Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.76 Durban 2022
http://dx.doi.org/10.17159/0379-4350/2021/v76a14
RESEARCH ARTICLE
Utilisation of bis-chloroacetamide derivative in the synthesis of new biologically active sulfide compounds
Norhan A Khalaf; Ehab Abdel-Latif*; Mohamed A Ismail; Heba M Metwally
Department of Chemistry, Faculty of Science, Mansoura University, Mansoura, Egypt
ABSTRACT
4-Aminobenzohydrazide (1) undergoes chloroacetylation twice, at the primary amine and hydrazide-NH2 functional groups. The conforming bis-chloroacetamide derivative 3 was reacted with different sulfur reagents (namely, 2-mercaptobenzothiazole, 6-amino-2-mercaptopyrimidin-4-ol, and 2-mercapto-4,6-dimethyl-nicotinonitrile) to give new bis-sulfide compounds 5, 7 and 9, respectively. The newly synthesised bis-chloroacetamide and corresponding sulfides were screened for anti-microbial and antioxidant potential. The sulfide derivative 7 exhibited the most potent activity against Staphylococcus aureus and Pseudomonas aeruginosa. It shows inhibition activities of 83.4% and 78.8%, respectively. Moreover, the sulfide derivative 7 showed the highest antioxidant activity with an inhibition ratio of 85.9%, which is close to L-ascorbic acid.
Keywords: 4-aminobenzohydrazide, antibacterial, antioxidantchloroacetamide, sulfide
INTRODUCTION
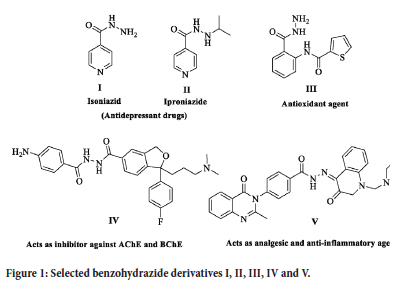
In recent years, a notable increase in bacterial infections has occurred.1 Resistance against bacterial and fungal infections - resulting from the abuse of antibacterial and antifungal drugs - poses a serious health hazard. The resistance of known antibacterial agents was the driving force for creating and modifying new antifungal and antibacterial medications.2,3 Nowadays, various pharmacological actions of benzohydrazide derivatives have given them great importance. For example, they have shown analgesic4 and anti-inflammatory properties.5 Along with their promising anti-microbial activities,6 benzohydrazide derivatives act as anti-cancer drugs.7 They also possess significant anti-tubercular,8 anti-HIV,9 and antioxidant10 activities. Benzohydrazide possesses some important drug features; for example, Isoniazid (I) and its derivative Iproniazid (II) (Figure 1) have an antidepressant action and are used in pharmacologic treatments for tuberculosis and psychosis.11 Several benzohydrazide compounds have been identified to possess a wide range of biological functions. 2-Aminobenzohydrazide derivative III (Figure 1) showed a greater than 90% scavenging percentage in their antioxidant activity experiment.12 In addition, a good inhibitory activity was shown by 4-amino escitalopram benzohydrazide derivative IV (Figure 1) against cholinesterase AChE and BChE.13 Furthermore, the most potent analgesic, ulcerogenic and anti-inflammatory activity was presented by quinazoline benzohydrazide derivative V (Figure 1).14
A well-known synthetic intermediate is chloroacetamide. It has received remarkable attention as this intermediate features various biological activities (for example, analgesic, antitumor, anti-microbial, antioxidant, hypoglycemic, and antipyretic) and applications in agriculture.15-24 Moreover, different 2-chloroacetamide compounds have been utilised in solid-state chemistry25 and were found to produce pharmacologically favourable compounds.26-29 Also, various transformations of chloroacetamide compounds could lead to the preparation of sulfur-containing compounds. Growth inhibition of Escherichia coli and Staphylococcus aureus was achieved by the most potent antibacterial compounds, sulfide derivatives.30
Based on the previous importance, various methods are described for the preparation of sulfides. The most common protocols include the addition of aryllithium or organocuprate to thiocarbonyl compounds, reduction of sulfoxides and sulfones;31 nucleophilic displacement of aryl and alkyl halides by thiols;32, 33 the treatment of alkyl halides with thiourea,34 or thiocarbonate;35 the reaction of halides with thiosilanes;36 Michael addition of thiols37 to α,β-unsaturated carbonyl compounds; intermolecular S-alkylation of thiols with alcohols;38 and the addition of thiols, or their anions to alkynes.39, 40 Because of various disadvantages encountered in the reported methodologies, such as use of hazardous and toxic solvents, difficulty in recovery of high boiling solvents, use of costly catalysts, etc., eco-friendly and more convenient methods have been developed.41 These comprise the use of catalytic phenylselenyl bromide,42 nickel,43 or native silica nanoparticle40 under solvent-free conditions, β-cyclodextrin in the presence of water and acetone or under catalyst-free conditions.44 In light of the previous statement, this study aims to prepare bis-sulfide derivatives from N-aryl bis-chloroacetamide and study the biological effects of these compounds as antioxidant and antibacterial agents.
