Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.76 Durban 2022
http://dx.doi.org/10.17159/0379-4350/2022/v76a02
RESEARCH ARTICLE
Synthesis and characterization of amine-functionalized supported phosphine catalysts and their application in ethylene oligomerization
Mzamo L. Shozi*; Xolelwa Zulu; Holger B. Friedrich
School of Chemistry and Physics, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
A series of phosphorus and nitrogen-based ethylene oligomerization ligands of the type Ph2PN(X)PPh2 where X = i-propyl, n-butyl, ethylbenzene and cyclohexyl substituents are reported. These ligands were functionalized to enable tethering on amino-silica. The free ligands, amino-silica and the tethered ligands were characterized by BET, XRD, IR, TGA and NMR. The catalysts were tested for ethylene oligomerization using Cr(acac)3 (acac = acetylacetonate) as the precursor and MMAO (modified methylaluminoxane) as the activator. The activity and selectivity of these catalysts to 1-octene was monitored at 45 bar ethylene in the temperature range of45-100 °C. The activity of the supported catalysts was comparable to their homogeneous counterparts, surpassing them in some cases and selectivities to 1-octene in the C8 products were as high as 99 wt%. The steric effect of the substituent on the ligand as well as that of the support was found to influence the activity and product distribution.
Keywords: 1-hexene, 1-octene, ethylene oligomerization, phosphine ligands, silica, supported catalysts
INTRODUCTION
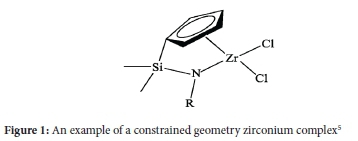
The drive in the oligomerization of ethylene to linear alpha olefins has been towards 1-hexene (1-C6) and 1-octene (1-C8). This is due to the increased demand and importance of these chemicals in the production of linear lower density polyethylene (LLDPE).1-4 There has been a growing interest in a class of catalysts, namely single-site catalysts (SSC) in olefin transformations. A sub-class of these compounds, constrained-geometry catalysts (CGCs), have emerged as important catalysts in olefin polymerization in that they offer interesting combinations of environments around the metal centre, which result in polymers with desired qualities such as high molecular weight, tacticity and degree of branching. This is because of the open nature of their reactive centre. The supported analogues of these CGCs are known and the latter have been investigated for ethylene polymerization and titanium and zirconium CGCs (Figure 1) find the most application for this purpose.5-7
Surface organometallic chemistry employed by Basset et al.8 produced a number of single-site catalysts on non-porous functionalized silica surfaces through residual silica pendant groups. The supported CGCs of this nature have been shown to exhibit good activity for ethylene polymerization which is mainly dependent on the nature of the support surface.9 McKittrick and Jones reported a method for preparing an isolated titanium-centred polymerization catalyst on porous silica.6,10,11 There are mainly three ways which have gained popular use for supporting organometallic compounds on silica in oligomerization catalysis viz. i) contacting the support with the co-catalyst, which in most cases has been methylaluminoxane (MAO); ii) the physisorption of the metal complex onto the support surface or a preformed complex that is designed to form covalent bonds between the ligand and the support surface and iii) immobilization of the complex via a covalent linkage between the ligand and the support using a multi-step grafting approach. The benefit of using this type of support has been reported to be that this method of supporting gives material that is less susceptible to the supported complex leaching due to MAO in the catalytic reaction.6
Amine-functionalized silica surfaces have been synthesized for a number of years for application in the fields of catalysis12,13 and adsorption chemistry14-16. These surfaces are of particular importance in catalysis because they create spatially isolated sites. This means that the amines on the surface can be strategically placed at minimum distances from each other, to avoid possible interactions that can occur between adjacent and neighbouring amines. The advantage of having these spatially isolated materials is that the amine sites, at relatively low loadings, behave as though they are isolated and uniform.15 In this work, the synthesis, characterization and the novel application of the grafting approach to tether functionalized PNP ligands is reported. These supported ligands were used for ethylene oligomerization. The effect of the amine density on the silica material and its interaction with the catalyst and how it affects the overall selectivity and activity of the PNP catalysts, compared to their homogeneous counterparts, is also reported.
