Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.76 Durban 2022
http://dx.doi.org/10.17159/0379-4350/2022/v76a04
RESEARCH ARTICLE
The analysis of alcohol content in hand sanitisers (in the Durban region) using gas chromatography-mass spectrometry during the COVID-19 pandemic
Kamini GovenderI; Sipho MdandaI; Sooraj BaijnathI; Hendrik Gerhardus KrugerI; Thavendran GovenderII; Tricia NaickerI,*
ICatalysis and Peptide Research Unit, School of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIDepartment of Chemistry, University of Zululand, KwaDlangezwa, South Africa
ABSTRACT
The COVID-19 pandemic has resulted in an unprecedented surge in the demand for alcohol-based hand sanitisers (ABHS). The Centre for Disease Control (CDC) and World Health Organisation (WHO) recommend alcohol, i.e., isopropanol or ethanol, at a 60-95% concentration in ABHS for sufficient antiviral protection. Consumers need to be vigilant of substandard hand sanitisers being marketed to the public. The frequent exposure of microorganisms to alcohol concentrations below the recommended range for infection prevention may lead to resistant mutations, and above the range may be ineffective. Therefore, this study aimed to verify the stated alcohol content in hand sanitisers from their respective labels. We analysed 50 hand sanitiser samples available to our region in Durban, KwaZulu-Natal, South Africa, using a Shimadzu GC-MS-QP2010 Ultra equipped with a Zebron ZB-wax capillary column. The hand sanitisers analysed had a range of 44-93% alcohol content. The data from our study also revealed that 32% (16) of hand sanitisers did not adhere to the stated alcohol indicated on their labels. 16% (8) contained >80% and 12% (6) contained <60%, while 6% (3) of the ABHS contained 1-propanol and ethyl acetate as contaminants, respectively. This study clearly emphasises manufacturers' exploitation of the pandemic and the need for stricter guidelines and regulations for consistency amongst ABHS manufacturers. The public should also be more alert to the % alcohol stated (ideal range 60-80%) on the sanitizer bottle and note one needs to rub their hands together until it feels dry.
Keywords: alcohol-content, ethanol, gas chromatography-mass spectrometry, hand sanitiser, 2-propanol (isopropanol)
INTRODUCTION
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in the COVID-19 pandemic, which has caused unprecedented challenges to the health care systems globally.1 According to the Worldometer stats released on the 14 December 2021 there were 271 379 514 cumulative COVID-19 estimated cases and approximately 5 332 977 deaths globally.2 As of 14 December 2021, South Africa had 3 180 785 COVID-19 estimated cases and approximately 90 148 deaths.2 Respiratory viruses can spread through various routes, including physical contact with infected patients, interaction with contaminated surfaces, and airborne transmission.3 Hands are easily contaminated by microorganisms found in droplets from sneezes, coughs, or direct contact with airborne pathogens.4 As a result, hand hygiene is critical during this pandemic because it can be used as an infection control approach to reduce COVID-19 transmission, both directly and indirectly.4 The current measures set in place to minimize COVID-19 transmission are preventative and supportive.1 One of the fundamental strategies for reducing transmission and contaminating pathogens is hand hygiene.1,5 Washing hands regularly is one of the measures used to reduce the spread of COVID-19.6 However, if soap and water are not available, hand sanitisers provide a suitable alternative since they are readily available, highly versatile, and have a quick and effective application.5 Currently, there is a global surge in the usage of hand sanitisers as a result of the COVID-19 pandemic.7,8 These hand sanitisers can be placed into non-alcohol-based hand sanitisers (NABHS) and ABHS.1,,5The main components of accepted alcohol-based hand sanitisers are isopropanol, ethanol, or a mixture of the alcohol as mentioned above.9-12
