Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.77 Durban 2023
http://dx.doi.org/10.17159/0379-4350/2023/v77a03
RESEARCH ARTICLE
A simple method for colourimetric determination of silver using a home-made double beam photometer
Masoud Saadati*
Department of Science, Farhangian University, Talegani Street, Tabriz, Iran
ABSTRACT
The present study proposes a simple and inexpensive photometric method for the selective determination of Ag(I). This method employed a home-made double beam photometer for the determination of silver ions based on an optode sensor prepared by immobilization of 5-(P-Dimethylaminobenzylidene)-rhodanine (PDR) on triacetyl cellulose (TAC) membrane. Two green LEDs as sources of light and light-dependent resistors (LDRs) as detectors were used in the proposed device. The LEDs are turned on by a programmable microcontroller that receives signals from light passed through the sensor, and the absorbance is calculated and displayed on a Liquid-crystal display (LCD) screen. With the optimum conditions, the calibration plot was linear in the concentration range of 0.5-6 μg mL-1. For five replicate determinations of 2 μg mL-1 Ag(I) and the related detection limits, the relative standard deviations were 2.29% and 0.25 μg mL-1, respectively. The method was applied successfully for determining silver in a silver sulphadiazine cream, a radiology effluent, and radiology film samples and the results obtained were in good agreement with those of the standard flame atomic absorption spectroscopy (FAAS) method.
Keywords: home-made photometer, light emitting diode,optical sensor, silver
INTRODUCTION
Using light emitting diodes (LEDs) for lighting is increasing, and several research works are being conducted on simple photometric techniques in different fields like clinical and environmental.1 The advantages of LED lights are long life, low price, and more energy efficiency, which results in replacing LED-based devices with the traditional spectrophotometer.2-3 An inexpensive and compact USB photometer based on a single interchangeable LED as a light source has been constructed and utilized to determine dye concentrations in time (characterization of a microreactor) and to determine the concentration of p-nitrophenol product in a kinetic study.4 In similar works, inexpensive and portable LED-based photometers were used to determine copper in sugarcane spirit5, urea in milk6, and a trace amount of nitrate in surface water and atmospheric liquids.7 Flow analyzers also use LED capabilities to make them portable and low cost with reduced waste generation8 and improve the sampling rate.9 Combining a set of LEDs as a radiation source and associate interference filters to narrow the selected wavelength developed simple and portable photometers that operate in the near IR spectral region and used for fuel quality control.10-11
LED has other characteristics and can be used simultaneously as a light source and detector in devices known as paired emitter-detector diodes (PEDDs) based photometers. LED as the light detector makes the device simpler because it does not require a power supply to generate photovoltaic output.12-13
Optical chemical sensors have some features, such as easy manufacturing, higher selectivity and sensitivity, and low price. Because ofthis, they are a suitable choice in process control and analysis of the environmental samples14 and heavy metals15 that require quick and easy techniques. Optical sensor-based methods have been used to determine sulfate,16 zinc,17 lead,18 and mercury19 ions in different environmental samples.
The present work reports a simple hyphenated approach combining a home-made double-beam LED-based photometer with triacetyl cellulose (TAC)-based sensor for colourimetric detection of silver ions in aqueous samples. The proposed device comprises green colour LEDs as a light source, light-dependent resistors (LDR) as detectors, and plastic semi-micro cuvettes as sensor membrane containers. Silver ion sensing membrane was prepared by immobilizing of 5-(P-Dimethylaminobenzylidene)-rhodamine (PDR) on a TAC membrane, and its colour change was determined using the devised photometer.
MATERIALS AND METHODS
Instruments
A Shimadzu UV-1650PC (Japan) spectrophotometer was employed to record all UV-Visible spectra, and a Shimadzu 2554 (Japan) spectrofluorimeter was used to record LEDs light emission spectra. A Shimadzu AA-6300 flame atomic absorption spectrophotometer was used as a standard method for comparing the results.
Reagents
All chemicals used in the study had the highest purity available or analytical reagent grade (Merck, Darmstadt, Germany). Double distilled water was used for preparing the solutions. A silver stock solution (1000 mg L-1) was prepared by dissolving 0.149 g of AgNO3 in water and making up to 100 mL in a volumetric flask. This solution was poured into a dark bottle for storage. Standard solutions were prepared on a daily basis by diluting the stock solution. A hydrogen phthalate buffer solution was developed by dissolving 1.88 g of sodium hydrogen phthalate in water and diluting it to 100 mL. The pH of the buffer solution was stabilized at 3.5 by adding 6 mol L-1 nitric acid, dropwise. A solution of PDR as a 0.024 μg mL-1 in ethylenediamine was prepared and used.