RESULTS AND DISCUSSION
Chemistry
The starting compound 4-aminobenzohydrazide (1) has been obtained by the reported experimental conditions through refluxing ethyl 4-aminobenzoate in hydrazine hydrate.45 The reactivity of 4-aminobenzohydrazide (1) towards chloroacetylation was tested by treatment with chloroacetyl chloride in DMF containing anhydrous potassium carbonate (Scheme 1). It was expected that a mono-substituted chloroacetamide product 2 would be obtained through chloroacetylation at the hydrazide function (-NH2). Unexpectedly, spectroscopic analyses indicated the formation of the bis-substituted chloroacetamide compound 3 through double chloroacetylation at both amino groups (primary aromatic amine and the amino group of hydrazide function. The structure of the previously obtained compound was confirmed by spectroscopic analyses, where the IR spectrum displayed absorptions at 3323, 3257, and 1685 cm-1, corresponding to the (N-H) and carbonyl (C=O) groups. The 1H NMR spectrum indicated the appearance of singlet signals at δ 4.20 and 4.28 ppm for the protons of two methylene groups, two doublet signals at δ 7.68 and 7.85 ppm for four aromatic protons, and three singlet signals at 5 10.33,10.43, and 10.57 ppm for the protons of three N-H groups.
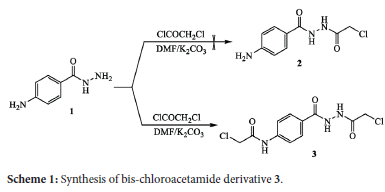
The 13C NMR spectrum confirmed the appearance of characteristic signals at δ 164.76, 165.12 and 165.44 ppm indicating the carbon atoms of three amidic carbonyl groups. It also showed the appearance of new signals at δ 41.00 and 43.64 corresponding to the two carbon atoms of the methylene groups.
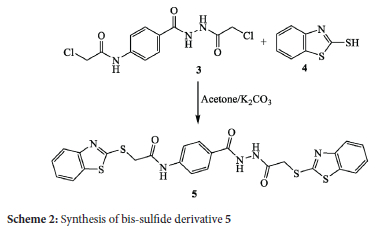
The chemical behaviour of the bis-chloroacetamide derivative 3 was tested against different types of nucleophilic sulfur reagents. Thus, stirring of bis-chloroacetamide compound 3 with 2-mercaptobenzothiazole (4) yielded the corresponding benzothiazole-2-yl sulfide derivative 5 in 60% yield (Scheme 2). The IR spectrum of sulfide 5 exhibited absorptions at 3326 and 3288 (N-H), 1677 and 1656 cm-1 (C=O). The 1H NMR spectrum indicated singlet signals at δ 4.28 and 4.42 ppm for the protons of methylene groups. The aromatic protons resonate as a multiplet and doublet in the range from δ 7.34 to 8.02 ppm. The protons of N-H groups resonate as three singlet signals at δ 10.38, 10.42 and 10.71 ppm. The 13C NMR spectrum exhibited twenty-three carbon signals for 25 carbon atoms and indicated the characteristic signals of methylene carbon atoms at δ 34.90 and 37.76 ppm.
