Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.76 Durban 2022
http://dx.doi.org/10.17159/0379-4350/2022/v76a08
RESEARCH ARTICLE
Comparison of laser nephelometric and HPLC techniques for efficient determination of solubility of ibuprofen and its 2-hydroxypropyl-β-cyclodextrin inclusion complex
Frank SsengoobaI, * ; Thashree MarimuthuIII; Eugene Ivan OlivierI; Patrick Hulisani DemanaII; Yahya E. ChoonaraIII
IDepartment of Pharmaceutical Sciences, Tshwane University of Technology, Pretoria, South Africa
IISchool of Pharmacy, Sefako Makgatho Health Sciences University, Ga-Rankuwa, Pretoria, South Africa
IIIWits Advanced Drug Delivery Platform Research Unit, Department of Pharmacy and Pharmacology, School of Therapeutic Sciences, University of the Witwatersrand, Parktown, South Africa
ABSTRACT
Poor drug solubility is a major problem that hinders the discovery and development of new drugs. There is a need for rapid and inexpensive techniques for acquiring reliable drug solubility data. In this study, the suitability of laser nephelometry for the determination of solubility was investigated using ibuprofen, as a model drug of low solubility, and its 2-hydroxypropyl-p-cyclodextrin inclusion complex (ic). This investigation involved the preparation of ibuprofen-ic-cyclodextrin (1:1) using coprecipitation and characterisation. Thermal analysis and assessment of molecular vibrations confirmed the existence of the inclusion complex. The shake flask testing method was employed and carried out in acidic and alkaline media, and solubility data were verified against high-performance liquid chromatography (HPLC). Results obtained via nephelometry showed relative enhanced solubility of ibuprofen in both acidic (0.565 mg ml-1) and basic (7.5 mg ml-1) media, respectively, which was enabled via inclusion complex formation. Relative to nephelometry data, HPLC results showed a similar trend with increased solubility values in acidic (0.454 mg ml-1) and basic (201.73 mg ml-1) media, respectively. The application of laser nephelometry proved to be a quick and relatively cost-effective technique for solubility measurements of the parent drug and its carrier system.
Keywords: cyclodextrin, ibuprofen, inclusion complex, laser nephelometry, solubility
INTRODUCTION
There is a pressing need to develop new drugs, especially for new diseases and those that are currently difficult to treat, such as cancer. The non-judicious use of antibiotics has led to antibiotic resistance, increasing the need to discover and develop new drugs.1-2 Large chemical libraries for hits and leads have been generated via combinatorial chemistry and automated screening methods.3 However, these approaches based on identifying active biological targets have resulted in drug candidates that are poorly water-soluble.4 A number of pharmacokinetic parameters are evaluated to understand the behaviour of lead molecules during drug discovery and development.5 Most importantly, these parameters are used to select candidates with ideal physical characteristics that could be developed into drugs.6 Amongst the physicochemical based criteria, aqueous solubility is important as it can direct drug absorption, contribute to bioavailability7 and is a key parameter used in the developmental process to categorise lead active pharmaceutical ingredients (API's) according to the Biopharmaceutics Classification System (BSC).8 Furthermore, almost 50% of newly discovered leads taken to the pre-formulation stages have poor aqueous solubility and low oral bioavailability.9-10 Moreover, an increasing number of compounds in the pipeline are representative of BSC Class II that possess high permeability coupled with low solubility11 It is therefore important for formulation scientists to devise techniques that could improve drug solubility.
The complexation of BCS class II drugs with cyclodextrins are well documented.12-14 Cyclodextrins are relatively safe natural oligosaccharides. These oligosaccharides are formed by transglycosylation during the process of starch degradation.15
Cyclodextrins are characterised by an inner lipophilic cavity with an outer hydrophilic surface that enables the preparation of inclusion complexes with several reported lipophilic drug molecules.16-17 Consequently, solubility measurements are also required during drug development and pre-formulation.
Owing to its importance, solubility must be determined early during drug discovery and development process. High-performance liquid chromatography (HPLC) is a conventional method to determine drug solubility.18-19 However, this method is expensive, labour intensive, and requires highly skilled personnel.20 Alternative methods for acquiring drug solubility data that allow for rapid analysis to reduce the possibility of formulation failure and wastage of resources are required.21-22
Laser nephelometry has been reported as one of the alternative techniques that can determine drug solubility.23-25 A nephelometer works on the principle that the intensity of light of scattered particles in suspension is indirectly used to calculate the number or the concentration of the particles in solution.23 Despite its proposed advantages over currently used techniques, limited information has been reported about screening the solubility of drugs with a nephelometer. Further, no literature could be identified that quantified increased drug solubility due to cyclodextrin complexation using laser nephelometry.