According to the WHO and CDC, the ABHSs should contain approximately 60-95% alcohol.1,4,9,13 The inactivation of viruses using alcohol is illustrated in Figure 1. Ideally, commercially available hand sanitisers should comply with the range above to provide effective antimicrobial activity. These hand sanitisers should also be manufactured using good manufacturing practices (GMP) standards.9 A South African Health Products Regulatory Authority (SAHPRA) license is not required for hand sanitisers since they do not contain substances listed in the Medicines and Related Substances Act, 1965 (Act 101 of 1965). Hand sanitisers are, however regulated under the foodstuffs, cosmetics, and disinfectants Act 54 of 1972 as amended (FCD Act), which aims to control the importation of food, disinfectants, and cosmetics as well as control the manufacturing and sale thereof. As such, alcohol-based hand sanitisers must comply with the South African National Standards (SANS) 289 and 490 as well as the Legal Metrology Act 9 of 2014.14 This act aims to provide the necessary maintenance and administration of legal metrology technical regulations to protect public health, the environment and also promote fair trade. However, none of these Acts have stipulated regulations that define the amount or type of alcohol as per the WHO/ CDC recommendation or the manufacturing conditions of hand sanitisers. Due to the crisis of COVID-19, the demand for sanitisers is skyrocketing; however, these products remain unregulated and unregistered manufacturers produce large volumes for consumption. Consumers are currently unprotected from 'fake' sanitisers, and there is no evidence that these hand sanitisers contain safe ingredients in the required concentrations.8
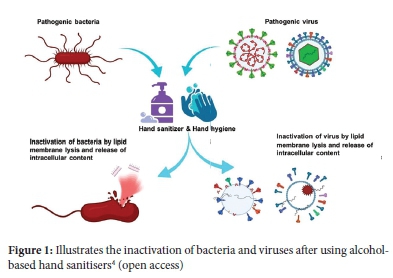
Another concern is that exposure to low alcohol levels in ABHS can increase alcohol tolerance, virulence, and pathogenicity of microorganisms evident in Enterococcus faecium 15 and Acinetobacter bauannii mutants found in hospitals.16 These nosocomial microorganisms are also associated with multi-drug resistance.15, 17 Bacteria acquire alcohol tolerance from gene mutations if coupled with extensive drug resistance this will create booms of robust nosocomial infections and eventually result in the formation of 'superbugs'.17 This was evident with enterococci since they increased the number of nosocomial enterococcal infections in Europe, Australia, and North America. They are adapting and acquiring resistance to alcohol-based hand sanitisers, the leading cause of sepsis.18,19 Additionally, the frequent exposure of microorganisms to alcohol concentrations below the recommended range for infection prevention may lead to resistant mutations.20 The U.S. food and drug administration (FDA) has recently recalled numerous hand sanitisers with immediate effect due to possible methanol contamination.10 Recent studies conducted by Puleng et al. 2021,21 Beradi et al. 2021,13 Timothy et al. 202122 and de Bruin and Korsten 20208 have indicated safety and health concerns regarding hand sanitisers. Another recent study (that was published while our work was under review) conducted by Yusuf 2021 discovered that majority of the commercially available ABHS products (in the Pretoria region) were sub-standard according to WHO recommendations (80±5%)23 for local production of hand rubs.
The rationale of our study is firstly to investigate the amount of the key active ingredients (ethanol or isopropanol) present within the hand sanitisers using gas chromatography mass spectrometric (GC-MS) . Secondly, verify the labels (alcohol content and or other ingredients) of commercially available hand sanitisers as part of a public awareness campaign to determine if the sanitisers meet the aforementioned WHO and CDC requirements to fully inactivate the corona virus.
MATERIALS AND METHODS
Chemicals and GC column
HPLC grade ethanol, isopropanol and acetonitrile were purchased from Merck (Germany). Ultrapure water was obtained from WaterPro PS Polishing Systems (Labconco, USA). 2 mL HPLC glass vials and caps were purchased from Agilent (USA).
Preparation of calibration standards
Stock solutions (10% v/v) of ethanol and isopropanol were prepared, with acetonitrile used as an internal standard (IS); 1 mL of the analyte and 200 ||L of the IS were added to a 10 ml volumetric flask which was made up to the mark using H2O. Ethanol and isopropanol calibration standards were prepared from 0.01-4%, respectively with a 2% internal standard (acetonitrile). Samples were capped and vortexed. All samples were injected in triplicate (n = 3).
Sample preparation
The samples were acquired from public sites and commercially available stores in Durban, South Africa. The sample preparations were conducted according to a similar method from an Agilent application note based on GC of hand sanitisers12. Accordingly, 2 mL HPLC glass vials (Agilent, USA) were utilized, 25 µL of each analyte was added, followed by 200 µL of acetonitrile (IS) and 775 µL of H2O. The samples were all vortexed prior to GC analysis. All samples were injected in triplicate (n = 3).