The detection device and analytical procedure
A schematic diagram of the constructed double-beam photocolourimeter applied for colourimetric determination of Ag(I) based on the sensing membrane is shown in Figure 1. A green-colour LED (5 mm diameter, Mingxue Optoelectronic) was employed for lighting, and an LDR (5 mm diameter, Xiamen Xinpulong Semiconductor Co.) was used as a detector in each channel of the photometer. The emission wavelength and the spectral bandwidth of the LED are 560 and 40 nm, respectively. The LED light passed through the plastic semi-micro cuvette with the sensing membrane, and the LDR detected the intensity of the transmitted light. The membrane was placed in the middle of the cuvette at an equal distance from the LED and the LDR and was held by a simple hand-made polymeric holder. The membrane entered the cuvette through the center slot of the holder. The LED was turned on and off sequentially using a switch, and after placing blank membranes in two cells, signals of LDRs were balanced using another switch, all controlled by a microcontroller processor. The absorbance of the sensing membrane was determined by replacing a blank membrane with a sample membrane, and the results were indicated on a Liquid-crystal display (LCD) screen. The proposed device is portable with five rechargeable serial batteries.
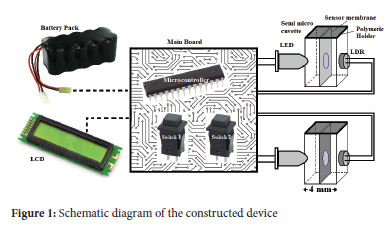
To perform absorbance measurement, the membranes were fixed on the phthalate buffer (pH 3.5) for 100 s to achieve equilibrium. The LEDs were turned on; two blank sensing optical membranes were placed into the sensor containers after standing for 1.5 min, followed by balancing of the two LDRs signals, and the absorbance of the analyte-containing sensor was then measured.
Optical membrane
The sensing optical membrane was prepared by immobilizing PDR on the TAC membrane. To produce the transparent TAC membrane, photographic film waste was treated with commercial sodium hypochlorite (0.01 mol L-1) to remove coloured gelatinous layers and irritated with grindstone (a 10x10 cm film for 5 min). Then, 5x1 cm slices of the membranes were prepared and treated with PDR (0.024 mol L-1) for eight minutes in ethylenediamine at an ambient temperature; the pieces were washed afterwards using water, and additional reagents were removed. The prepared colourimetric sensing membrane was stored in water for later use.
Sample preparation
Firstly, 10 mL of 6 mol L-1 nitric acid was added into a beaker containing 0.5 g of radiology film sample. The mixture was filtered after 10 minutes, and the separated solution was made up to 100 mL in a volumetric flask.
Secondly, 2 g of the silver sulphadiazine cream sample was transferred into a 100 mL beaker containing 1 mL of perchloric acid (60%), 10 mL of nitric acid (65%), and 40 mL of distilled water. The mixture was heated in a water bath for 30 min at 100 °C and cooled to 50 °C after dissolution. After adding 5 mL of hydrogen peroxide, the mixture was slowly heated to 200 °C to decrease the volume, followed by dilution to 50 mL.
Thirdly, 50 mL of radiology effluent sample was transferred into a 250 mL beaker containing 2.5 mL of hydrogen peroxide (30%), 10 mL of sulphuric acid (0.5 mol L-1), and 10 mL of nitric acid (1 mol L-1) and mixed thoroughly. The mixture was heated, and when the solution was almost dry, 1 mL of nitric acid (1 mol L-1) was added to the beaker, and the beaker-wall was washed with distilled water. The prepared solution was then filtered, and the remaining was poured into a 100 mL graduated flask, filled with distilled water to the mark.