A comparative solubility analysis of laser nephelometer and HPLC was performed in this study for ibuprofen, a BSC class II, non-steroidal anti-inflammatory drug and its 2-hydroxypropyl-P-cyclodextrin (HPßCD) complex to investigate the applicability of laser nephelometry in determining drug solubility.
METHODS
Materials
Ibuprofen (>98), HPßCD (Lot number 090M0140V), reagents, and buffer salts were of analytical grade and were procured from Sigma-Aldrich.
Synthesis of Ibuprofen -ic- HPßCD
Initially, the HPßCD solution was prepared (6.65 g; 20 ml water) and heated at 75 °C (10 min) to ensure complete dissolution. A coprecipitation method was then adapted for the study and involved the dropwise addition of ibuprofen solution (1.0 g; 2.5 ml acetone) to a stirring solution of HPBCD (6.65 g; 20 ml water), and after which the solutions were stirred vigorously, at 75 °C until complete solvent evaporation.26 The resulting powder was then screened through a 212 μιη aperture sieve (Labotec test sieve, Johannesburg, South Africa) and stored in an amber glass bottle at 25 °C for further analysis.
Physical mixture of ibuprofen and HPßCD
A mortar was charged with 1:1 ibuprofen: HPβΟ) and mixed using a pestle for 10 mins. The resulting physical mixture was sieved through a 212 μιη screen and remixed. It was stored in an amber glass bottle at 25 °C for further analysis.
Characterisation
Four samples were characterised: neat ibuprofen, ibuprofen-HPßCD complex, physical mixture and neat HPβΟ). Differential Scanning Calorimetry (DSC) studies were recorded on a Shimadzu 60-A model calorimeter (Shimadzu, Kyoto, Japan). The samples (2-5 mg) were heated from 20 to 500 °C at 10 °C min-1 under constant nitrogen flow in aluminium pans. Thermograms were referenced against an empty aluminium pan. Infrared spectra (4000 to 550 cm-1) were acquired on the Alpha-P Bruker FTIR spectrometer (Bruker OPTIK GmbH, Ettlingen, Germany) mounted with an ATR diamond crystal and analysed using OPUS 6.5 software.
Assessment of solubility via nephelometric methods
Solubility was determined using a nephelometer (BMG LABTECH NEPHELOstar Galaxy, Ortenburg, Germany) as per the method established by Bevan and Lloyd. 23 Two methods were derived for the measurement of solubility of the drug and the inclusion complex after serial dilution. Based on previous reports23-27, a low solubility method was employed for neat ibuprofen diluted solutions, while a modified high solubility method 28 was employed for the complex (ibuprofen -ic- HPßCD).
A working stock solution of ibuprofen (30 mg ml-1; 100% DMSO) was prepared and sonicated for 25 mins. The stock solution was then serially diluted with pure DMSO and PBS (pH 7.4) in a Costar® 96-Well plate to generate concentrations for the nephelometric graphs in the range of 30-0.15 mg ml-1. A stock solution (10 mg ml-1 1% DMSO/PBS) was prepared for the complex. Serial dilution was performed to generate concentrations ranging from 10-0.01 mg ml-1. The resulting diluted solutions were analysed at ambient temperature, and nephelometric readings were taken every 1.5 hours over 24 hours.
Assessment of solubility via HPLC methods
Ibuprofen and complex in acidic medium
For each sample, ibuprofen (200 mg) and the complex (400 mg) were weighed, and 4 ml of PBS (pH 2.5) was pipetted into the flask. The resulting suspensions were then shaken for 48 hours at 25±2 °C at 120 rpm until saturation was achieved. After that, samples were subjected to filtration using a 0.45 μm MS® nylon syringe filter and then analysed.
Ibuprofen and complex in alkaline medium
A similar method was employed as per the analysis in the acidic medium, with the exception that ibuprofen (200 mg) and complex (1000 mg) were respectively weighed and 4 ml (0.1M PBS; pH 7.4) was pipetted into the flask to form the suspensions.
Instrumentation and chromatographic conditions
Experiments were performed on a Waters™ HPLC system (New York, USA) comprising of a model 600E System Controller, 717 Plus Autosampler and 486 Absorbance Detector. The stationary phase was a reverse-phase Gemini 5μ C18 110A (250 χ 4.60 mm) column (Phenomenex, Randburg, South Africa).