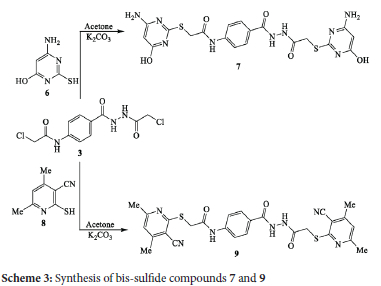
The reaction of one molar amount of bis-chloroacetamide derivative 3 with two molar amounts of 6-amino-2-mercaptopyrimidin-4-ol (6) and/or 2-mercapto-4,6dimethyl nicotinonitrile (8) in acetone and anhydrous K2CO3 yielded the corresponding bis-sulfide derivatives 7 and 9 in 79% and 71% yields, respectively (Scheme 3). The reaction proceeded via attack of the sulfur nucleophile on bis-chloroacetamide derivative 3 and substitution of the chlorine atom. The IR spectrum of bis-sulfide compound 7 (as an example) exhibited absorptions at 3475, 3336 and 3228 cm-1 for the O-H and NH2 functions. The 1H NMR spectrum displayed singlet signals at δ 4.98 and 5.02 ppm for the protons of pyrimidine-H and a broad singlet at δ 6.57 ppm for the protons of amino groups.
Biological activity of the prepared bis-sulfide compounds
Antibacterial activity
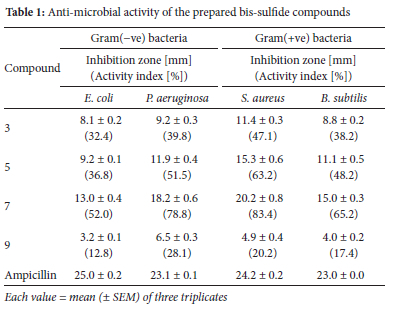
The bis-chloroacetamide compounds and their corresponding bis-sulfide compounds were examined as antibacterial agents. Two types of Gram(+ve) bacteria (Staphylococcus aureus and Bacillus subtilis) and two types of Gram(-ve) bacteria (Escherichia coli and Pseudomonas aeruginosa) were selected for this study. The methodology of the applied antibacterial assay was carried out as previously described in the literature.30 A primary investigation of the antibacterial activity of the prepared sulfide derivatives (Table 1) indicated that the bis-sulfide derivative 7, linked to the pyrimidine ring system, is the most active compound against S. aureus and P. aeruginosa bacteria with relatively inhibitory activity indices of 83.4% and 78.8%, respectively. It also showed relatively moderate activity against B. subtilis and E. coli with activity indices of 65.2% and 52.0% (inhibition zones of 15 and 13 mm), respectively. The bis-sulfide derivative 5, with the benzothiazole moiety, showed moderate antibacterial activity against S. aureus with an activity index of 62.2%. It also showed significant activity against Pseudomonas aeruginosa with an activity index of 51.5% (inhibition zone of 11.9 mm). On the other hand, compounds 3 and 9 are the least active compounds against all tested bacteria.
Antioxidant assay
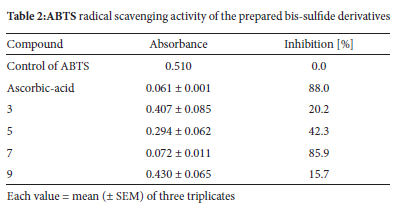
The pro-oxidant activities of the produced compounds were tested using the ABTS assay. The antioxidant potential of the prepared sulfide derivatives 5, 7, and 9 was investigated (Table 2). The results indicated that the sulfide derivative 7, bearing pyrimidine rings, showed antioxidant activity with the highest percentage inhibition (85.9%), which is close to the reference, ascorbic acid (88.0%). The sulfide derivatives 5 and 9, and bis-chloroacetamide compound 3 exhibited weak antioxidant activity.
CONCLUSION
Chloroacetylation of 4-aminobenzohydrazide (1) was carried out at both amino groups to give bis-chloroacetamide reagent 3. Nucleophilic substitution of the mobile chlorine atom of compound 3 with thiol reagents led to the synthesis of bis-sulphide derivatives 5, 7 and 9. Antibacterial activities of the new bis-sulfide derivatives were screened against S. aureus, B. subtilis, E. coli, and P. aeruginosa. The bis-sulphide derivative 7 containing the pyrimidine ring showed significant antibacterial and antioxidant potentialities.
EXPERIMENTAL
Melting points were determined on the Gallenkamp apparatus. The IR spectroscopic analysis was recorded on a Thermo Scientific Nicolet iS10 FTIR spectrometer. 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were obtained in DMSO-d6 using JEOL's spectrometer. The Perkin-Elmer 2400 analyser was used to determine the elemental analyses.