Gas Chromatography-mass spectrometry (GC-MS) detection conditions
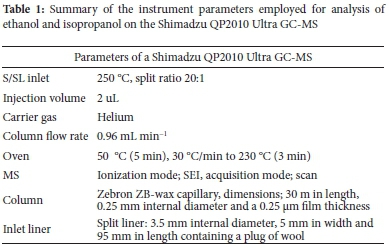
GC-MS was utilised to analyse and quantify alcohol content in hand sanitisers. A GC-MS-QP2010 Ultra (Shimadzu, Japan) equipped with a MS detector and an AOC-20i auto injector (Shimadzu, Japan) coupled to an Edwards E2M1.5 Rotary Vacuum Pump (Edwards, United Kingdom). A Zebron ZB-wax capillary GC column (Phenomenex, USA) was used for GC analysis with the following dimensions 30 m in length, 0.25 mm internal diameter, and a 0.25 µm film thickness. Helium baseline 5.0 was used as a carrier gas (Afrox, South Africa) at a column flow rate of 0.96 mL min-1 and total flow of 23 mL min-1. The ion source temperature was 200 °C; the interface temperature and injection port temperature were both 250 °C. The acquisition mode was set on scan and the ionization mode was standard electron impact ionisation (SEI). The column oven was initially held at a temperature of 50 °C for five minutes thereafter it was increased to 230 °C at a ramping rate of 30 °C min-1 and held at that temperature for 3 minutes. The total run time was 14 minutes. The injection volumes were 0.2 µL utilising a 10 µL syringe (Shimadzu, Japan) which was injected into a split/spitless injector. The injection mode was split injection with a split ratio of 20:1 and a split liner with the following dimensions: 3.5 mm internal diameter, 5 mm in width and 95 mm in length containing a plug of wool. The GC analytical parameters are displayed in Table 1.
Data acquisition and quantitation
The quantitation and data acquisition were conducted using the GCMS solution version 4.45 software. A correlation coefficient (r2) of greater than 0.999 was obtained for both the ethanol and the isopropanol calibration curves.
Data analysis
Results were analysed on Microsoft* Excel*. All ABHS samples were analysed in triplicate (n = 3), and the data were represented as the mean ± relative standard deviation (RSD).
RESULTS AND DISCUSSION
The fundamental and accepted constituents in hand sanitisers are ethanol and isopropanol.24 GC is recommended by U.S pharmacopeia to ascertain alcohol concentrations since GC provides qualitative and quantitative analysis of the respective alcohols.24 To ensure the efficacy of hand sanitisers supplied to consumers, the concentrations of the aforementioned alcohols must comply with their commercial labels and WHO as well as CDC recommended alcohol requirements (60-95%).1,13,25 It should be noted that other impurities or alcohols (that are deemed toxic if ingested) can be present in hand sanitisers, resulting in substandard ABHS.24 As such, this study determined the alcohol content in 50 commercial ABHS. The samples represent sanitisers found in Durban, South Africa, either from a commercial source or provided at a mall/shop entrance which has become a mandatory requirement before entry. Calibration curves of ethanol and isopropanol using acetonitrile as the internal standard were conducted on a Zebron ZB-wax capillary GC column. The limits of detection (LODs) for ethanol and isopropanol on the GC were both 0.001% and the limits of quantitation (LOQs) were 0.01%, respectively. The calibration curves concentrations ranged from 0.01%-4% (v/v) Figures 2 and 3).
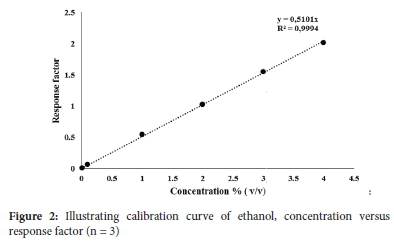
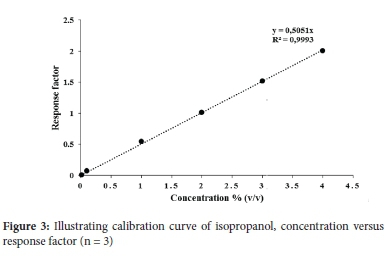
The form in which hand sanitisers are administered also affects its efficiency in killing the Coronavirus.11 Alcohol based hand rubs are found in three forms foam, liquid or gel according to the WHO, an alcohol based hand rub must contain alcohol in order to either suppress or inactivate the growth of microorganisms.25 Eight hand sanitiser samples were manufactured according to the following regulations and guidelines; WHO guidelines and ISO 9001:2015 (Sample 20), SANS 490 and SANS 51276 (sample 25), SANS 5261 for zero bacterial growth (samples 44, 46, 48), WHO and CDC guidelines of recommended 60% ethanol (samples 9, 42). Sample 5 indicated it was food grade approved on its label, and its bacterial efficacy was tested whereby it was capable of killing 99.99% of bacteria in one minute. The samples specifications are displayed in Figure 4 and Table S1 in the supplementary section.