For the acidic medium, elution (1 ml/min) with mobile phase (20 mM phosphate buffer; pH 2.5) and acetonitrile (250:750 %v/v) at 230 nm was executed.29 For the alkaline medium, the mobile phase comprised of 60:40 acetonitrile:0.1M phosphate buffer (pH 7.4) and isocratic elution (0.8 ml min-1) at 260 nm was carried out.30 Two runs were performed on day 0, followed by a single run on days 1 and 3 for both samples. Solubility was calculated as the average of these runs. HPLC data were recorded and stored using Clarity software version 5.0.3.180 (DataApex, Kent, United Kingdom). HPLC results obtained were then analysed from which standard curves were plotted, and values were expressed as means ± SD. The methods were revalidated.
Ibuprofen and complex standard curve preparation Standard curves were generated by preparing a 4 mg ml-1 stock solution of ibuprofen and its complex in both media (acidic and alkaline) in acetonitrile. Serial dilutions were then prepared to generate standard curves ranging from 0.004 to 3.6 mg ml-1.
Ibuprofen and complex sample preparation
For ibuprofen, a 1 ml sample (both acidic and alkaline media) was directly analysed by HPLC. For the complex in the acidic medium, the sample was diluted to prepare a 50:50 %v/v acetonitrile and analysed. For the complex in the alkaline medium, 1 ml of the filtrate was diluted 100 with acetonitrile and analysed by HPLC. Solubility determinations were performed in triplicate on three different days.
RESULTS AND DISCUSSION
The coprecipitation method was successfully carried out and yielded ibuprofen-ic-HPßCD (Figure 1A), which was subsequently characterised by thermal analysis and FTIR spectroscopy. After that, dissolution experiments were carried out for pristine and complexed ibuprofen. Figure 1(B-C) shows digital images from the dissolution experiment of pristine ibuprofen and ibuprofen-ic-HPßCD, respectively, in acidic media. The observations were indicative of the poor dissolution of ibuprofen and the complex when dissolved in the acidic medium (n = 3).
DSC analysis
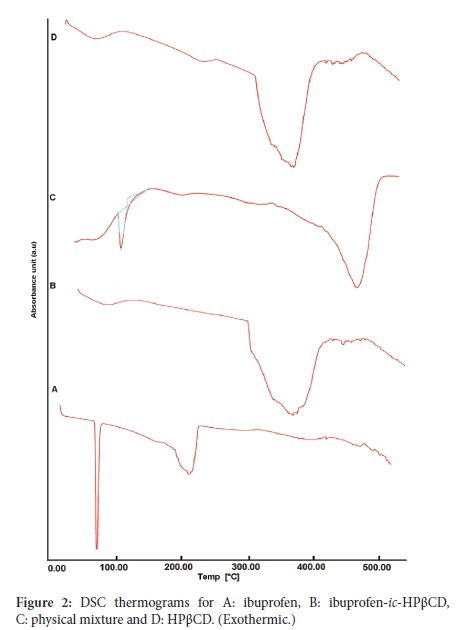
The thermogram for neat ibuprofen displayed a melting endotherm at 78 °C, indicative of the inherent crystallinity of the neat drug (Figure 2A). The complete formation of ibuprofen-ic-HPßCD was evident by the absence of the characteristic melting endotherm (Figure 2B). Similar observations have been found for ibuprofenic-HPßCD) formulations.31 The thermogram of pristine HPßCD displayed a broad endotherm indicative of dehydration (Figure 2D). A similar peak of dehydration was displayed in (Figure 2B). Relative to Figure 2A, a similar melting endotherm of relatively lower intensity was depicted for the physical mixture (Figure 2C). This lowering of the endotherm can be attributed to a dilution effect in the physical mixture (Figure 2C).
FTIR analysis
The formation of ibuprofen-ic-HPßCD was also confirmed by FTIR analysis based on the significant shifting and/or signal attenuation. Figure 3 presents the FTIR spectra of pristine ibuprofen, ibuprofen-HPßCD complex, physical mixture and pristine HPßCD. Significant changes were depicted in the 1500-1700 cm-1 range (Figure 3). Relative to the strong C=O band at 1660 cm-1 for the pristine drug (Figure 3A), Figure 3B displayed the C=O band that was greatly reduced in intensity and slightly shifted to a higher wavenumber which supported the formation of ibuprofen-zc-HPßCD.