EXPERIMENTAL
Materials and instrumentation
Unless otherwise stated, all reactions were carried out using conventional Schlenk techniques under an inert atmosphere of pre-dried nitrogen. THF was dried using sodium and benzophenone and distilled under inert atmosphere. These chemicals were commercially available and they were used as received: 3,3,3-triphenylpropionic acid (Aldrich), 3-aminopropyltriethoxysilane (Aldrich), 1,1,1,3,3,3-hexamethyldisilizane (Aldrich), pyridinium dichromate (Fluka), Pluronic F-68 (Aldrich), sodium metasilicate (Aldrich), 3-iodobenzoic acid (Fluka), di-tertbutyl carbonate (Aldrich), 4-methylamino pyridine, chloro diphenylphosphine, 97% (Aldrich), lithium aluminium hydride, 1.0 M in THF (Aldrich), N- chloro succinamide, 97% (Aldrich), methylmagnesium chloride (Aldrich).
X-ray diffraction patterns were collected using a Phillips PW 1730/10 diffractometer, using Co Ka radiation on a long line focus operating with an amperage of 25 mA and a voltage of 40 kV. TEM images were obtained using a JEOL JEM 1010 and samples were placed on a copper micro grid. The samples were analyzed for porosity measurements on an Accelerated Surface Area and Porosity (ASAP 2020) instrument at 77 K. The samples were pre-treated by heating under N2 at 90 °C for 1 h then at 350 °C for 4 h. Surface area measurements were done using a Micromeretics Gemini instrument. Prior to analysis, approximately 0.20 g of sample was degassed overnight under N2 at 200 °C. Thermogravimetric analysis was performed on a Universal V4.5A TA SDT Q600 instrument. Samples were heated under air from 30 to 1000 °C at a rate of 10 °C min-1. The organic loading was measured by determining the weight loss from 200 to 650 °C. Solution NMR spectra were recorded on a Bruker 400 MHz Ultrashield spectrometer. Solid-state NMR (CP-MAS) spectra were recorded on a Bruker DSX 600 MHz spectrometer. Samples were spun in 7-mm zirconia rotors at 5 kHz. Typical 13C CP-MAS parameters were 10 000 scans, a 90° pulse length of 4 µs, and a delay of 4 s between scans. The 29Si CP-MAS parameters were 2000 scans, a 90° pulse length of 5 is, and a delay of 10 s between scans.
Ligand synthesis
Homogeneous ligands
The bis(diphenylphosphino)amine (PNP) ligands 1-4 were synthesized according to literature procedures or modification thereof.17,25 Full details are given in the supplementary information.
Heterogeneous ligands
Amino-functionalized SBA-15 was synthesized according to literature procedures.10,18 Prior to tethering the ligands onto the SBA-15, they were functionalized according to modification of literature procedures.19 Full details are given in the supplementary information. The supported ligands are denoted as S1-S4.
Catalyst testing
Prior to each run, a Parr® reactor was heated to the desired temperature. To eliminate water, oxygen and oxygenated impurities the reactor was evacuated using a high vacuum pump. The reactor was then filled with nitrogen. The reactor was then charged with solvent. Thecatalytic mixture (combination of Cr(acac)3, MMAO-3A and ligand solutions) was then charged to the reactor. A constant flow of ethylene i.e. 45 bar was discharged and maintained through a pressure control flowmeter. After 30 minutes the ethylene flow was stopped and the reactor cooled to room temperature, followed by venting. The reaction was quenched using ethanol. Nonane was used as an internal standard in the liquid fraction. The liquid samples were analyzed using GC-FID equipped with a PONA column. The solid polyethylene obtained was dried in an oven at 110 °C overnight and then weighed.
RESULTS AND DISCUSSION
Synthesis and characterization of the ligands
Homogeneous ligands
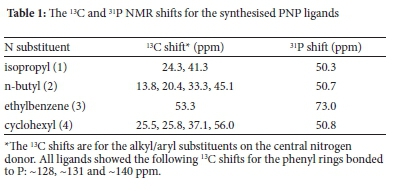
The mixed heteroatomic PNP ligands were synthesized by reacting the amine and phosphine chlorides of the type Ph2PCl (Scheme 1). This reaction takes place in basic medium in which triethylamine is used as a base. The resulting chloride salt is removed from the solution and the resulting ligands are purified. Table 1 shows the 13C and 31P NMR shifts of the prepared ligands.