Preparation of 2-chloro-N-(4-(2-(2 chloroacetyl) hydrazinecarbonyl)-phenyl)acetamide (3)
A mixture of 4-aminobenzohydrazide (0.30 g, 2 mmol), 2-chloroacetyl chloride (0.16 mL, 2 mmol) and potassium carbonate (0.28 g, 2 mmol) was stirred in 20 mL DMF for 4 h. The mixture was poured into ice water and subjected to neutralisation by diluted HCl. The solid was filtered and subjected to recrystallisation from ethanol to furnish the bis-chloroacetamide compound 3.
Yield 57%, m.p. 240-241°C. IR (v/cm-1): 3323, 3257 (N-H), 1685, 1655 (C=O); 1 H NMR (δ/ppm): 4.20 (s, 2H, CH2), 4.28 (s, 2H), 7.68 (d, J = 8.50 Hz, 2H), 7.85 (d, J = 8.50 Hz, 2H), 10.33 (s, 1H), 10.43 (s, 1H), 10.57 (s, 1H). 13C NMR (δ/ppm): 41.00, 43.64, 118.65 (2C), 127.19, 128.72 (2C), 128.72, 141.70, 164.76, 165.12, 165.44.
Analysis Calculated for C11H11Cl2N3O3 (303.02): C, 43.44; H, 3.65; N, 13.82%. Found: C, 43.28; H, 3.56; N, 13.95%.
General methodology for the production of bis-sulfide compounds 5 and 7
A mixture of bis-chloroacetamide compound 3 (0.15 g, 0.5 mmol), 2-mercaptobenzothiazole (0.17 g, 1 mmol) or 6-amino-2-mercaptopyrimidin-4-ol (0.14 g, 1 mmol) and potassium carbonate (0.14 g, 1 mmol) was stirred in 20 mL acetone for 4 h. The mixture was poured into ice water, and the solid was collected and subjected to recrystallisation from ethanol to produce the targeted sulfides 5 and 7.
2-(Benzothiazol-2-ylthio)-N-(4-(2-(2-(benzothiazol-2-ylthio)acetyl)-hydrazinecarbonyl)phenyl)acetamide (5)
Yield 63%, m.p. 195-196 °C; IR (v/cm-1): 3326, 3288 (N-H), 1677, 1656, 1625 (C=O); 1H NMR (δ/ppm): 4.28 (s, 2H), 4.42 (s, 2H), 7.36 (q, J = 7.00 Hz, 2H), 7.40-7.50 (m, 2H), 7.68 (d, J = 8.50 Hz, 2H), 7.80 (d, J = 8.00 Hz, 1H), 7.80-7.89 (m, 3H), 8.01-8.02 (dd, J = 8.00, J2 = 4.00 Hz, 2H), 10.38 (s, 1H), 10.42 (s, 1H), 10.71 (s, 1H). 13C NMR (δ/ppm): 34.90, 37.76, 118.41 (2C), 121.08, 121.18, 121.84, 121.91, 124.55 (2C), 126.39, 126.43, 127.26, 127.29, 128.50, 134.77, 134.80, 141.78, 152.50, 152.52, 164.55, 165.71, 165.76, 165.91, 165.97. Analysis calculated for C25H19N5O3S4 (565.04): C, 53.08; H, 3.39; N, 12.38%. Found: C, 52.87; H, 3.34; N, 12.48%.
2-((4-Amino-6-hydroxypyrimidin-2-yl)thio)-N-(4-(2-(2-((4-amino-6-hydroxypyrimidin-2-yl)thio)acetyl)hydrazine-1-carbonyl)phenyl)acetamide (7)
Yield 75%, m.p. 318-319°C. IR (v/cm-1): 3457, 3336, 3228 (OH and NH2), broad centered at 1633 (C=O). 1H NMR (δ/ppm): 3.84 (s, 2H), 3.98 (s, 2H), 4.98 (s, 1H, pyrimidine-H), 5.02 (s, 1H, pyrimidine-H), 6.57 (broad s, 4H, NH2), 7.66 (d, J = 8.50 Hz, 2H), 7.82 (d, J = 8.50 Hz, 2H), 10.09 (s, 1H), 10.40 (s, 1H), 10.42 (s, 1H). Analysis calculated for C19H19N9O5S2 (517.10): C, 44.10; H, 3.70; N, 24.36%. Found: C, 44.23; H, 3.66; N, 24.42%.
