Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.77 Durban 2023
http://dx.doi.org/10.17159/0379-4350/2023/v77a01
RESEARCH ARTICLE
Trace nickel analysis in water samples via paper-based devices coupled with co-precipitation
Abdellah MuhammedI; Ahmed HussenII,*; Takashi KanetaIII
IDepartment of Chemistry, College of Natural Sciences, Wollo University, Dessie, Ethiopia
IICenter for Environmental Science, College of Natural and Computational Sciences, Addis Ababa University, Addis Ababa, Ethiopia
IIIDepartment of Chemistry, Graduate School of Natural Science and Technology, Okayama University, Okayama, Japan
ABSTRACT
A simple, fast, low-cost and sensitive microfluidic paper-based analytical device (u-PADs) integrated with the co-precipitation enrichment procedure was developed to analyse Ni(II) in tap and mineral water samples. The impacting factors, including pH, centrifugation (5 min at 4000 rpm), and amounts of reagents were optimized. The limit of detection of 0.009 mg L-1 and linear range of 0.03-2.00 mg L-1 were achieved with good intra- and inter-day precision (4.7 and 5.6% RSD, respectively). The recovery tests were conducted by spiking tap and mineral water samples and analyzed using the u-PADs after co-precipitation enrichment. The results obtained by the proposed method were validated by inductively coupled plasma-optical emission spectrometry (ICP-OES). The recoveries of the present method and ICP-OES were ranged from 92.4-106.8% and 92.9-97.2%, respectively. The two sets of (u-PADs and ICP-OES) results were in good agreement, as a paired t-test indicated no significant differences. The proposed method could be utilized for analyzing trace levels of Ni(II) in water samples in developing countries where the availability of conventional analytical instruments are significant problems.
Keywords: co-precipitation, dimethylglyoxime, microfluidic paper-based analytical device, nickel ion enrichment, water analysis
INTRODUCTION
Ni is a transition element extensively distributed in the environment, air, water, and soil deriving from natural sources and anthropogenic activity.1 Ni is an important constituent of several steel alloys,2 however its functional role as a trace element for animals and human beings has not been recognized. The environmental pollution from Ni may be due to industry, the use of liquid and solid fuels, and municipal and industrial waste. The Ni contact can cause various harmful effects on human health, such as allergy, cardiovascular and kidney diseases, lung fibrosis, and lung and nasal cancer.1 The World Health Organization has set a 0.07 mg L-1 limit for Ni in drinking water.3 Consequently, fast and reliable monitoring of Ni is an important requirement for drinking water, particularly in developing countries.
The conventional analytical methods for the determination of Ni including inductively coupled plasma optical emission spectrometry (ICP-OES),4 electrothermal atomic absorption spectrometry,5 HPLC/ UV-VIS spectrophotometer,6,7 voltammetry8 and flame atomic absorption spectrometry9,10 are very expensive and have limited accessibility in most developing countries. Thus, low cost, portable, simple, sensitive and easily accessible devices are required in developing countries for environmental monitoring.
Microfluidic paper-based analytical devices (μ-PADs) have a fascinating potential to address the demands of developing countries for environmental monitoring of heavy metals.11-13 However, the colourimetric |-PADs that have been so far developed for Ni assay have limitations to complying with the guideline established by the WHO because of low sensitivity.14-16 Therefore, the use of an enrichment procedure is required prior to Ni assay using |-PADs to improve the analytical sensitivity of the paper-based method.
Different enrichment techniques have been reported for conventional analytical methods. These include liquid-liquid extraction,17 cloud point extraction,6,18 solid-phase extraction (SPE)7,19-22 and co-precipitation.23 Recently Ninwong, et al.24 demonstrated a paper-based method coupled with heating pre-concentration for the determination of Ni in water samples to enhance the analytical signal. However, the use of heating would incur complexity and time in the procedure. The liquid-liquid extraction, cloud point extraction and SPE procedures are labour-intensive, time and reagent consuming, and require a large volume of samples.25,26 Among the cited procedures, co-precipitation stands out for its advantages in its implementation, such as simplicity, speed, low solvent consumption, and ability to achieve high enrichment factors.23,26-28 Co-precipitation of Ni ion using Mg(OH)2 has been previously demonstrated as a sample preparation technique prior to analysis with the conventional method.23 In the co-precipitation procedure, a precipitate is formed by combining a carrier element and a suitable inorganic ligand.9 The co-precipitation can be associated with metal adsorption on the precipitate surface or metal incorporation onto the precipitate structures.27 Centrifugation process provides the separation of the co-precipitated part from the supernatant, and the precipitate can be then dissolved in acids.10,28 Aluminium hydroxide (Al(OH)3) is readily available, inexpensive and has a higher enrichment factor over the other carrier elements.28 In paper-based methods, co-precipitation with Al(OH)3 has not been previously demonstrated for Ni determination. Recently, we have demonstrated the assay of Cu ions via combining colourimetric U-PADs with co-precipitation.29
In this study, we report an assay of Ni ions using colourimetric μ-PADs coupled with co-precipitation enrichment technique. The co-precipitation enrichment under optimized condition allowed detection of Ni(II) in tap and mineral water samples at trace level lower than the WHO guideline value (0.07 mg L-1). The results obtained by the proposed method were found to be in a good agreement with the ICP-OES measurements.