Heterogeneous ligands
Synthesis of SBA-15
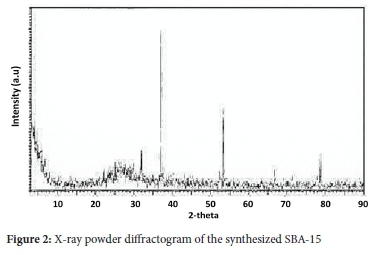
Figure 2 shows the X-ray powder diffractogram of the synthesized SBA-15. The diffraction pattern shows three intense peaks 20 values of 37, 53 and 78°. The XRD pattern obtained is characteristic of the 2D hexagonal structure as is reported in literature.20 The physisorption measurements revealed that the SBA-15 had a surface area of602 g m-2, pore volume of 0.04 cm3 g-1 and pore diameter of 153 nm, showing the material to be microporous.
Synthesis and characterization of site-isolated amine-functionalized silica surfaces
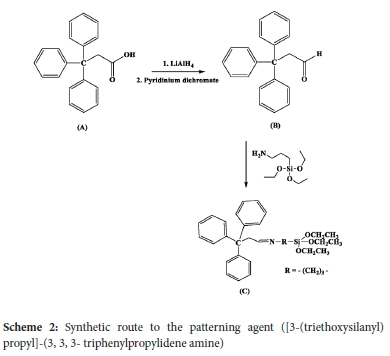
The patterning agent that was used in this work for tethering on the silica surface was ([3-(triethoxysilanyl) propyl]-(3,3,3-triphenylpropylidene) amine) and was synthesized as shown in Scheme 2. This patterning agent was chosen because of its bulkiness. The bulkiness aids in the synthesis of isolated amine-functionalization, in that the size of the agent strategically blocks the attachment of other groups in close proximity and thereby avoids the possible interactions between the surface and the amine groups.
The patterning agent was grafted onto the SBA-15 as shown in Scheme 3 (i). After contact, the silica surface is left with silanol groups that were determined by IR to have not reacted with the patterning agent. To prevent further reactions of these unreacted silanol groups, the surface was capped using hexamethyldisilizane (HDMS) (Scheme 3 (ii)). The bulky trityl groups on the capped surface were then hydrolyzed, under acidic conditions. The hydrolysis removes the bulky groups (Scheme 3 (iii)). As a result of this treatment, the silica surface forms surface silanols, which were capped by a second aliquot of HDMS (Scheme 3 (iv)).
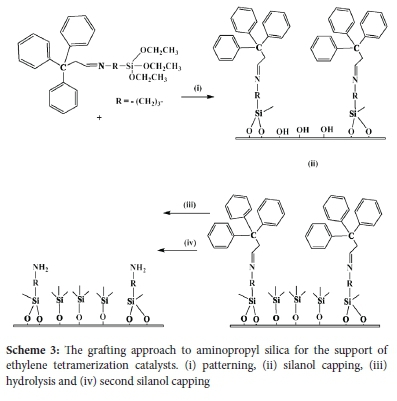
After incorporation of the patterning agent onto the SBA-15, the surface decreased to 590 g m-2. The grafting approach was also followed by FT-IR. Upon hydrolysis there were major changes observed in the surface of the material. The characteristic FT-IR band of the imine at 1546 cm-1, which was previously observed, disappeared. The peaks assigned to the aromatic C-H were still present, though greatly reduced. The diagnostic N-H peak at 3500-3200 cm-1 was observed as a very weak band. This is probably due to the relatively low amine loading on the material. The only peaks that can be considered strong are the ones that are due to the aliphatic C-H bands of the propyl linker in the material.
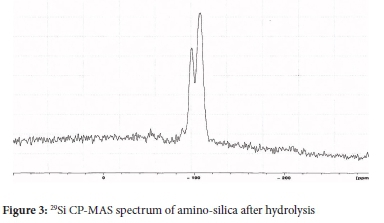
Solid-state 29Si CP-MAS NMR was also used to analyse amine-functionalized silica surfaces (Figure 3). After hydrolysis, the resonances for the singly bonded SiOH groups are not observed. Instead, a well-resolved resonance for the geminal and hydrogen bonded SiOH are observed. It can be concluded that the bonding in the material is through both the double and single surface to silane linkages, with very few single linkages.21,22
Coupling of functionalized PNP ligands to aminosilica
The functionalized PNP ligands were reacted with amine functionalized silica in appropriate ratios to give the desired ligand:metal ratios for application in ethylene tetramerization. The successful linkage of these ligands on the amine functionalized silicas was confirmed by FT- IR. The C-N stretching band was observed at 1156 cm-1 and simultaneously the disappearance of NH2 (1551 and 3301 cm-1) stretching bands in amino-silica was observed, which confirmed the substitution reaction between the surface linkers and the functionalized ligands.