RESULTS AND DISCUSSION
Preparation of optical membrane and LED selection
PDR is a chemical dye, practically insoluble in water and soluble in organic solvents, and reacts with metal ions in an acidic solution, resulting in insoluble metal-PDR complexes. On the other hand, PDR as a reagent with amino group could be linked chemically with triacetyl cellulose.20 Because of these characteristics, it is used to prepare optical sensors.21 Response mechanism of the PDR based optode sensor to metal ions was proposed in the literature.22 As shown by the absorption spectrum of PDR fixed on the TAC membrane; the highest absorbance wavelength was at 450 nm. In addition, a new peak appeared at 530 nm with an Ag-PDR complex on the surface of the membrane. As a light source, a green LED with a continuous spectrum between 520 and 605 nm (λ max = 560 nm) was used.
Irritation of membrane surface
Results have shown that the membrane surface can affect dye adsorption characteristics, and the smooth surface of the membrane cannot adsorb adequate PDR. Irritation of the membrane surface using sandpaper increases the adsorbing efficiency because the irritated surface possesses several tiny pores to maintain the sensing reagent. Sandpapers are available in grit size ranges which are used to clear surfaces by removing a layer or making the surface smoother or adding to the roughness of a surface. Usually, the grit size is marked on the paper as a number that has an inverse relationship with the particle size. Nine membranes were irritated with three different types of sandpaper, and the results obtained for conventional spectrophotometry, and the proposed LED-LDR-based methods are presented in Table 1. While the macro grit sandpapers demonstrate a higher response rate, there is lower repeatability than the micro grits, which results in higher surface non-uniformity.
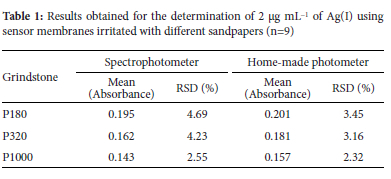
Most importantly, given the high precision of the LED-LDR-based detection system for macro grits, it outperformed the spectrophotometers, and overall, the results are entirely satisfactory for the proposed method. It can be said that using an LED-based detection system overcomes the decreased reproducibility and repeatability. It was also observed that divergence of the light emitting from a LED compared to a conventional spectrophotometer leads to a notably larger area of sensor membrane being in contact with the emitted light.
Due to the results obtained, sandpaper assigned by the P1000 code was selected as irritating sandpaper in this work. A Simpler design and lower price of the introduced method mixed with improved repeatability and precision of the result may overcome other weaknesses and makes the method competitive with commercial spectrophotometers.
Parameters in the sensor preparation
PDR is a hydrophobic dye and must be dissolved in a sufficient organic solvent to immobilize on the TAC. For this purpose, five membranes irritated according to the procedure described, placed in a 0.1 mol L-1 KOH solution for 14 hours, and treated with 10 mL of PDR (0.015 mol L-1) in five solvents (dimethylformamide, tetrahydrofuran, acetonitrile, ethanol, and ethylenediamine). The results obtained showed that using ethylenediamine as a solvent yields the highest outcomes.
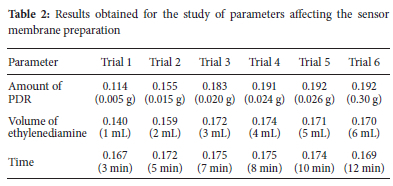
The effect of the concentration of PDR in ethylenediamine, the volume of the ethylenediamine, and the role of time in PDR immobilization on the membrane were also studied (Table 2). As seen, the sensitivity increases with the PDR amount up to 0.024 g and remains constant thereafter. On the other hand, the chemical effect of the ethylenediamine on triacetyl cellulose and deformation of the membrane results in decreases in absorbance after 10 min. Eventually, dilution of the PDR solution in high amounts of ethylenediamine leads to a decrease in the response. Therefore, to achieve the best results, the optical membrane should be treated for 7-8 minutes with 0.024 g PDR in 4 mL of ethylenediamine.
Optimizations of the parameters in Ag-PDR complex formation
The effect of the pH of the solution, buffer type, and buffer concentration on the Ag-PDR complex formation was examined. The treated and activated membranes were washed in 2 μg mL-1 Ag (I) in the pH range of 3.0-7.0. After washing with distilled water and drying, the complex and signal on the surface of the membranes were determined. Based on the obtained results, the highest response of the sensor was achieved with a pH of 3.5, which was then selected for deeper investigation. The same procedure was repeated in 0.1 mol L-1 acetate, phosphate, borate, sodium hydrogen phthalate, and citrate buffer solutions at pH = 3.5, and the best result was obtained with phthalate buffer solution. To achieve the highest buffer concentration, its effect was examined in the 0.02-0.50 mol L-1 range. According to the findings obtained, there was a slight increase in the sensitivity up to 0.10 mol L-1, which then remained unchanged until 0.40 mol L-1, followed by a slight decrease. To avoid the desorption of PDR because of the growth in the solution's organic characteristics, 0.10 mol L-1 phthalate was adopted as the best concentration.