The ABHS comprised of 22 gel and 28 liquid-based hand sanitisers, of which three contained isopropanol and 46 contained ethanol as the key active ingredient, while one contained 1-propanol. Sixteen of the ABHS did not comply with the indicated alcohol percentages on their labels (refer to Figure 4, Figures S1-S4 and Table S1).
The results from this study indicated that the liquid ABHS had a range of 49-93%, and the gel ABHS had approximately 44-87%. A study conducted by Gunter et al., 2010 21 indicated that liquid-based hand sanitisers were more effective than foam and gel-based hand sanitisers since they require short application times (<30 seconds). Gel applications were slow compared to liquid-based hand sanitisers.11,26
The GC results indicated that Sample 23 (gel based hand sanitiser) had only 44% alcohol content. This is of great concern since it is well below the required range for infection control. This was also observed in a study conducted by Berardi et al., 2020, whereby gels contained approximately 40% alcohol and these ABHS should be used for cosmetic use only and not as a biocide.13
In our study, approximately 68% of commercially available hand sanitisers comply with the aforementioned recommended range of alcohol (60-95%). In contrast, the remainder, 32%, did not adhere to the regulations above and alcohol percent indicated on their respective labels (four were above and six were below the specified amounts, refer to Figure 4 and Table S1). Interestingly, this study found that 16% (4 gel and 4 liquid ABHS) contained >80%. None of the ABHS samples analysed in our study contained more than 95% alcohol, which is an imperative finding as above this, would not result in adequate denaturation of the virus. According to a review conducted by Villa and Russo 2021, hand sanitisers require a mixture of water and alcohol as key active ingredients to achieve effective protein denaturation of viruses.5 It should be noted that if the alcohol concentration >95% and water is excluded from the sanitiser it is not efficacious in killing pathogens since water is required for protein denaturation.5,22
Herein this study, the data revealed that 12% (3 gels and 3 liquid ABHS) contained <60%. This is of great concern since a minimum of 60% alcohol is needed to act as a microbicide, virucide, or bactericide.4,10,11 The CDC also recommends a minimum concentration of 60% alcohol in ABHS. In our results (Figure 4), the general trend is that the samples contained around 60% alcohol. If the alcohol content in hand sanitisers is below the aforementioned requirement, this will limit the growth of pathogens instead of killing them.10 As such, these conditions allow for the creation of highly virulent 'superbugs.17,20
The US FDA allows ethanol and isopropanol as the key active ingredients for ABHS, and ingredients such as 1-propanol and methanol are classified as toxic.8b As such, they recently recalled hand sanitisers based on possible methanol contamination.8b Hand sanitiser sample 3 contained neither ethanol nor isopropanol; however it contained 1-propanol, and sample 33 contained trace amounts of 1-propanol (refer to Figures S5-S7). In additonan, 1-propanol is a toxic ingredient if ingested.7b,8d,16 It also causes mild central nervous system depression (narcosis) and skin irritation.8d,22 Ethyl acetate was present in hand sanitiser sample 24 (refer to Figures S8-S9), a skin irritant; repeated long-term exposure can affect the skin, liver, kidney and cause central nervous system depression.23 In a toxicology study conducted by Timothy et al., 2021, technical grade ethanol was employed to produce alcohol-based hand rubs; however, Health Canada recalled numerous hand sanitiser products due to ethyl acetate contamination.17 This is of great concern as these substandard hand sanitisers are harmful to unaware consumers. If consumers are constantly exposed to unregulated alcohol-based products containing toxic contaminants, this will result in adverse secondary toxic effects on our health.17 However, the public should not misconstrue that all ABHS has false labelling. To avoid substandard ABHS production, higher governing authorities must implement stricter and consistent manufacturing guidelines.