Preparation of 2-((3-cyano-4,6-dimethylpyridin-2-yl)thio)-W-(4-(2-(2-((3-cyano-4,6-dimethyl-pyridin-2-yl)thio)acetyl) hydrazinecarbonyl)phenyl)-acetamide (9):
To a suspension of bis-chloroacetamide derivative 3 (0.15 g, 0.5 mmol), and potassium carbonate (0.14 g, 1 mmol) in 20 mL acetone, 2-mercapto-4,6-dimethylnicotinonitrile (0.16 g, 1 mmol) was added. The mixture was refluxed for 4 hours and then poured into ice water. The produced solid was collected and recrystallised from ethanol.
Yield 71 %, m.p. 282-283°C IR (v/cm-1): 3320, 3263 (N-H), 2215 (C=N), 1681, 1652 (C=O). 1H NMR (δ/ppm): 2.36 (s, 3H, CH3), 2.39 (s, 3H, CH3), 2.40 (s, 3H, CH3), 2.51 (s, 3H), 4.10 (s, 2H), 4.17 (s, 2H), 7.08 (s, 1H, pyridine-H), 7.12 (s, 1H, pyridine-H), 7.66 (d, J = 8.50 Hz, 2H), 7.81 (d, J = 8.50 Hz, 2H), 10.25 (s, 1H), 10.33 (s, 1H), 10.58 (s, 1H). 13C NMR (δ/ppm): 19.61 (2C), 24.19, 24.28, 31.75, 34.97, 103.60, 103.66, 115.00 (2C), 118.30 (2C), 120.49, 120.57, 126.89, 128.49 (2C), 142.08, 152.51 (2C), 159.79, 160.19, 161.32, 161.67, 164.80, 166.55, 166.61. Analysis Calculated for C27H25N7O3S2 (559.15): C, 57.94; H, 4.50; N, 17.52%. Found: C, 57.73; H, 4.38; N, 17.80%.
SUPPLEMENTARY MATERIAL
Supplementary information for this article is provided in the online supplement. This contains spectral characterization of title compounds, copies of IR, 1H NMR and 13C NMR spectra (Figures S1-S11).
ORCID IDs
Ehab Abdel-Latif: https://orcid.org/0000-0003-4437-6635
REFERENCES
1. Ulusoy N, Kiraz M, Küçükbasmac Ö.. New 6-(4-bromophenyl)-imidazo[2,1-b]thiazole derivatives: synthesis and anti-microbial activity. Monatsh Chem. 2002;133(10):1305-1315. https://doi.org/10.1007/s007060200108 [ Links ]
2. Kaplancikli ZA, Turan-Zitouni G, Revial G, Guven K. Synthesis and study of antibacterial and antifungal activities of novel 2-[[(benzoxazole/ benzimidazole-2-yl)sulfanyl] acetylamino]thiazoles. Arch Pharm Res. 2004;27(11):1081-1085. https://doi.org/10.1007/BF02975108 [ Links ]
3. Singh N. U.S. Sharma1, N. Sutar1, S. Kumar, U.K. Sharma1. Synthesis and anti-microbial activity of some novel 2-amino thiazole Derivatives. J Chem Pharm Res. 2010;2:691-698. [ Links ]
4. Deep A, Jain S, Sharma PC, Phogat P, Malhotra M. Synthesis of 2-(aryl)-5-(arylidene)-4-thiazolidinone derivatives with potential analgesic and anti-inflammatory activity. Med Chem Res. 2012;21(8):1652-1659. https://doi.org/10.1007/s00044-011-9679-0 [ Links ]
5. Jarapula R, Gangarapu K, Manda S, Rekulapally S. Synthesis, in vivo anti-inflammatory activity, and molecular docking studies of new isatin derivatives. Int J Med Chem. 2016;2016:2181027. https://doi.org/10.1155/2016/2181027. [ Links ]
6. Manivannan R, Shopna R. Anti-microbial and anti-inflammatory activity of new 4-methoxy-3-(methoxymethyl)phenol and (E)-N'-(5-bromo-2-methoxybenzylidene)-4-methoxy benzohydrazide isolated from calotropisgigantean white. Nat Prod Sci. 2017;23(1):69-74. https://doi.org/10.20307/nps.2017.23.1.69 [ Links ]
7. Katiyar A, Hegde M, Kumar S, Gopalakrishnan V, Bhatelia KD, Ananthaswamy K, Ramareddy SA, De Clercq E, Choudhary B, Schols D, et al. Synthesis and evaluation of the biological activity of N'-[2-oxo-1,2dihydro-3H-indol-3-ylidene]benzohydrazides as potential anticancer agents. RSC Advances. 2015;5(56):45492-45501. https://doi.org/10.1039/C5RA01528F [ Links ]