Investigation of the LED light stability
When LED is turned on, its' intensity decreases by 3-4% in the initial moments and stabilizes after a few minutes. Variation in the absorbance of a standard sample solution in different standing times after turning on the LED was studied. The results indicated that the absorbance remained unchanged following 2.5 min. Thus, all determinations were performed following 2.5 min with the LED on.
Response time, stability, and lifetime
Figure 2 shows the changes in Ag-PDR complex formation on the membrane surface by measuring the absorption after the given membrane/Ag(I) solution contact time. As can be seen, the sensor membrane reached the maximum signal after 10 min in different Ag(I) concentrations.
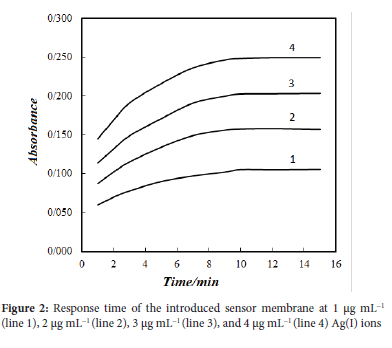
The repro ducibility of the sensor membrane was investigated through five determinations for the seven different membranes on different days. The relative standard deviation (RSD) for the determination of 2 μg mL-1 solution of Ag(I) was 3.67% which indicated a satisfactory day-to-day reproducibility.
With the prepared membrane in the open air stable for one hour, studies have indicated that the absorbance of the prepared membrane after storing it in distilled water for 30 days has no significant change compared to the newly prepared membranes. Oxidation of the reagent in the air is a possible cause of the instability that even causes the reagent to change colour from yellow to red.
Regeneration of the sensor membrane
Regeneration or recovery of a sensor membrane means leaving the analyte out of the membrane surface so it can be used again. This operation is performed by immersing used membranes in a solution of a sufficient regeneration agent. In this work, some anions, including I-, EDTA, SCN-, CN- and S2O3-, were studied as regeneration agents and found based on the experiments that Cyanide, with a regeneration time of less than one minute at a low concentration (0.001 mol L-1), is the optimum reagent for this objective.
The repeatability of the sensor, the number of sensor regeneration, was performed by successive runs on a single sensor.23 It was found that the result was repeatable until ten continuous implementations, and the RSD of sensor response for 2 μg mL-1 was 2.58%.
Analytical parameters
Quantitative characteristics of the introduced method were studied with optimized analysis conditions to evaluate the performance. There was a linear calibration graph in the 0.5-6 μg mL-1 range of Ag(I) concentration with a correlation equation of Y = 0.0513X + 0.0512 (in this equation, X is Ag(I) concentration (mol L-1)) and an R2 value of 0.9985. The relative standard deviation (RSD) calculated for nine replicates of 2 μg mL-1 of Ag(I) was 2.29%. Based on the IUPAC definition (CLOD = 3Sb/m, where Sb represents the standard deviation of blank, and m denotes the slope of the calibration line), the limit of detection (LOD) was equal to 0.25 μg mL-1. For the standard spectrophotometric method, the linearity ofthe method was in the 0.35 μg mL-1 of Ag(I) range. The RSD (n=9) of the method for 2 μg mL-1 Ag(I) and the estimated LOD value were 3.44% and 0.20 μg mL-1, respectively. The sensor membranes used in this comparison were prepared by the same procedure.
Method selectivity
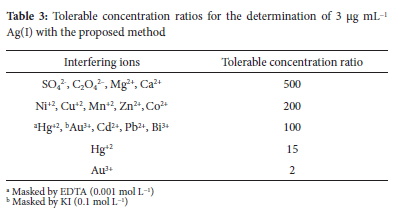
In order to study the method selectivity, the effect of some foreign ions on the determination of 3 μg mL-1 Ag(I) was examined. The tolerance limit of interfering ions, which gives an error of less than ±5% in absorbance reading, is given in Table 3. The results obtained show that no interference was observed from most of the ions except from Au3+ and Hg2+, which were minimized by using EDTA (0.001 mol L-1) and KI (0.1 mol L-1) as masking agents, respectively.