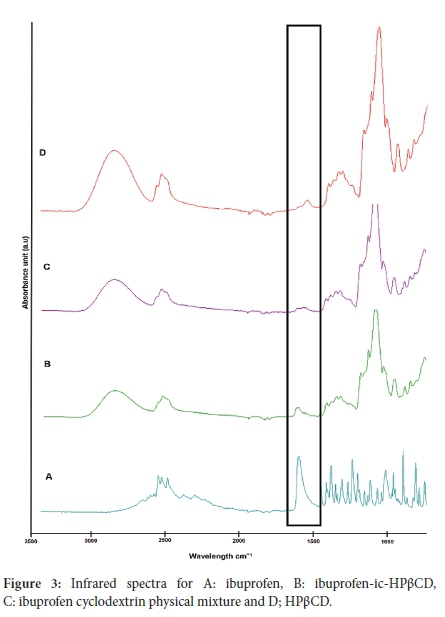
The minor shift depicted for C=O indicated interactions between carbonyl groups in ibuprofen and hydroxyl groups in HPßCD owing to intermolecular hydrogen bonding. These interactions are in agreement with the studies performed by Nazan and Begum, who reported similar shifts in ibuprofen peaks due to interaction with -cyclodextrins.32 A weaker stretching vibration at ~1500 cm-1 was indicative of C=C (phenyl ring) stretching for pristine ibuprofen (Figure 3A), and similar changes to reduced intensity were observed for the inclusion complex (Figure 3B), which further supported the inclusion of ibuprofen within the cavity of HPßCD. In Figure 3D, broad O-H stretching between 3000 and 3600 cm-1 were ascribed to the hydroxyl moieties on the pristine HPßCD units. Similar molecular vibrations were expected and observed for the inclusion complex (Figure 3B). However, due to the overlapping bands in the region of 1000 to 1200 cm-1, there are no characteristics bands that support the formation of ibuprofen-zc-HPßCD. The FTIR spectrum of the physical mixture of ibuprofen (Figure 3C) reflects the mixture of ibuprofen and HPßCD. Overall FTIR analysis confirmed that ibuprofen-zc-HPßCD was successfully prepared.
Determination of solubility using Laser Nephelometry
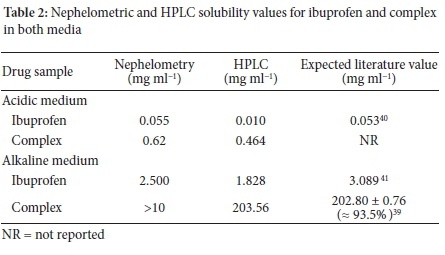
The solubility of ibuprofen in the acidic medium was 0.055 mg ml-1 over 24 h (Figure 4A and B). In addition, the graph displayed an erratic pattern in the graphs plotted at different time intervals.
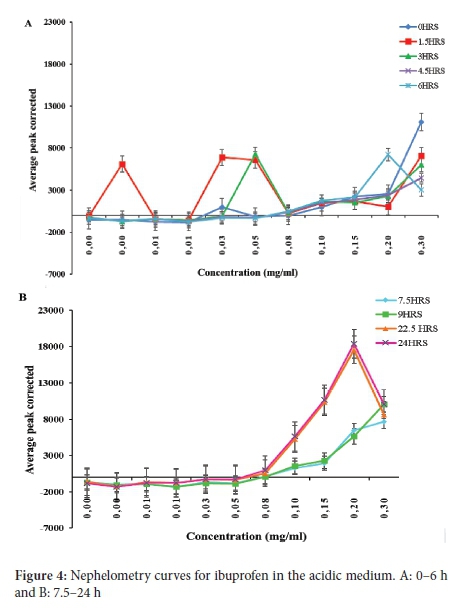
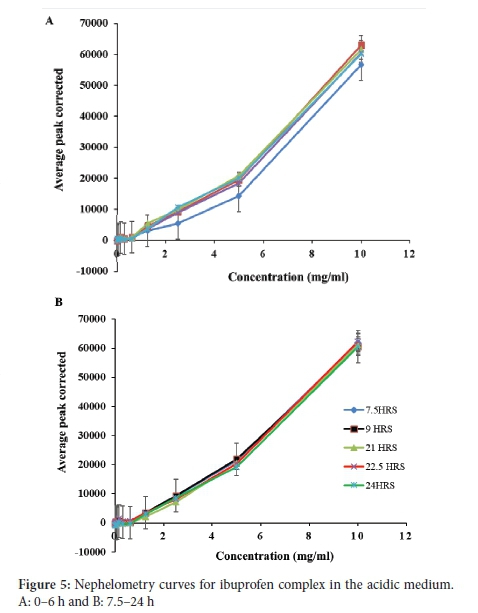
The solubility of the ibuprofen-HPßCD complex was found to be 0.620 mg ml-1 (Figure 5). This value on the graph represents the highest rise in solubility of the drug over 24 h. Figure 6 also displayed a similar and uniform pattern for graphs plotted at different time intervals. The unpredictable pattern of the solubility graph for ibuprofen in the acidic medium is because ibuprofen is an acidic drug. In the acidic medium, it exhibits poor and erratic solubility.33 In the acidic solution, the ibuprofen molecules remain neutral, and little ionisation occurs. Moreover, this is supported by the Henderson-Hasselbach equation, where it has been established that the degree of ionisation of an acidic drug is dependent on the pH of the dissolution medium and the pKa of the drug. In the acidic medium, the solubility of ibuprofen was found to be 0.055 mg ml-1 (Figure 5). These values are in good agreement with the value reported in the literature of 0.056 mg ml-1 at 25 °C for ibuprofen.34 Moreover, ibuprofen is described as practically insoluble in water.35 The value obtained in this study is < 0.1 mg ml-1, which is in agreement with that assigned by Stegemann et al. for a drug defined as practically insoluble.10 The solubility of the complex in acidic medium was 0.62 mg ml-1 (Figure 5A and B), which indicated a high rise in solubility congruent to the solubility point of the drug, the point at which the drug