EXPERIMENTAL
Reagents and chemicals
The reagents used in this study were of analytical grade and were used as received. The solutions were prepared with deionized water purified using an Elix water purification system (Millipore Co., Ltd., Molsheim, France). The following chemicals were obtained from Wako Pure Chemical Industries (Osaka, Japan): Cobalt(II) nitrate hexahydrate, sodium fluoride, ammonium acetate, magnesium sulfate, barium chloride dehydrate, aluminium nitratenonahydrate, acetone, phosphate buffer, sodium acetate, acetic acid, and standard solution of copper, zinc and calcium. The standard solutions of nickel and manganese were obtained from Kishida Chemicals (Osaka, Japan). Sodium carbonate, nitric acid and dimethylglyoxime (DMG) were purchased from Kanto chemical co. Inc. (Tokyo, Japan), Junsei Chemical Co., Ltd. (Tokyo, Japan) and Katayama's Chemical (Osaka, Japan), respectively. Methanol and Iron(III) chloride anhydrous were obtained from Sigma-Aldrich (MO, USA) and Alfa Aesar (WH, USA), respectively. The mineral water sample was purchased from a local store at the Okayama University, Japan. The tap water samples were obtained from an outlet at the Analytical Chemistry Group, Department of Chemistry, Graduate School of Natural Science and Technology, Okayama University, Okayama, Japan.
Equipment and apparatus
The pH test paper (Toyo roshi, Tokyo, Japan) was used for rough pH measurements. The pH meter (model D-52, HORIBA, Kyoto, Japan) was used for precise pH measurements. The centrifugal machine (Model 3740, KUBOTA, Tokyo, Japan) was employed to separate the precipitate part from the supernatant. The |i-PADs were fabricated on a sheet of filter paper (200 x 200 mm, Chromatography Paper 1CHR, WhatmanTM, GE Healthcare Lifesciences, UK) by printing with a wax printer (ColorQube 8580N, Xerox, CT, USA). The printed sheets were heated using a drying machine (ONW-300S, AS ONE, Tokyo, Japan) to penetrate wax to the backside of the sheets. The desktop scanner (Canon MG 6300 Series, Tokyo, Japan) was employed to capture images of the |-PADs that were dried before scanning. Inductively coupled, plasma-optical emission spectrometry (ICP-OES) (Model VISTA-PRO, Seiko Electronics, Tokyo, Japan) was also used to determine Ni(II).
Ni ion enrichment procedure
The procedure developed in the present study for the μ-PAD assay coupled with co-precipitation is presented in Figure 1. The sample/ standard solution (14 mL) was used for the co-precipitation procedure. Based on optimized experimental conditions, 40 μL of 1 M Al(NO3)3 and 180 μL of 1 M Na2CO3 were added to the 15-mL centrifuge tube containing aliquots of sample solution (Figure 1A). The added Na2CO3 adjusted the pH to 9.39 and produced a cloudy suspension of Al(OH)3 (Figure 1B). The resulting solution was tuned up and down for mixing, followed by centrifugation at 4000 rpm for 5 min and the white precipitate of Al(OH)3 settled at the bottom of the clear supernatant (Figure 1C). The supernatant was decanted, and any residual water was removed with a micropipette. The precipitate was dissolved with 12 |L of 6 M HNO3 followed by neutralization with 16 μL of 4 M sodium acetate to increase the pH above 3. Ten μL of the sample solution was loaded on the sample zone of the μ-PADs for the Ni assay (Figure 1E).
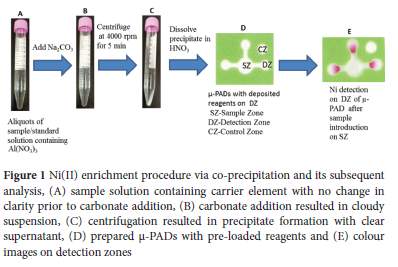
Design and fabrication of the μ-PAD
The μ-PAD design and dimensions are described in Figure 1D, and our previously reported design, dimensions and techniques were used for device fabrication.29 The pattern of the μ-PADs was drawn using Microsoft Office PowerPoint 2010. The μ-PADs consisted of a sample zone attached to three channels that led to three detection zones for triplicate measurements, and one control zone (Figure 1D). The μ-PADs have a height of 17 mm, a width of 20 mm, and a sample zone diameter of 6 mm at the center; there are also channels with a width of 2 mm and a length of 3 mm along with detection zones with diameters of 4 mm. The designed μ-PADs were printed using a wax printer on a sheet of filter paper (200 x 200 mm), followed by heating at 120 °C for 2 min in a drying machine to melt the wax ink to create a hydrophobic barrier as described in previous studies.12,30 33 The bottom of the printed paper was covered with clear packing tape to avoid leakage of the solutions through the bottom of the μ-PADs during the analysis.
