019.115828. [ Links ]
Received 12 November 2022
Revised 26 March 2023
Accepted 26 March 2023
* To whom correspondence should be addressed Email: raalsayari@nu.edu.sa