precipitates out of solution.36 This value is higher than that obtained for ibuprofen in the acidic medium as a result of complexation with cyclodextrin. Complexation increased the solubility of ibuprofen 12-fold in comparison to the drug alone. Moreover, complexation increased ibuprofen's stability, which is evident by the similar and uniform pattern displayed by the graphs plotted at different time intervals.37 This further signifies that due to the increased stability of the ibuprofen by complexation, the same amount of the drug was dissolving at different time intervals.38
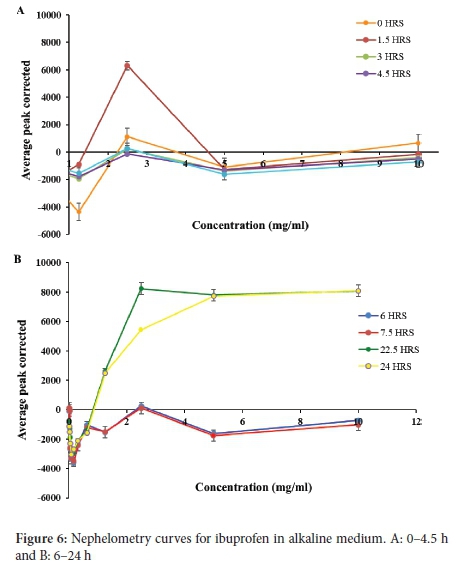
In the alkaline medium, the solubility of ibuprofen was found to be 2.500 mg ml-1 (Figure 6A and B). The graphs for solubility measured at different time intervals displayed a similar pattern with an initial exponential increase in solubility up to 2.5 mg ml-1 and a uniform decrease up to 5 mg ml-1 for all graphs. The value of ibuprofen obtained in the alkaline medium is 45 times higher than that of the drug in the acidic medium, indicating that the solubility of ibuprofen, an acidic drug is higher in the alkaline medium than in the acidic medium. This solubility is because, at high pH values (alkaline), a weak acidic drug such as ibuprofen undergoes deprotonation and is ionised, creating negative ions responsible for its increased solubility.
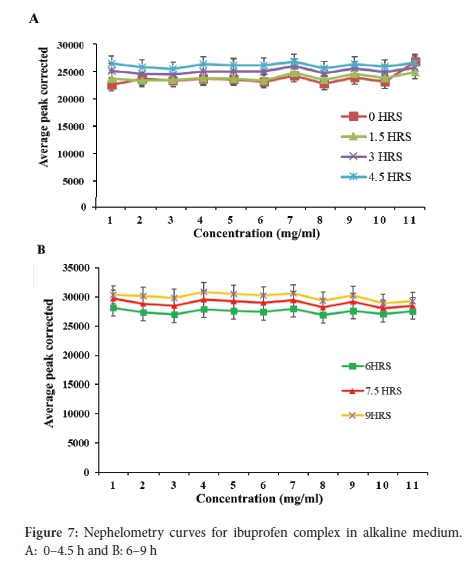
The ibuprofen-HPßCD complex in alkaline medium (Figure 7A and B) graphs over a period of 24 hours displayed straight lines without any visible increase in solubility. The graphs also displayed a similar pattern throughout the 24 hours, indicating that the stock solution's solubility was >10 mg ml-1. The straight lines without any visible increase in solubility observed for ibuprofen-HPßCD complex in alkaline medium (Figure 7) indicate that the solubility of the ibuprofen complex is greater than the concentration of the original stock solution (10 mg ml-1) used for the study. Considering ibuprofen is an acidic drug, it displays a high solubility in the alkaline medium as a result of ionisation. Moreover, complexing it with HPßCD significantly increased its solubility. Therefore, ionisation and complexation of ibuprofen are responsible for the synergistic increase in solubility of ibuprofen in the alkaline medium. The drug was fully soluble at all concentrations, including the original stock solution concentration. In order to quantify the solubility of ibuprofen complex, a stock solution greater than 10 mg ml-1 would have to be prepared. Similarly, Celebioglu et al. reported that the solubility of ibuprofen increased after complexation with 2-hydroxypropyl beta cyclodextrin.31
Determination of solubility via HPLC methods
The samples were analysed in triplicates for the drug and complex using HPLC. Four runs were performed for each sample (n = 4). The amount of the dissolved drug was then calculated from the standard curve using the line equation (Table 1). See supplementary information for HPLC calibration data.