Ni(II) assay and image processing
Previously reported methods24,31,15 were used with some modifications to prepare the μ-PADs for the Ni(II) assay. The assay is based on the formation of a chelate compound between Ni(II) and dimethylglyoxime (DMG) at alkaline pH.24 Under optimized experimental conditions, the μ-PADs were prepared by adding 1 μL of 1 M NaF one time into the sample zone to mask iron ion (Fe(III)) effectively, 0.4 μL of a 0.1 M acetate buffer solution (pH 5.6) once to the detection zones and 0.25 μL of a 2% DMG solution thrice to the detection zones. Ten μL of a sample/ standard solution was introduced to the sample zone after drying the reagent solutions on the μ-PAD (Figure 1E). Ni(II) immediately forms a visible pink colour in the detection zones when the sample/standard solution flows into the channels and enters the detection zones (Figure 1E). The devices were then allowed to dry at room temperature, and then a desktop scanner was used to capture images developed on the μ-PADs for quantitative analysis. The scanned images of the μ-PADs were stored in JPG format at 600 dpi resolution. The mean colour intensity was measured using ImageJ software. Accordingly, the background colour and the blank signal, were removed by adjusting the hue and brightness window in the image colour threshold until only the pink colour was visible. The images were then converted to a grayscale and inverted to achieve greater intensity, and the mean colour intensity was measured. The mean colour intensity values were proportional to the analyte concentrations.
RESULTS AND DISCUSSION
Optimization of parameters
Number of deposition of DMG solution effect on the intensity
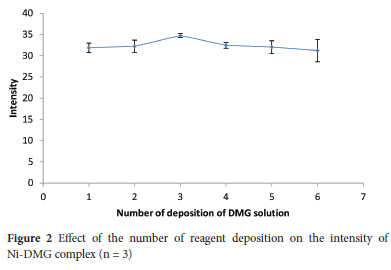
DMG dissolved in acetone was used for the colourimetric assay of Ni(II) using μ-PADs after Ni(II) enrichment with the co-precipitation procedure. To evaluate the effect of the number of reagent deposition, 0.25 μL of 2% (m/v) DMG solution was deposited onto the detection zones from 1 to 6 times. The colour intensity of the Ni-DMG complex was a bit increased with an increase in the number of depositions to 3 times deposition and then steadily declined (Figure 2). The intensity decrement at a higher number of depositions could be ascribed to the high hydrophobicity of DMG, preventing the penetration of aqueous sample solutions into the detection zones. Thus, the increased hydrophobicity associated with the increasing number of depositions could hinder aqueous sample solution penetration into the detection reservoir. Thereby a three-time deposition of 0.25 μL DMG solution was chosen in the subsequent experiments.
pH effect on the intensity
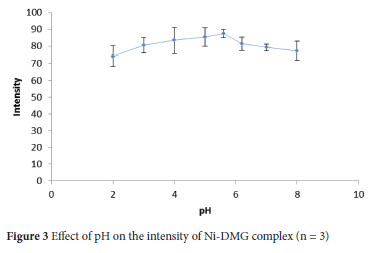
A pH is one of the prominent parameters affecting the mean colour intensity of images developed on μ-PADs as the complexation reaction of Ni(II) with DMG is pH-dependent.15,24,31 Thus, the pH of the detection zone influences the colour intensity of the complex between Ni(II) and DMG. Solutions containing 0.5 mg L-1 Ni(II) were introduced into the μ-PADs prepared by acetate buffer acidified with hydrochloric acid, acetate buffer and phosphate buffer with values of pH ranging from 2 to 8 to determine the optimum pH for Ni assay. The mean colour intensity of the Ni-DMG complex increased to pH 5.6 and then decreased with increasing pH (Figure 3). Thus, pH 5.6 was selected to achieve optimum intensity in the subsequent Ni(II) assay.
Optimization of Ni(II) enrichment via co-precipitation
The μ-PADs have been developed for Ni assay, but the method has limitations in sensitivity to detect Ni concentrations at or below the WHO limit of 0.07 mg L-1.14,15,24 Therefore, in this study, the co-precipitation technique for the enrichment of Ni prior to its determination in water samples using |-PADs was investigated. Several parameters affecting the performance of the co-precipitation, such as volumes of reagents, pH, centrifugation time and rate, were optimized to obtain the best experimental conditions with optimum intensity.