Catalyst testing
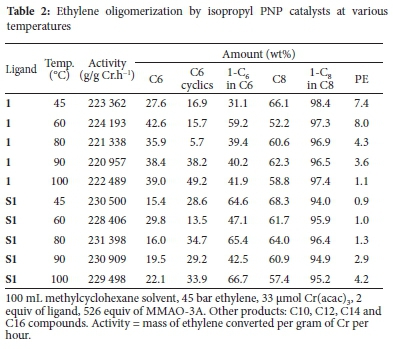
Bollmann et al.23 reported that oligomerization catalysts performed much better in aliphatic solvents due to higher ethylene solubility, hence the choice of methylcyclohexane as a solvent in this work. The catalyst's activity in this solvent is, however, enhanced at the expense of a slightly lower selectivity to 1-C8 in the C8 fraction. Table 2 shows the activity of the homogeneous isopropyl system 1 together with its supported equivalent S1 at various temperatures. The change in temperature did not have a pronounced effect on the catalyst activity. The highest activity of 1 was at 60 °C, while that of S1 was at 80 °C. The selectivities to 1-C6 and 1-C8 were notably different for both 1 and S1. The increase in temperature was accompanied by an increase in the selectivity to C6 cyclics at the expense of C8 fraction. The selectivity to 1-C8 in the C8 fraction for S1 was, however, comparable to that obtained for its homogeneous counterpart..
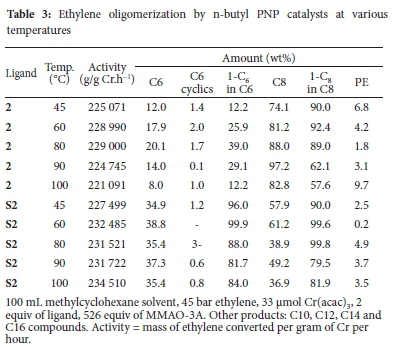
To compare the effect of a branched alkyl substituent (isopropyl) to one that is unbranched, the n-butyl PNP ligand 2 and its heterogeneous counterpart S2 were tested (Table 3). The catalyst activities with increasing temperature were comparable to those obtained for the isopropyl catalysts (Table 2). Catalyst 2 achieved its highest activity at 80 °C, which decreased with further increase in temperature to 100 °C. On the other hand, S2 showed increasing activity up to the maximum temperature. These results suggest catalyst deactivation at elevated temperatures, which seems to be remedied by the presence of the support.
Catalysts which contain ligands that have a single carbon spacer between the N-atom and a phenyl group were shown by Killian et al.24 to lead to an increase in overall alpha olefin selectivity. The combination of a N-alkyl group and alpha branching on this moiety results in these improved results. Catalysts 3 and S3 allowed for the investigation of this class of ligands that have both the a-branching as well as an aryl group within the substituent and the results are shown in Table 4. The authors cited above found that releasing the steric strain as a result of the presence of the methylene spacer between the N-atom and the phenyl group had little effect on the product composition, although the catalyst productivity did improve by approximately 30%. In the system reported in this work, the most active catalyst of this set was 3 at 45 °C. This activity decreased with an increase in temperature.
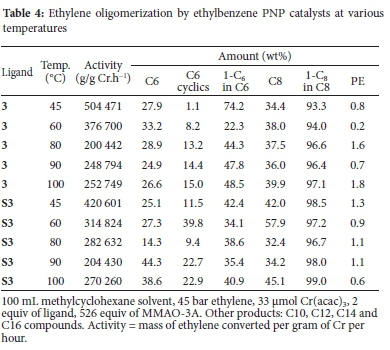
The results shown by these catalysts were particularly interesting because there was an observed interchange between these two catalysts in terms of activity and selectivity to C8 (1- C8). For example, from 45 to 80 °C the catalytic activity of 3, although it decreases with the increase in temperature, was superior to that of S3. However, from 80 °C, the activity of 3 is surpassed by that of S3. In terms of selectivity to C8 (1-C8), from 45 to 80 °C, an increase is seen over 3 while a decrease in selectivity over S3 is observed. From 80 to 100 °C both these catalysts show a similar selectivity trend and an increase in C8 (1-C8) selectivity is observed.