Analytical applications
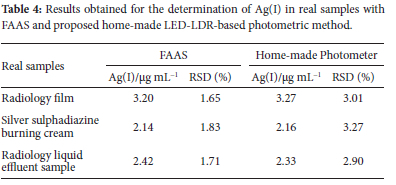
The method accuracy was examined using the introduced sensor membrane to determine silver sulphadiazine burning cream, silver in a radiology film, and radiology effluent samples. The outcomes of the introduced procedure were compared to those of flame atomic absorption (FAAS) methods (Table 4). Five replicate samples were analysed separately using FAAS and optical sensor membrane-based methods. The results showed that the methods were not significantly different and showed good agreement.
CONCLUSIONS
In this work, a simple and cheap photometric approach was developed to the selective determination of Ag(I) in aqueous samples using a homemade double beam photocolourimeter composed of green colour LEDs as a source of light and light-dependent resistors (LDR) as a detector. The determination procedure was performed using a sensor membrane based on the immobilization of PDR on a TAC membrane to generate an Ag-PDR colouring complex. The results showed that the proposed method has a good selectivity towards silver ions, and the devised detection system has good capability to overcome the usual disadvantage of low repeatability of sensor membranes. The introduced method was used to determine Ag(I) in some real samples. The outcomes obtained are in good agreement with those obtained by the FAAS.
ACKNOWLEDGEMENT
The author thanks the Research Council of Farhangian University for the financial and moral support.
ORCID ID
Masoud Saadati - https://orcid.org/0000-0002-1937-389X
REFERENCES
1. Bui DA, Hauser PC, Analytical devices based on light-emitting diodes--a review of the state-of-the-art. Anal Chim Acta, 2015;853:46-58. https://doi.org/10.1016/j.aca.2014.09.044 [ Links ]
2. Mohammad K, Zekry A, Abouelatta-Ebrahim M. LED Based Spectrophotometer can compete with conventional one. Int J Eng Technol. 2015;4(2):309-407. https://doi.org/10.14419/ijet.v4i2.4504. [ Links ]
3. Chen P, Wang H, Lin L, et al., editors. A practical portable photometer using LEDs as inspection light source. 2017 IEEE International Instrumentation and Measurement Technology Conference (I2MTC). 2017;1-6. https://doi.org/10.1109/I2MTC.2017.7969714 [ Links ]
4. Glotz G, Kappe CO. Design and construction of an open source-based photometer and its applications in flow chemistry. React Chem Eng. 2018;3(4):478-486. https://doi.org/10.1039/C8RE00070K. [ Links ]
5. Suarez WT, Gabriel WL, de Alvarenga Junior BR, et al. A Simplistic Portable LED-Based Photometer for In Situ Determination of Copper in Sugarcane Spirits. Food Anal Methods. 2018;11(12):3324-3330. https://doi.org/10.1007/s12161-018-1306-y. [ Links ]
6. Suarez WT, de Alvarenga Junior BR, de Oliveira Krambeck Franco M, et al. In Situ Determination of Urea in Milk Employing a Portable and Low-Cost LED Photometer. Food Anal Methods. 2018;11(4):1149-1154. https://doi.org/10.1007/s12161-017-1087-8. [ Links ]
7. Khoshmaram L, Saadati M, Sadeghi F. Magnetic solid-phase extraction and a portable photocolourimeter using a multi-colour light emitting diode for on-site determination of nitrite. Microchem J. 2020;152:104344. https://doi.org/10.1016/j.microc.2019.104344. [ Links ]
8. Pessoa-Neto O, Santos V, Vicentini F, et al. A low-cost automated flow analyzer based on low temperature co-fired ceramic and LED photometer for ascorbic acid determination. Open Chem. 2014;12(3):341-347. https://doi.org/10.2478/s11532-013-0377-2. [ Links ]
9. Santos FG, Pereira AC, Cruz SM, et al. Development of a multicommuted flow analysis procedure for simultaneous determination of sulfate and chloride in petroleum coke employing a homemade syringe pump and a LED-based photometer. Anal Methods. 2015;7(11):4769-4779. https://doi.org/10.1039/C5AY00565E. [ Links ]