Concentration of carbonate and carrier element effect on the intensity
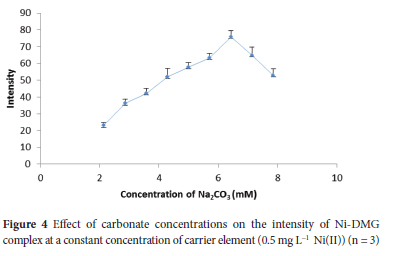
Aluminium hydroxide Al(OH)3 precipitate is easily coagulated when sodium carbonate (Na2CO3) is used, and controlling pH gradient using sodium carbonate is simple and convenient.28 Thereby, in the present study, Na2CO3 was used as a hydroxide ion source and for pH adjustment in the co-precipitation procedure. In this study, it was observed that Al(OH)3 precipitate settled rapidly and easily separated from the matrix solution after centrifugation for a brief time. The concentrations of Na2CO3 ranging from 2.14 to 7.86 mM were preliminarily investigated at a constant 1.43 mM Al(NO3)3 using a sample containing 0.5 mg L-1 Ni(II) to evaluate its effect on intensity. Intensity increased with increasing concentration to 6.43 mM and then sharply declined at higher concentrations (Figure 4). The declining in intensity at higher concentrations was due to the decreasing in precipitate size, which could be insufficient to collect Ni (II). On the other hand, for lower concentrations of Na2CO3, the pH was less than 8.95, which could be due to insufficient hydroxide ion. Consequently, co-precipitation of Ni could decrease down to the concentration gradient.
The results indicated that a relatively proportional amount of Na2CO3 and Al(NO3)3 need to be used to achieve quantitative co-precipitation of Ni(II) with Al(OH)3. The different concentrations of Na2CO3 and Al(NO3)3 at a constant concentration ratio of 4.5 were investigated using a sample containing 0.5 mg L-1 Ni(II) to achieve optimum colour intensity. A precipitate was not observed at concentrations less than 6.43 mM Na2CO3 and 1.43 mM Al(NO3)3, which could be due to less basic conditions as the pH was less than 9 (Table 1). Al(OH)3 precipitate formation was dependent on pH and the working pH range was narrow and a quantitative result was obtained at pH 9.54 (Table 1).
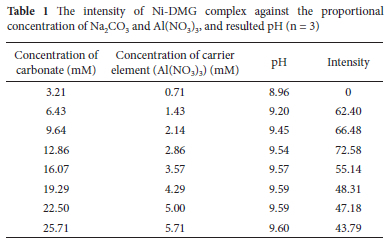
The amount of the precipitate, Al(OH)3, increased with the concentration of Na2CO3 and Al(NO3)3. The colour intensity was increased to concentrations of 12.86 mM Na2CO3 and 2.86 mM Al (NO3)3 and then declined at higher concentrations.
The higher concentrations of Na2CO3 and Al(NO3)3 required higher volumes of HNO3 to dissolve Al(OH)3, which in turn required higher volumes of sodium acetate for partial acid neutralization. Thus, an increase in the volume of 6 M HNO3 and 4 M sodium acetate diluted the sample solution in the subsequent μ-PAD assays so that a gradual decrease in the colour intensity was observed at concentrations higher than 12.86 and 2.86 mM Na2CO3 and Al(NO3)3, respectively, (Table 1). Therefore, the amount of precipitate should be as small as possible to obtain a higher enrichment factor. The maximum colour intensity was obtained at concentrations of 12.86 and 2.86 mM Na2CO3 and Al(NO3)3, respectively, and hence these concentrations were used for the subsequent experiments.
Precipitate digestion and pH adjustment
The resulted precipitate after centrifugation and removal of supernatant was dissolved in the minimum amount of 6 M HNO3 (12 μL) to get a higher enrichment factor. The high concentration of HNO3 (6 M) completely suppressed Ni-DMG interaction on the μ-PADs. Thereby, pH adjustment of the sample solution was carried out using sodium acetate to achieve the desired Ni-DMG interaction.
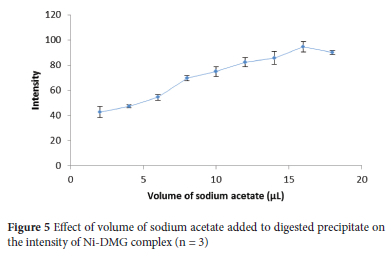
Thus, volumes of 4 M sodium acetate ranging from 2 to 18 μL were investigated using a sample solution containing 0.5 mg L-1 Ni(II) to set a volume with optimum intensity. The intensity steadily increased with increasing volume to 16 μL and then declined (Figure 5). There by, 16 μL of 4 M sodium acetate was chosen for the subsequent experiments to raise the pH to 3 with optimum intensity. Minimum volumes of 6 M HNO3 (12 μL) and 4 M sodium acetate (16 μL) were added to the precipitate for precipitate dissolution and pH adjustment, respectively, to optimize the enrichment factor (EF).