CONCLUSION
There is currently a paucity of literature on the most effective alcohol concentration in hand sanitisers from governing authorities; there is merely a range stated (60-95%). As such, this study employed a Shimadzu GC-MS-QP2010 Ultra coupled with Zebron ZB-wax capillary GC column to analyze 50 ABHS. Our study also discovered the use of two toxic contaminants (1-propanol and ethyl acetate) in three samples. Consumers must be wary and vigilant regarding the alcohol concentrations in ABHS because substandard products could be marketed to the public. The usage of ABHS with lower alcohol levels is not virucidal and can also result in the development ofhighly virulent "superbugs" with resistant mutations. Hand sanitiser manufacturers should ideally comply with the alcohol content indicated on their product labels and display which regulations/guidelines were followed during their production process. It is imperative that South Africa implement stricter regulations to eliminate the use of substandard hand sanitisers as long-term exposure can cause deleterious health effects. The public should also be more alert to the % alcohol stated (ideal range 60-80%) on the sanitizer bottle and note one needs to rub their hands together until it feels dry.
Acknowledgements
This study was made possible through financial support from the School of Health Sciences, University of KwaZulu-Natal, and the National Research Foundation (NRF) of South Africa.
SUPPLEMENTARY MATERIAL
Supplementary information for this article is provided in the online supplement.
ORCID IDs
Kamini Govender - https://orcid.org/0000-0002-1610-3456
Sipho Mdanda - https://orcid.org/0000-0003-0146-0538
Sooraj Baijnath - https://orcid.org/0000-0001-7860-1779
Gert Kruger - https://orcid.org/0000-0003-0606-2053
Thavendran Govender - https://orcid.org/0000-0003-2511-2503
Tricia Naicker - https://orcid.org/0000-0002-7134-6258
REFERENCES
1. Golin AP, Choi D, Ghahary A. Hand sanitizers: A review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am J Infect Control. 2020 Sep;48(9):1062-1067. [ Links ]
2. Worldometer, Covid-19 Corona Virus Pandemic. 2021. https://www.worldometers.info/coronavirus/country/south-africa (accessed 10 October 2021). [ Links ]
3. Dhand R, Li J. Coughs and Sneezes: Their Role in Transmission of Respiratory Viral Infections, Including SARS-CoV-2. Am J Respir Crit Care Med. 2020 Sep 1;202(5):651-659. [ Links ]
4. Jing JLJ, Pei Yi T, Bose RJC, McCarthy JR, Tharmalingam N, Madheswaran T. Hand Sanitizers: A Review on Formulation Aspects, Adverse Effects, and Regulations. Int J Environ Res Public Health. 2020 May 11;17(9):3326. [ Links ]
5. Villa C, Russo E. Hydrogels in Hand Sanitizers. Materials (Basel). 2021 Mar 24;14(7):1577. [ Links ]
6. Beiu C, Mihai M, Popa L, Cima L, Popescu MN. Frequent Hand Washing for COVID-19 Prevention Can Cause Hand Dermatitis: management Tips. Cureus. 2020 Apr 2;12(4):e7506. [ Links ]
7. Mary B, Jacolin M, Aaron U, William M, Walter A comparison of measurement methods for alcohol-based hand sanitizers. National Institute of Standards and Technology February 2021;(Internal Report 8342):63; [ Links ]
8. Bruin W, Korsten L. South Africans Aren't being protected from fake sanitisers: What needs to be done. https://theconversation.com/south-africans-arent-being-protected-from-fake-sanitisers-what-needs-to-be-done-148128 (accessed 9 September 2021). [ Links ]
9. Boyce JM, Pittet D. Healthcare Infection Control Practices Advisory Committee. Society for Healthcare Epidemiology of America. Association for Professionals in Infection Control. Infectious Diseases Society of America. Hand Hygiene Task Force. Guideline for Hand Hygiene in Health-Care Settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002 Dec;23(12) Suppl:S3-S40. [ Links ]
10 Sissons B. How Much alcohol, or ethanol, should hand sanitizer contain? https://www.medicalnewstoday.com/articles/how-much-alcohol-should-hand-sanitizer-contain#efficacy (accessed October 10 2021) [ Links ]