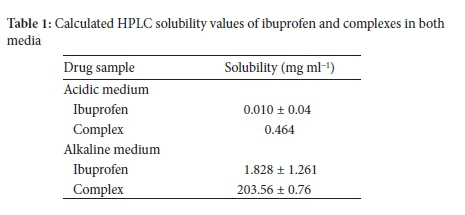
The solubility of ibuprofen in the acidic medium was 0.010 mg ml-1. This value is close to that obtained by nephelometry and is similar to that reported in the literature, which describes ibuprofen as a practically insoluble drug. The solubility of the complex in the acidic medium is close to that obtained by nephelometry and indicates a 46-fold increase in solubility relative to the parent drug owing to complexation. The solubility of ibuprofen in the alkaline medium was predictably greater than that obtained in the acidic medium, indicating that ibuprofen (an acidic drug) would be ionised in the alkaline medium exhibiting a higher solubility relative to dissolution in the acidic medium. The solubility value of the complex in alkaline medium was the highest obtained, indicating a synergistic increase of ionisation and complexation. This value corresponds to the result reported by Silberberg. He found the solubility of ibuprofen to have increased in the alkaline medium as a result of complexation.39
Comparison of nephelometric and HPLC methods
In comparison, there was a general correlation between the results obtained by nephelometry and HPLC analysis for all drugs (Table 2). The formation of the inclusion complex led to a significant increase in the solubility of ibuprofen. Using the trendline equation, the solubility of ibuprofen and its complex in the acidic medium were 0.010 and 0.464 mg ml-1, respectively. Additionally, the calculated values for ibuprofen and complex in the alkaline medium were 1.828 and 203.56 mg ml-1, respectively.
CONCLUSION
Numerous studies documented the application of cyclodextrins to increase the solubility of ibuprofen. However, limited papers provide quantified values of this solubility. Moreover, none has been reported on measuring the solubility of complexed systems utilising nephelometry. The results obtained from this study indicate that laser nephelometry provides a rapid, simple and cost-effective method that could be employed as an alternative method to determine the drug solubility early in drug development and in pre-formulation stages.
CONFLICT OF INTERESTS
Authors have no conflict of interest to declare.
ACKNOWLEDGEMENTS
Financial support through the Incentive Funding for Rated Researchers (IPRR) Programme (Reference number: IFR2011041200016) of the South African National Research Foundation (NRF) and the Tshwane University of Technology is recognised by the authors.
SUPPLEMENTARY MATERIAL
Supplementary information for this article is provided in the online supplement.
ORCID IDs
Frank Ssengooba - https://orcid.org/0000-0002-6861-0687
Thashree Marimuthua - https://orcid.org/0000-0003-1487-5273
Eugene Ivan Oliviera - https://orcid.org/0000-0002-1949-2493
Patrick Hulisani Demanaa - https://orcid.org/0000-0001-6359-0499
Yahya E. Choonaraa - https://orcid.org/0000-0002-3889-1529
REFERENCES
1. Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Nisar MA, Alvi RF, Aslam MA, Qamar MU, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645-1658. https://doi.org/10.2147/IDR.S173867. [ Links ]
2. Mohs RC, Greig NH. Drug discovery and development: role of basic biological research. Alzheimer's & Dementia: Transl. Res. Clin. Interv. 2017;3(4):651-657. https://doi.org/10.1016/j.trci.2017.10.005. [ Links ]
3. Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, Porter CJH. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65(1):315-499. https://doi.org/10.1124/pr.112.005660. [ Links ]
4. Dahlin JL, Walters MA. The essential roles of chemistry in high-throughput screening triage. Future Med Chem. 2014;6(11):1265-1290. https://doi.org/10.4155/fmc.14.60. [ Links ]
5. Dunnington K, Benrimoh N, Brandquist C, Cardillo-Marricco N, Di Spirito M, Grenier J. Pharmacokinetics and adverse effects of drugs-mechanisms and risks factors. In: N. Malangu, editor. Application of pharmacokinetics in early drug development, London: IntechOpen, 2018; pp 5-75. [ Links ]
6. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3-26. https://doi.org/10.1016/S0169-409X(00)00129-0. [ Links ]
7. Kerns EH. High throughput physicochemical profiling for drug discovery. J Pharm Sci. 2001;90(11):1838-1858. https://doi.org/10.1002/jps.1134. [ Links ]
8. Hoelke B, Gieringer S, Arlt M, Saal C. Comparison of nephelometric, UV-spectroscopic, and HPLC bethods for high-throughput determination of aqueous drug solubility in microtiter plates. Anal Chem. 2009;81(8):3165-3172. https://doi.org/10.1021/ac9000089. [ Links ]
9. Boyd BJ, Bergström CAS, Vinarov Z, Kuentz M, Brouwers J, Augustijns P, Brandl M, Bernkop-Schnürch A, Shrestha N, Préat V, et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur J Pharm Sci. 2019;137:104967. https://doi.org/10.1016/j.ejps.2019.104967. [ Links ]
10. Stegemann S, Leveiller F, Franchi D, de Jong H, Lindén H. When poor solubility becomes an issue: from early stage to proof of concept. Eur J Pharm Sci. 2007;31(5):249-261. https://doi.org/10.1016/j.ejps.2007.05.110. [ Links ]
11. Keserü GM, Makara GM. The influence of lead discovery strategies on the properties of drug candidates. Nat Rev Drug Discov. 2009;8(3):203-212. https://doi.org/10.1038/nrd2796. [ Links ]
12. Saokham P, Muankaew C, Jansook P, Loftsson T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules. 2018;23(5):1161. https://doi.org/10.3390/molecules23051161. [ Links ]
13. Felton LA, Popescu C, Wiley C, Esposito EX, Lefevre P, Hopfinger AJ. Experimental and computational studies of physicochemical properties influence NSAID-cyclodextrin complexation. AAPS PharmSciTech. 2014;15(4):872-881. https://doi.org/10.1208/s12249-014-0110-2. [ Links ]
14. Miranda GM, Santos V, Bessa JR, Teles YCF, Yahouédéhou S, Goncalves MS, Ribeiro-Filho J. Inclusion complexes of non-steroidal anti-inflammatory drugs with cyclodextrins: A systematic review. Biomolecules. 2021;11(3):361. https://doi.org/10.3390/biom11030361. [ Links ]
15. Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98(5):1743-1754. https://doi.org/10.1021/cr970022c. [ Links ]
16. Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilisers. Adv Drug Deliv Rev. 2007;59(7):645-666. https://doi.org/10.1016/j.addr.2007.05.012. [ Links ]
17. Loftsson T, Masson M. Cyclodextrins in topical drug formulations: theory and practice. Int J Pharm. 2001;225(1/2):15-30. https://doi.org/10.1016/S0378-5173(01)00761-X. [ Links ]
18. Pan L, Ho Q, Tsutsui K, Takahashi L. Comparison of chromatographic and spectroscopic methods used to rank compounds for aqueous solubility. J Pharm Sci. 2001;90(4):521-529. https://doi.org/10.1002/1520-6017(200104)90:43.0.CO;2-B. [ Links ]
19. Sou T, Bergström CAS. Automated assays for thermodynamic (equilibrium) solubility determination. Drug Discov Today Technol. 2018;27:11-19. https://doi.org/10.1016/j.ddtec.2018.04.004. [ Links ]
20. Dai WG, Pollock-Dove C, Dong LC, Li S. Advanced screening assays to rapidly identify solubility-enhancing formulations: high-throughput, miniaturisation and automation. Adv Drug Deliv Rev. 2008;60(6):657-672. https://doi.org/10.1016/j.addr.2007.10.017. [ Links ]
21. Dehring KA, Workman HL, Miller KD, Mandagere A, Poole SK. Automated robotic liquid handling/laser-based nephelometry system for high throughput measurement of kinetic aqueous solubility. J Pharm Biomed Anal. 2004;36(3):447-456. https://doi.org/10.1016/j.jpba.2004.07.022. [ Links ]
22. Stukelj J, Svanbäck S, Agopov M, Löbmann K, Strachan CJ, Rades T, Yliruusi J. Direct measurement of amorphous solubility. Anal Chem. 2019;91(11):7411-7417. https://doi.org/10.1021/acs.analchem.9b01378. [ Links ]
23. Bevan CD, Lloyd RS. R.S. Lloyd A high-throughput screening method for the determination of aqueous drug solubility using laser nephelometry in microtiter plates. Anal Chem. 2000;72(8):1781-1787. https://doi.org/10.1021/ac9912247. [ Links ]