Centrifugation rate and time effect on the intensity
In the present study, the centrifugation process was used to properly settle the suspension of Al(OH)3 as a precipitate and to separate the precipitated part from the supernatant.28 The effect of centrifugation rate (revolution per minute of centrifuge machine, rpm) on colour intensity was investigated in 1000-4000 rpm. The intensity of the Ni-DMG complex increased with increasing centrifugation rate (Figure 6A), and hence a centrifugation rate of 4000 rpm was chosen for the subsequent experiments.
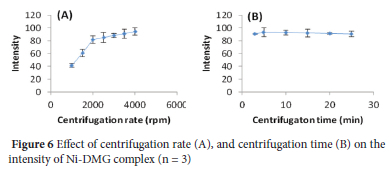
Similarly, the centrifugation time impact on the analytical signal was also studied in the range of 3-25 min. The intensity increased to 5 min and then slightly decreased with increasing centrifugation time (Figure 6B). Thereby, a centrifugation time of 5 min at 4000 rpm was selected for the subsequent experiments.
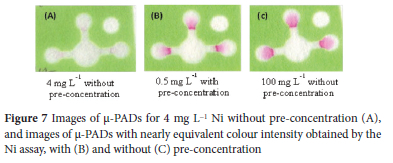
In this study, four ppm Ni(II) (Figure 7A) was the least attempted concentration without pre-concentration, which demonstrated that μ-PADs analysis for Ni assay needs to be accompanied by pre-concentration technique to determine Ni(II) at lower concentration levels to comply with the environmental guideline. Thus, the co-precipitation procedure developed in this study improved the analytical sensitivity for μ-PAD assay of Ni(II) at trace levels with enrichment factor (EF) of 200. The EF was defined as the ratio of the concentration without pre-concentration (100 mg L-1) to the concentration with pre-concentration (0.5 mg L-1) where their colour intensity is nearly equivalent. Thus, the 0.5 mg L-1 Ni(II) sample with pre-concentration (Figure 7B) showed a colour intensity nearly equivalent to 100 mg L-1 Ni(II) without pre-concentration (Figure 7C), giving the EF of a 200fold.
Analytical features
A series of working standard solutions of Ni(II) (0.03, 0.05, 0.1, 0.2, 0.5, 1 and 2 mg L-1) were prepared from a stock of 1000 mg L-1 Ni standard solution and analyzed using the μ-PADs after Ni(II) enrichment based on the optimized conditions to plot a calibration curve (Figure 8). The relationship between the logarithm of Ni(II) concentration and the logarithm of the colour intensity was linear in the range of 0.03-2.00 mg L-1 with a correlation coefficient of 0.997.
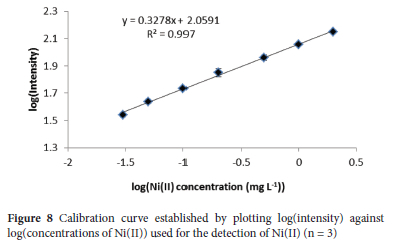
The limit of detection (LOD) and limit of quantification (LOQ) were 0.009 and 0.03 mg L-1, using concentration equivalents of three times the standard deviation for the colour intensities of a spiked blank sample (n = 9) and 3.3LOD, respectively. The concentrations above 2 mg L-1 showed colour intensity saturation, which is consistent to previous studies.31,34 The relative standard deviation (%RSD) of a standard solution at a concentration of 0.2 mg L-1 was 4.7% for intra-day (repeatability) and 5.6% for inter-day (reproducibility) (n = 9).
The analytical features obtained in this study were compared with the reported values,14-16,24,33,34 as presented in Table 2. The LOD was one to three orders of magnitude lower than the previous studies. The present enrichment technique decreased the measurable concentration to 0.03 mg L-1 which is the lowest value among the reported values. The results of the present study showed that the coupling of μ-PADs with enrichment technique is useful for trace level determination of Ni(II) in water samples.
Interference and stability study of μ-PADs
Water samples usually contain various common metal ions. An interference study was conducted under optimized experimental conditions to evaluate the effects of other ions and the analytical applications of the co-precipitation procedure for Ni assay in water samples.
Fe(III) interferes the detection of Ni by forming complexes with DMG, and hence NaF is used as a masking agent to prevent the interference effect of Fe(III).24 Thus, in this study, Fe(III) showed a slightly reddish-brown signal on detection zones of |-PADs in the absence of NaF. When NaF was deposited on the sample zone of μ-PADs, loading of 10 μL of 1000 mg L-1 Fe(III) on the μ-PADs did not show a signal, which confirmed that NaF effectively masked Fe(III) in the determination of Ni(II).