11 Charmary JV. How Much Alcohol Do You Really Need in Hand Sanitizer? https://www.forbes.com/sites/jvchamary/2020/07/31/coronavirus-alcohol-based-hand-sanitiser/?sh=7c53bee03a6f (accessed 10 October 2021) [ Links ]
12 U.S. Food and Drug Administration. Is Your hand sanitizer on FDA's list of products you should not use? https://www.fda.gov/consumers/consumer-updates/your-hand-sanitizer-fdas-list-products-you-should-not-use (accessed 10 October 2021). [ Links ]
13. Berardi A, Cenci-Goga B, Grispoldi L, Cossignani L, Perinelli DR. Analysis of commercial hand sanitisers amid CoViD-19: Are we getting the products that we need? AAPS PharmSciTech. 2020 Oct 15;21(7):286. [ Links ]
14. Omarjee M. Regulatory and Licensing Requirements for Sanitisers. https://ctfa.co.za/wp-content/uploads/2020/09/Presentation_regulatory-and-licensing-requirements_sanitisers_02092020_Momeena.pdf (accessed 9 September 2021). [ Links ]
15. Pidot SJ, Gao W, Buultjens AH, Monk IR, Guerillot R, Carter GP, Lee JYH, Lam MMC, Grayson ML, Ballard SA, et al. Increasing tolerance of hospital Enterococcus faecium to handwash alcohols. Sci Transl Med. 2018 Aug 1;10(452):eaar6115. https://doi.org/10.1126/scitranslmed.aar6115. [ Links ]
16. Edwards J, Patel G, Wareham DW. Low concentrations of commercial alcohol hand rubs facilitate growth of and secretion of extracellular proteins by multidrug-resistant strains of Acinetobacter baumannii. J Med Microbiol. 2007 Dec;56(Pt 12):1595-1599. [ Links ]
17. Perera M. Is There Any Commonality between Transmition of Antimicrobial Resistant Genes and Alcohol Tolerant Genes from One Bacterium to Another. Academia Letters; 2021. p. 2. [ Links ]
18 Schreiber M. Some bacteria are becoming 'more tolerant' of hand sanitizers, study finds. https://www.npr.org/sections/goatsandsoda/2018/08/02/635017716/some-bacteria-are-becoming-more-tolerant-of-hand-sanitizers-study-finds (accessed 9 September 2021). [ Links ]
19 Pinholt M, 0stergaard C, Arpi M, Bruun NE, Schenheyder HC, Gradel KO, Segaard M, Knudsen JD, Network DCB; Danish Collaborative Bacteraemia Network (DACOBAN). Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006-2009: a population-based cohort study. Clin Microbiol Infect. 2014 Feb;20(2):145-151. [ Links ]
20. Assefa D, Melaku T. Commercial Hand Sanitizers use amid COVID-19 Pandemic: the Concerns of Antimicrobial Resistance. Infect Drug Resist. 2021 Jun 10;14:2183-2185. [ Links ]
21. Matatiele P, Southon B, Dabula B, Marageni T, Poongavanum P, Kgarebe B. Monitoring quality of alcohol-based hand sanitizers used in Johannesburg area during the Covid-19 pandemic. [Preprint]. 2021. Sci Rep https://doi.org/10.21203/rs.3.rs-612413/v1. [ Links ]
22. Tse TJ, Purdy SK, Shen J, Nelson FB, Mustafa R, Wiens DJ, Reaney MJT. Toxicology of alcohol-based hand rubs formulated with technical-grade ethanol. Toxicol Rep. 2021 Apr 2;8:785-792. [ Links ]
23. Yusuf AA. Determination of alcohols in hand sanitisers: are off-the-shelf hand sanitisers what they claim to be? S Afr J Sci. 2021;117(11/12). https://doi.org/10.17159/sajs.2021/9328. [ Links ]
24. Zhang J. Hand sanitizer analysis using the Agilent 8860 Gc Configured with a flame ionization detector. USA: Agilent Technologies, Inc.; 2020. p. 1-8. [ Links ]
25. Gold NA, Mirza TM, Avva U. Statpearls. Treasure Island (FL): StatPearls Publishing LLC; 2021. Alcohol Sanitizer. [ Links ]
26. Kampf G, Marschall S, Eggerstedt S, Ostermeyer C. Efficacy of ethanol-based hand foams using clinically relevant amounts: a cross-over controlled study among healthy volunteers. BMC Infect Dis. 2010 Mar 26;10(1):78. [ Links ]
27. National Center for Biotechnology Information. Pubchem Compound Summary for Cid 1031, Propanol. https://pubchem.ncbi.nlm.nih.gov/compound/Propanol. (accessed 10 October 2021). [ Links ]
28. National Center for Biotechnology Information. Pubchem compound summary for cid 8857, ethyl acetate. https://pubchem.ncbi.nlm.nih.gov/compound/Ethyl-acetate. (accessed 10 October 2021). [ Links ]
Received 11 August 2021
Revised 01 December 2021
Accepted 01 December 2021
* To whom correspondence should be addressed. Email: Naickert1@ukzn.ac.za
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