8. Tatipamula VB, Vedula GS. Anti-microbial and anti-tubercular activities of isolates and semi synthetic derivatives of lichen Ramalina leiodea (Nyl.) Nyl. J Serb Chem Soc. 2019;84(6):555-562. https://doi.org/10.2298/JSC180924003T [ Links ]
9. Randhawa H, Kamboj A, Saluja AK. Synthesis, pharmacological evaluation and computational studies of some novel hydrazine derivatives of thiophene chalcone as anti-microbial and antioxidant agents. World J Pharm Res. 2014;3:3146-3159. [ Links ]
10. el-Sabbagh OI, Rady HM. Synthesis of new acridines and hydrazones derived from cyclic |3-diketone for cytotoxic and antiviral evaluation. Eur J Med Chem. 2009 Sep;44(9):3680-3686. https://doi.org/10.1016/j.ejmech.2009.04.001 [ Links ]
11. Waldman SD. Antidepressants. In: Waldman SD, editor. Pain Review. Philadelphia: WB Saunders, 2009, p. 640-646. https://doi.org/10.1016/B978-1-4160-5893-9.00345-2 [ Links ]
12. Kausar N, Murtaza S, Arshad MN, Rashid R, Asiri AM, Javid N, Asim MH, Ashraf Z. Synthesis, characterisation, biological evaluation and molecular docking studies of N-functionalized derivatives of 2-aminobenzohydrazide. J Mol Struct. 2020;1210:128042. https://doi.org/10.1016/j.molstruc.2020.128042 [ Links ]
13. Nisa MU, Munawar MA, Iqbal A, Ahmed A, Ashraf M, Gardener QA, Khan MA. Synthesis of novel 5-(aroylhydrazinocarbonyl)escitalopram as cholinesterase inhibitors. Eur J Med Chem. 2017 Sep 29;138:396-406. https://doi.org/10.1016/j.ejmech.2017.06.036 [ Links ]
14. Saravanan G, Alagarsamy V, Prakash CR. Synthesis, analgesic, anti-inflammatory and ulcerogenic properties of some novel N'-((1-(substituted amino)methyl)-2-oxoindolin-3-ylidene)-4-(2-(methyl/phenyl)-4-oxoquinazolin-3(4H)-yl)benzohydrazide derivatives. Drug Discov Ther. 2012 Apr;6(2):78-87. https://doi.org/10.5582/ddt.2012.v6.278 [ Links ]
15. S. Apostolov, D. Vastag, B. Matijevic J. Nakomcic, A. Marinkovic. Studying retention behavior lipophilicity and pharmacokinetic characteristics of N-substituted Phenyl-2-chloroacetamides. Contemp Mater. 2014;1:101-110. https://doi.org/10.7251/COMEN1401101A [ Links ]
16. Berest GG, Voskoboynik OY, Kovalenko SI, Antypenko OM, Nosulenko IS, Katsev AM, Shandrovskaya OS. Synthesis and biological activity of novel N-cycloalkyl-(cycloalkylaryl)-2-[(3-R-2-oxo-2H-[1,2,4]triazino[2,3-c] quinazoline-6-yl)thio]acetamides. Eur J Med Chem. 2011;46(12):6066-6074. https://doi.org/10.1016/j.ejmech.2011.10.022 [ Links ]
17. Vastag G, Apostolov S, Matijevic B. Prediction of lipophilicity and pharmacokinetics of chloroacetamides by chemometric approach. Iran J Pharm Res. 2018;17(1):100-114. [ Links ]
18. Demir Özkay Ü, Özkay Y, Can ÖD. Synthesis and analgesic effects of 2-(2-carboxyphenylsulfanyl)-N-(4-substitutedphenyl) acetamide derivatives. Med Chem Res. 2011;20(2):152-157. https://doi.org/10.1007/s00044-010-9300-y [ Links ]
19. Ertan T, Yildiz I, Ozkan S, Temiz-Arpaci O, Kaynak F, Yalcin I, Aki-Sener E, Abbasoglu U. Synthesis and biological evaluation of new N-(2-hydroxy-4(or 5)-nitro/aminophenyl)benzamides and phenylacetamides as antimicrobial agents. Bioorg Med Chem. 2007 Mar 1;15(5):2032-2044. https://doi.org/10.1016/j.bmc.2006.12.035 [ Links ]