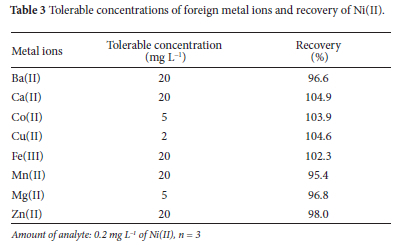
The selectivity of the proposed co-precipitation procedure was evaluated under optimized conditions by adding metal ions at 2, 5, and 20 mg L-1 (10 to 100 times) to 0.2 mg L-1Ni(II) solution (Table 3). Ten-times higher concentrations of foreign ions showed no interference in the present analytical method. The criterion for interference was an intensity value varying by more than 5% from the expected value for a 0.1 mg L-1 Ni(II) solution.36 Thus, the tolerance limit is the largest interfering ion concentration causing a relative error smaller than ±5%.
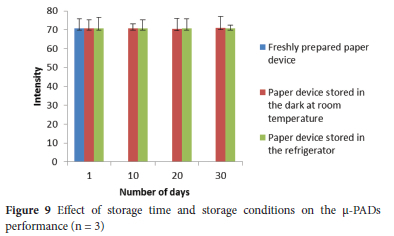
The storage stability of the proposed method was examined for consecutive 1, 10, 20 and 30 days. The tested μ-PADs were stored in the dark at room temperature and in the refrigerator at 4 °C. The μ-PADs in both storing conditions showed a nearly consistent response with that of freshly prepared μ-PADs throughout the whole examined days (Figure 9), which demonstrated that the prepared |-PADs are stable and do not require a refrigerator for storing the device for about a month.
Recovery study
The proposed method was applied to detect Ni+2 in spiked tap and mineral water samples to evaluate its potential application and reliability. Thus, aliquots of tap and mineral water samples were spiked at 0.05 and 0.1 mg L-1 Ni+2 and subjected to the co-precipitation procedure for the subsequent determination of Ni+2 using the μ-PADs. The spiked Ni+2 was quantitatively recovered from the samples using the developed method with recovery values of 92.4-106.8% (Table 4). The reliability of the results obtained by the proposed method was validated by inductively coupled plasma-optical emission spectroscopy (ICP-OES). ICP-OES exhibited recoveries of 92.9-97.2%, which is in good agreement with the results ofthe u-PADs as paired t-tests showed no significant differences between them. The proposed method could be applicable for the analysis of Ni+2 in water samples at trace levels, particularly in resource-limited settings.
CONCLUSION
In this study, a simple, low-cost, fast, selective and sensitive method was presented to detect trace Ni(II) in water samples. Ni(II) enrichment via co-precipitation was successfully applied prior to Ni(II) assay using u-PADs. The enrichment factor as high as 200-fold was achieved and resulted in a significant improvement in the sensitivity of the u-PADs. The Ni(II) enrichment technique coupled with u-PADs permitted lower LOD and LOQ of 0.009 and 0.03 mg L-1, respectively, with a wider linear dynamic range of 0.03-2.00 mg L-1. The proposed method was successfully applied to spiked water samples and the results obtained were in good agreement with ICP-OES results. Therefore, the combination of μ-PADs with the enrichment technique has great potential to extend the applications of u-PADs in environmental monitoring without need for sensitive conventional analytical instruments, particularly in developing countries.
ACKNOWLEDGEMENTS
The authors gratefully thank the Division of Instrumental Analysis, Department of Instrumental Analysis & Cryogenics, Advanced Science Research Center, Okayama University for the ICP-OES measurements.
CONFLICT OF INTEREST
The authors declare that they have no competing interests
ORCID IDs
Abdellah Muhammed: https://orcid.org/0000-0002-7172-4642
Ahmed Hussen: https://orcid.org/0000-0003-4303-2013
Takashi Kaneta: https://orcid.org/0000-0001-9076-3906
REFERENCES
1. Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A. Nickel: Human health and environmental toxicology. Int J Environ Res Public Health. 2020 Jan 21;17(3):679. https://doi.org/10.3390/ijerph17030679 [ Links ]
2. Hashemi-Moghaddam H. A selective flotation-spectrophotometric method for the determination of nickel using dimethylglyoxime. J Braz Chem Soc. 2011;22(6):1056-1060. https://doi.org/10.1590/S0103-50532011000600008 [ Links ]
3. WHO. Guidelines for drinking-water quality, 4th ed. WHO 2011;396-397. [ Links ]
4. Takei T, Shimizu F, Morishita K, Kondo M. Analysis of trace elements by ICP-OES and XRF in pulp and paper industry. Tappi J. 2020;74(7):737-743. https://doi.org/10.2524/jtappij.74.737 [ Links ]