10. Paiva EM, Rohwedder JJR, Pasquini C, et al. Method for building a portable near infrared photometer based on LEDs and interference filters chosen by a spectral variable selection algorithm. Microchem J. 2019;146:842-849. https://doi.org/10.1016/j.microc.2019.01.074. [ Links ]
11. Dantas HV, Barbosa MF, Pereira A, et al. An inexpensive NIR LED Webcam photometer for detection of adulterations in hydrated ethyl alcohol fuel. Microchem J. 2017;135:148-152. https://doi.org/10.1016/j.microc.2017.08.014. [ Links ]
12. Seetasang S, Kaneta T. Portable two-color photometer based on paired light emitter detector diodes and its application to the determination of paraquat and diquat. Microchem J. 2021;171:106777. https://doi.org/10.1016/j.microc.2021.106777. [ Links ]
13. Seetasang S, Kaneta T. Development of a miniaturized photometer with paired emitter-detector light-emitting diodes for investigating thiocyanate levels in the saliva of smokers and non-smokers. Talanta. 2019;204:586-591. https://doi.org/10.1016/j.talanta.2019.06.024. [ Links ]
14. Najlaoui D, Echabaane M, Khélifa AB, et al. Development of a selective and highly sensitive optical chemical sensor for determination of sulfaguanidine based on a novel mixed isopolymolybdates. Optik. 2021;231:166507. https://doi.org/10.1016/j.ijleo.2021.166507. [ Links ]
15. Firooz AR, Movahedi M, Sabzyan H. A new selective optode for the determination of iron(III) based on the immobilization of morin on triacetylcellulose: A combined experimental and computational study. Mater Sci Eng C. 2019;94:410-416. https://doi.org/10.1016/j.msec.2018.09.031. [ Links ]
16. Masadome T, Ishikawa H. Determination of Sulfate Ion with 2-Aminoperimidine Hydrobromide Using an Optode Based on Tetrabromophenolphthalein Ethyl Ester Membrane. Anal Sci. 2018;34(3):383-385. https://doi.org/10.2116/analsci.34.383. [ Links ]
17. Alian E, Semnani A, Firooz A, et al. A Novel Sensitive Bulk Optode Based on 5-Br Salophen as an Ionophore for Determination of Zinc Ion in Real Samples. J Braz Chem Soc. 2018;29. https://doi.org/10.21577/0103-5053.20170165. [ Links ]
18. Nur Y, Rohaeti E, Darusman L. Optical Sensor for the Determination of Lead(II) Based On Immobilization of Dithizone onto Chitosan-Silica Membrane. Indones J Chem. 2017;17:7. https://doi.org/10.22146/ijc.23560. [ Links ]
19. Shahamirifard SA, Ghaedi M. Design and construction of a new optical solid-state mercury(ii) sensor based on PVC membrane sensitized with colloidal carbon dots. New J Chem. 2017;41(20):11533-11545. https://doi.org/10.1039/C7NJ02421E. [ Links ]
20. Kostov Y, Tzonkov S, Yotova L, Krysteva M. Membranes for optical pH sensors. Anal Chim Acta. 1993;280(1):15-19. https://doi.org/10.1016/0003-2670(93)80235-D [ Links ]
21. Ludlow JW, Guikema JA, Consigli RA. Use of 5-(4-dimethylaminobenzylidene)rhodanine in quantitating silver grains eluted from autoradiograms of biological material. Anal biochem. 1986;154(1):104-09. https://doi.org/10.1016/0003-2697(86)90502-6. [ Links ]
22. Sorouraddin MH, Saadati M, Aghaei A. An optode membrane for determination of gold using a simple light-emitting diode-based device. Monatsh Chem. 2011;142:439-445. https://doi.org/10.1007/s00706-011-0476-8 [ Links ]
23. Dashtian K, Zare-Dorabei R. Preparation and characterization of a novel optical chemical sensor for determination of trace amounts of Praseodymium ion by UV/Vis spectrophotometry. Sens Actuators B Chem. 2017;242:586-594. https://doi.org/10.1016/j.snb.2016.11.087 [ Links ]
Received 03 September 2022
Revised 29 December 2022
Accepted 3 January 2023
* To whom correspondence should be addressed Email: m.saadati@cfu.ac.ir