20. Bogdanovic A, Lazic A, Grujic S, Dimkic I, Stankovic S, Petrovic S. Characterisation of twelve newly synthesised N-(substituted phenyl)-2-chloroacetamides with QSAR analysis and antimicrobial activity tests. Arh Hig Rada Toksikol. 2021;72(1):70-79. https://doi.org/10.2478/aiht-2021-72-3483 [ Links ]
21. Jawed H, Shah SUA, Jamall S, Simjee SU. N-(2-hydroxy phenyl) acetamide inhibits inflammation-related cytokines and ROS in adjuvant-induced arthritic (AIA) rats. Int Immunopharmacol. 2010;10(8):900-905. https://doi.org/10.1016/j.intimp.2010.04.028 [ Links ]
22. Kaldrikyan MA, Grigoryan LA, Melik-Ogandzhanyan RG, Arsenyan FG. Synthesis and antitumor activity of some benzofuryl-substituted 1,2,4-triazoles. Pharm Chem J. 2009;43(5):242-244. https://doi.org/10.1007/s11094-009-0287-y [ Links ]
23. Hirashima A, Yoshii Y, Eto M. Synthesis and biological activity of 2-aminothiazolines and 2-mercaptothiazolines as octopaminergic agonists. Agric Biol Chem. 1991;55:2537-2545. [ Links ]
24. Okamoto H, Kato S, Kobutani T, Ogasawara M, Konnai M, Takematsu T. Herbicidally active N-(1-arylethenyl)-2-chloroacetamides bearing an alkyloxyalkyl moiety. Agric Biol Chem. 1991;55(11):2737-2743. https://doi.org/10.1271/bbb1961.55.2737 [ Links ]
25. Zaragoza F, Stephensen H. Solid-Phase Synthesis of substituted 4-scyl-1,2,3,4-tetrahydroquinoxalin-2-ones. J Org Chem. 1999;64(7):2555-2557. https://doi.org/10.1021/jo982070i [ Links ]
26. Sirasani G, Andrade RB. Sequential one-pot cyclizations: concise access to the ABCE tetracyclic framework of strychnos alkaloids. Org Lett. 2009;11(10):2085-2088. https://doi.org/10.1021/ol9004799 [ Links ]
27. Kaoudi T, Miranda LD, Zard SZ. An easy entry into berbane and alloyohimbane alkaloids via a 6-exo radical cyclization. Org Lett. 2001 Oct 4;3(20):3125-3127. https://doi.org/10.1021/ol016424v [ Links ]
28. Evans MJ, Cravatt BF. Mechanism-based profiling of enzyme families. Chem Rev. 2006 Aug;106(8):3279-3301. https://doi.org/10.1021/cr050288g [ Links ]
29. Shaul M, Abourbeh G, Jacobson O, Rozen Y, Laky D, Levitzki A, Mishani E. Novel iodine-124 labeled EGFR inhibitors as potential PET agents for molecular imaging in cancer. Bioorg Med Chem. 2004;12(13):3421-3429. https://doi.org/10.1016/j.bmc.2004.04.044 [ Links ]
30. Abdel-Latif E, Fahad MM, El-Demerdash A, Ismail MA. Synthesis and biological evaluation of some heterocyclic scaffolds based on the multifunctional N-(4-acetylphenyl)-2-chloroacetamide. J Heterocycl Chem. 2020;57(8):3071-3081. https://doi.org/10.1002/jhet.4012 [ Links ]
31. Pattenden G, Urch CJ. Alcohols, halogeno-compounds, and ethers. In: Pattenden, G, editor. General and synthetic methods. Volume 12. London: Royal Society of Chemistry 1987;203-248. https://doi.org/10.1039/9781847556240 [ Links ]
32. Lakouraj MM, Movassagh B, Fadaei Z. Synthesis of organic sulfides via Zn/ AlCl3 system in aqueous media. Synth Commun. 2002;32(8):1237-1242. https://doi.org/10.1081/SCC-120003615 [ Links ]
33. Movassagh B, Lakouraj MM, Fadaei Z. Synthesis of organic sulfides from disulfides using a Zn/AlCl3 system in aqueous media. J Chem Res. 2000;2000(7):350-351. [S]. https://doi.org/10.3184/030823400103167615 [ Links ]