24. Petereit AC, Saal C. Solubility-What is the solubility of my compound? Assessing solubility for pharmaceutical research and development compounds. Am Pharm Rev. 2011;14(5):68. [ Links ]
25. Saunders KC. Automation and robotics in ADME screening. Drug Discov Today Technol. 2004;1(4):373-380. https://doi.org/10.1016/j.ddtec.2004.11.009. [ Links ]
26. Hedges AR. Industrial applications of cyclodextrins. Chem Rev. 1998;98(5):2035-2044. https://doi.org/10.1021/cr970014w. [ Links ]
27. Goodwin JJ. Rationale and benefit of using high throughput solubility screens in drug discovery. Drug Discov Today Technol. 2006;3(1):67-71. https://doi.org/10.1016/j.ddtec.2005.03.001. [ Links ]
28. Chen TM, Shen H, Zhu C. Evaluation of a method for high throughput solubility determination using a multi-wavelength UV plate reader. Comb Chem High Throughput Screen. 2002;5(7):575-581. https://doi.org/10.2174/1386207023330075. [ Links ]
29. Kumar SA, Debnath M, Rao JS. D.G. Sankar A new RP-HPLC stability indicating method development and validation for simultaneous estimation of ibuprofen & famotidine in bulk as well in pharmaceutical dosages form by using PDA detector. Int J Pharm Sci Res. 2014;5(9):3829. [ Links ]
30. Jahan MS, Islam MJ, Begum R, Kayesh R. A. Rahman A study of method development, validation, and forced degradation for simultaneous quantification of paracetamol and ibuprofen in pharmaceutical dosage form by RP-HPLC method. Anal Chem Insights. 2014;9:75-81. [ Links ]
31. Celebioglu A, Uyar T. Fast dissolving oral drug delivery system based on electrospun nanofibrous webs of cyclodextrin/ibuprofen inclusion complex nanofibers. Mol Pharm. 2019;16(10):4387-4398. https://doi.org/10.1021/acs.molpharmaceut.9b00798. [ Links ]
32. Nazan S, Begum S. Fabrication and characterisation of supramolecular assembly between ibuprofen and betacyclodextrins. Asian J. Pharm. Sci. 2013;3(1):47-56. [ Links ]
33. Al Omari MM, Daraghmeh NH, El-Barghouthi MI, Zughul MB, Chowdhry BZ, Leharne SA, Badwan AA. Novel inclusion complex of ibuprofen tromethamine with cyclodextrins: physico-chemical characterisation. J Pharm Biomed Anal. 2009;50(3):449-458. https://doi.org/10.1016/j.jpba.2009.05.031. [ Links ]
34. Kocbek P, Baumgartner S, Kristl J. Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs. Int J Pharm. 2006;312(1-2):179-186. https://doi.org/10.1016/j.ijpharm.2006.01.008. [ Links ]
35. Le VN, Leterme P, Gayot A, Flament MP. Influence of granulation and compaction on the particle size of ibuprofen-development of a size analysis method. Int J Pharm. 2006;321(1-2):72-77. https://doi.org/10.1016/j.ijpharm.2006.05.010. [ Links ]
36. Wassvik C. Computational analysis of aqueous drug solubility-Influence of the solid state [Doctoral dissertation]. Acta Universitatis Upsaliensis, Upsala, Sweden. 2006. [ Links ]
37. Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6(2):E329-E357. https://doi.org/10.1208/pt060243. [ Links ]
38. ffiadon T, Pawlaczyk J, Szafran B; T. HLAdoN. J.A.N. Pawlaczyk, B. Szafran Stability of Ibuprofen in its inclusion complex with ß-cyclodextrin. J Incl Phenom Macrocycl Chem. 2000;36(1):1-8. https://doi.org/10.1023/A:1008046724527. [ Links ]
39. Silberberg M. Cyclodextrin as a drug carrier increasing drug solubility. Sci J Lander Coll Arts Sci. 2017;11(1):5. [ Links ]
40. Shaw LR, Irwin WJ, Grattan TJ, Conway BR. The effect of selected water-soluble excipients on the dissolution of paracetamol and ibuprofen. Drug Dev Ind Pharm. 2005;31(6):515-525. https://doi.org/10.1080/03639040500215784. [ Links ]
41. Al Masum MA, Islam SA, Reza MS. Enhancement of solubility and dissolution characteristics of ibuprofen by solid dispersion technique. Dhaka Uni. J Pharm Sci. 2012;11(1):1-6. [ Links ]
Received 14 December 2020
Revised 03 September 2021
Accepted 03 September 2021
* To whom correspondence should be addressed Email: SsengoobaF@tut.ac.za
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