5. Amirkavei M, Dadfarnia S, Shabani AMH. Dispersive liquid-liquid microextraction based on solidification of floating organic drop for simultaneous separation/preconcentration of nickel, cobalt and copper prior to determination by electrothermal atomic absorption spectrometry. Quim Nova. 2013;36(1):63-68. https://doi.org/10.1590/S0100-40422013000100012 [ Links ]
6. Ali S, Ali IR. Cloud point extraction and determination of Nickel (II) ions complex in real samples using new azo reagent. Adv. Sci. 2020;1(2):7-18. [ Links ]
7. Song X, Pang J, Wu Y, Huang X. Development of magnetism-reinforced in-tube solid phase microextraction combined with HPLC for the sensitive quantification of cobalt(II) and nickel(II) in environmental waters. Microchem J. 2020;159:105370. https://doi.org/10.1016/j.microc.2020.105370 [ Links ]
8. Pokpas K, Jahed N, McDonald E, Bezuidenhout P, Smith S, Land K, Iwuoha E. Graphene-AuNP enhanced inkjet-printed silver nanoparticle paper electrodes for the detection of nickel (II)-dimethylglyoxime [Ni (dmgH2)] complexes by adsorptive cathodic stripping voltammetry (AdCSV). Electroanalysis. 2020;32(12):3017-3031. https://doi.org/10.1002/elan.202060379 [ Links ]
9. Divrikli Ü, Elçi L. Determination of some trace metals in water and sediment samples by flame atomic absorption spectrometry after coprecipitation with cerium (IV) hydroxide. Anal Chim Acta. 2002;452(2):231-235. https://doi.org/10.1016/S0003-2670(01)01462- [ Links ]
10. Saracoglu S, Divrikli U, Soylak M, Elci L. Determination of copper, iron, lead, cadmium, cobalt and nickel by atomic absorption spectrometry in baking powder and baking soda samples after pre-concentration and separation. J Food Drug Anal. 2002;10:188-194. https://doi.org/10.38212/2224-6614.2749 [ Links ]
11. Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem. 2010 Jan 1;82(1):3-10. https://doi.org/10.1021/ac9013989 [ Links ]
12 Muhammed A, Hussen A, Redi M, Kaneta T. Remote investigation of total chromium determination in environmental samples of the kombolcha industrial zone, Ethiopia, using microfluidic paper-based analytical devices. Anal. Sci. 2021;37:1-7. https://doi.org/10.2116/analsci.20P303 [ Links ]
13. Muhammed A, Hussen A, Kaneta T. Speciation of chromium in water samples using microfluidic paper-based analytical devices with online oxidation of trivalent chromium. Anal Bioanal Chem. 2021 May;413(12):3339-3347. https://doi.org/10.1007/s00216-021-03274-y [ Links ]
14. Nurak T, Praphairaksit N, Chailapakul O. Fabrication of paper-based devices by lacquer spraying method for the determination of nickel (II) ion in waste water. Talanta. 2013 Sep 30;114:291-296. https://doi.org/10.1016/j.talanta.2013.05.037 [ Links ]
15. Li F, Hu Y, Li Z, Liu J, Guo L, He J. Three-dimensional microfluidic paper-based device for multiplexed colorimetric detection of six metal ions combined with use of a smartphone. Anal Bioanal Chem. 2019 Sep;411(24):6497-6508. https://doi.org/10.1007/s00216-019-02032-5 [ Links ]
16. Sun X, Li B, Qi A, Tian C, Han J, Shi Y, Lin B, Chen L. Improved assessment of accuracy and performance using a rotational paper-based device for multiplexed detection of heavy metals. Talanta. 2018 Feb 1;178:426-431. https://doi.org/10.1016/j.talanta.2017.09.059 [ Links ]
17. Erulas FA. Sensitive determination of nickel at trace levels in surface water samples by slotted quartz tube flame atomic absorption spectrometry after switchable solvent liquid-phase microextraction. Environ Monit Assess. 2020;192(5):272-277. https://doi.org/10.1007/s10661-020-8208-3. [ Links ]
18. Safavi A, Abdollahi H, Hormozi Nezhad MR, Kamali R. Cloud point extraction, preconcentration and simultaneous spectrophotometric determination of nickel and cobalt in water samples. Spectrochim Acta A Mol Biomol Spectrosc. 2004 Oct;60(12):2897-2901. https://doi.org/10.1016/j.saa.2004.02.001 [ Links ]
19. Alaa SA, Amirah SA. Study of the solid phase extraction and spectrophotometric determination of nickel using 5-(40-chlorophenylazo)-6-hydroxypyrimidine-2,4-dione in environmental samples. J Saudi Chem Soc. 2011;16(4):451-460. https://doi.org/10.1016/j.jscs.2011.02.018 [ Links ]
20. Shakerian F, Chelongar Y, Shabani AMH, Dadfarnia S. Mixed hemimicelles solid phase extraction based on sodium dodecyl sulphate-coated nano-magnets Fe3O4 for the simultaneous separation and preconcentration of cobalt and nickel. Microchem J. 2019;146:234-238. https://doi.org/10.1016/j.microc.2019.01.004 [ Links ]