34. Takagi K. A facile synthesis of sulfides using S-aryl-isothiuronium intermediates. Chem Lett. 1986;15(8):1379-1380. https://doi.org/10.1246/cl.1986.1379 [ Links ]
35. Degani I, Fochi R, Regondi V. S,S-Dialkyl dithiocarbonates as a convenient source of alkanethiolate anions in phase-transfer-catalysis systems: an improved synthesis of organic sulfides. Synthesis. 1983;1983(8):630-632. https://doi.org/10.1055/s-1983-30450 [ Links ]
36. Ando W, Furuhata T, Tsumaki H, Sekiguchi A. Useful application of thiosilanes for preparation of symmetrical and unsymmetrical dialkyl sulfides. Synth Commun. 1982;12(8):627-631. https://doi.org/10.1080/00397918208061893 [ Links ]
37. Movassagh B, Shaygan P. Michael addition of thiols to a,|3-unsaturated carbonyl compounds under solvent-free conditions. ARKIVOC. 2006;7(12):130-137. https://doi.org/10.3998/ark.5550190.0007.c15 [ Links ]
38. Zaragoza F. (Cyanomethyl) trimethylphosphonium iodide as reagent for the intermolecular S-alkylation of thiols with alcohols. Tetrahedron. 2001;57(25):5451-5454. https://doi.org/10.1016/S0040-4020(01)00447-1 [ Links ]
39. Corma A, González-Arellano C, Iglesias M, Sánchez F. Efficient synthesis of vinyl and alkyl sulfides via hydrothiolation of alkynes and electron-deficient olefins using soluble and heterogenized gold complexes catalysts. Appl Catal A Gen. 2010;375(1):49-54. https://doi.org/10.1016/j.apcata.2009.12.016 [ Links ]
40. Banerjee S, Das J, Santra S. Native silica nanoparticle catalyzed anti-Markovnikov addition of thiols to inactivated alkenes and alkynes: a new route to linear and vinyl thioethers. Tetrahedron Lett. 2009;50(1):124-127. https://doi.org/10.1016/j.tetlet.2008.10.110 [ Links ]
41. Movassagh B, Soleiman-Beigi M. Synthesis of sulfides under solvent-and catalyst-free conditions. Monatsh Chem. 2009;140(4):409-411. https://doi.org/10.1007/s00706-008-0043-0 [ Links ]
42. Manarin F, Roehrs JA, Prigol M, Alves D, Nogueira CW, Zeni G. Regioand stereoselective synthesis of vinyl sulfides via PhSeBr-catalyzed hydrothiolation of alkynes. Tetrahedron Lett. 2007;48(28):4805-4808. https://doi.org/10.1016/j.tetlet.2007.05.076 [ Links ]
43. Ananikov VP, Orlov NV, Beletskaya IP. Efficient and convenient synthesis of p-vinyl sulfides in nickel-catalyzed regioselective addition of thiols to terminal alkynes under solvent-free conditions. Organometallics. 2006;25(8):1970-1977. https://doi.org/10.1021/om051105j [ Links ]
44. Schneider CC, Godoi B, Prigol M, Nogueira CW, Zeni G. Highly stereoselective one-pot procedure to prepare unsymmetrical bis-and tris-chalcogenide alkenes via addition of chalcogens to alkynes. Organometallics. 2007;26(17):4252-4256. https://doi.org/10.1021/om070229o [ Links ]
45. Ahmed WS, Mahmood AAR, Al-Bayati RI. Synthesis and evaluation of anti-microbial activity of new imides and Schift bases derived from ethyl-4-amino benzoate. Orient J Chem. 2018;34:2477-2486. https://doi.org/10.13005/ojc/340533 [ Links ]
Received 31 January 2022
Revised 22 June 2022
Accepted 12 August 2022
* To whom correspondence should be addressed Email: ehabattia00@gmx.net