21. Gazda DB, Fritz JS, Porter MD. Determination of nickel(II) as the nickel dimethylglyoxime complex using colorimetric solid phase extraction. Anal Chim Acta. 2004;508(1):53-59. https://doi.org/10.1016/j.aca.2003.11.044 [ Links ]
22. Nakhaei JM, Jamali MR, Sohrabnezhad S, Rahnama R. In-syringe solvent-assisted dispersive solid phase extraction followed by flame atomic absorption spectrometry for determination of nickel in water and food samples. Microchem J. 2019;144:88-92. https://doi.org/10.1016/j.microc.2018.08.063 [ Links ]
23. Luana SM, Ívero PS, Raquel CM, Ana Rita AN, Erik GPS, Clarice DBA. Coprecipitation magnesium (II) hydroxide as a strategy of pre-concentration for trace elemental determination by microwave-induced plasma optical emission spectrometry. Spectrochim Acta Part B At Spectrosc. 2020;169:1-6. https://doi.org/10.1016/j.sab.2020.105899 [ Links ]
24. Ninwong B, Ratnarathorn N, Henry CS, Mace CR, Dungchai W. Dual sample pre-concentration for simultaneous quantification of metal ions using electrochemical and colourimetric assays. ACS Sens. 2020;5(12):3999-4008. https://doi.org/10.1021/acssensors.0c01793 [ Links ]
25 Busa LSA, Mohammadi S, Maeki M, Ishida A, Tani H, Tokeshi M. Advances in microfluidic paper-based analytical devices for food and water analysis. Micromachines (Basel). 2016 May 9;7(5):86. https://doi.org/10.3390/mi7050086 [ Links ]
26. Mohammadi SZ, Shamspur T, Baghelani YM. Determination of copper, nickel, manganese and cadmium ions in aqueous samples by flame atomic absorption spectrometry after simultaneous coprecipitation with Co(OH)2. Arab J Chem. 2019;12(7):1751-1757. https://doi.org/10.1016/j.arabjc.2014.11.054 [ Links ]
27. Bader N, Benkhayal AA, Zimmerman B. Co-precipitation as a sample preparation technique for trace element analysis: an overview. Int. J. Chem. Sci. 2014;12(2):519-525 [ Links ]
28. Doner G, Ege A. Determination of copper, cadmium and lead in seawater and mineral water by flame atomic absorption spectrometry after coprecipitation with aluminum hydroxide. Anal Chim Acta. 2005;547(1):14-17. https://doi.org/10.1016/j.aca.2005.02.073 [ Links ]
29. Muhammed A, Hussen A, Kaneta T. Microfluidic paper-based analytical devices coupled with co-precipitation enrichment show improved trace analysis of copper ions in water samples. Anal. Sci. 2021;38:123-130. https://doi.org/10.2116/analsci.21P215 [ Links ]
30. Alahmad W, Uraisin K, Nacapricha D, Kaneta T. A miniaturized chemiluminescence detection system for a microfluidic paper-based analytical device and its application to the determination of chromium( iii ). Anal Methods. 2016;8(27):5414-5420. https://doi.org/10.1039/C6AY00954A [ Links ]
31. Mentele MM, Cunningham J, Koehler K, Volckens J, Henry CS. Microfluidic paper-based analytical device for particulate metals. Anal Chem. 2012 May 15;84(10):4474-4480. https://doi.org/10.1021/ac300309c [ Links ]
32 Rattanarat P, Dungchai W, Cate DM, Siangproh W, Volckens J, Chailapakul O, Henry CS. A microfluidic paper-based analytical device for rapid quantification of particulate chromium. Anal Chim Acta. 2013 Oct 24;800:50-55. https://doi.org/10.1016/j.aca.2013.09.008 [ Links ]
33. Cate DM. Developing paper-based analytical devices for particulate metals in welding fume. [PhD thesis,]. Colorado State University, Colorado, USA, 2015. [ Links ]
34. Rattanarat P, Dungchai W, Cate D, Volckens J, Chailapakul O, Henry CS. Multilayer paper-based device for colorimetric and electrochemical quantification of metals. Anal Chem. 2014 Apr 1;86(7):3555-3562. https://doi.org/10.1021/ac5000224 [ Links ]
35. Cate DM, Noblitt SD, Volckens J, Henry CS. Multiplexed paper analytical device for quantification of metals using distance-based detection. Lab Chip. 2015 Jul 7;15(13):2808-2818. https://doi.org/10.1039/C5LC00364D [ Links ]
36. Ojeda CB, De Torres AG, Rojas FS, Pavon JC. Fluorimetric determination of trace amounts of gallium in biological tissues. Analyst. 1987;112(11):1499-1501. https://doi.org/10.1039/AN9871201499 [ Links ]
Received 01 March 2022
Revised 01 October 2022
Accepted 16 December 2022
* To whom correspondence should be addressed Email: ahmed.hussen29@aau.edu.et