In terms of C6 products, it can be seen that at the lower temperatures, 3 is more active and its selectivity to 1-C6 is higher than that for S3. The support influence in S3 is noticeable for the C6 products, where the selectivity to C6 cyclics is higher than those of 3. Similar observations were made previously, where an increase in C6 cyclics was seen when a supported PNP ligand with a phenyl functional group was used.1
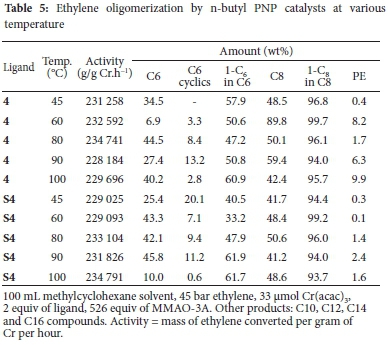
Kuhlmann et al.25 had highlighted that exchanging of the relatively small methyl group on the N-atom of the PNP ligand to a cycloalkyl group led to a significant increase in the 1-C6 selectivity within the C6 fraction. This was achieved through the suppression of the formation of C6 cyclic products. The basicity and the size of the cyclohexyl group on the N-atom has been shown by Blann et al.26 to enhance and increase catalytic activity. The increased bulk around the nitrogen led to an increase in total alpha olefin selectivity. In an attempt to achieve a similar result, the cyclohexyl PNP ligand 4 and its heterogeneous counterpart S4 were tested in ethylene oligomerization and the results are shown in Table 5. The highest activity by 4 was achieved at 80 °C, which, as with 1, 2 and 3 decreases with further increase in temperature. Contrary to this, the activity of S4 increased with increasing temperature up to 100 °C. With increasing temperature, there was also an increase in 1-C6 in the C6 fraction, while the selectivity to the C6 cyclics decreased over both catalysts.
CONCLUSION
The supported phosphine catalysts that were synthesized were tested for ethylene tetramerization and showed good activity and selectivity to 1-C8. The role of the support has been found to be mainly in enhancing the catalyst stability at higher temperatures. There is evidence that the steric influence of the support competes with the electronic influence of the substituent on the central donor N-atom. The supported catalysts showed improved selectivity to 1-C8 at increased temperatures when the selectivity to 1-octene of the homogeneous counterparts was shown to be affected negatively. In most cases in the set of catalysts that were studied, it was observed that the heterogenized catalysts are comparable in activity and selectivity to their homogeneous counterparts, and in a few cases, they surpass the homogeneous catalyst. Another advantage of the supported catalysts is that they suppress the formation of cyclic products, which leads to a desirable increase in the C8 fraction and ultimately 1-C8 The steric effect of the support has been shown to override the electronic and steric effect of the substituents on the central donor atom and thus enhance catalyst integrity at higher temperatures. From the results, the interactions of the supports, which are steric in nature, can be represented in terms of the strength of influence on catalyst productivity as S3 > S4 > S2 > S1. In terms of catalyst stability at elevated temperatures, the influence of the support can be represented as S4 > S2 > S3 > S1. Thus, a possible trend, less electron donating substituents give a more stable catalyst.
SUPPLEMENTARY MATERIAL
Supplementary information for this article is provided in the online supplement.
ORCID IDs
Mzamo Shozi - https://orcid.org/0000-0001-7666-2543
Xolelwa Zulu - https://orcid.org/000-0001-6951-8391
Holger Friedrich - https://orcid.org/0000-0002-1329-0815
REFERENCES
1. Shozi ML, Friedrich HB. Heterogenization of some PNP ligands for the oligomerization of ethylene. S Afr J Chem. 2012;65:214-222. [ Links ]
2. Na Y, Dai S, Chen C. Direct synthesis of polar-functionalized linear low-density polyethylene (LLDPE) and low-density polyethylene (LDPE). macromolecules. 2018;51(11):4040-4048. https://doi.org/10.1021/acs.macromol.8b00467 [ Links ]
3. Nabizadeh N, Zohuri GH, Khoshsefat M, Ramezanian N, Ahmadjo S. Ethylene/1-Hexene copolymerization and synthesis of LLDPE/nanocarbon composite through in situ polymerization. Polym Sci Ser B. 2018;60(1):122-129. https://doi.org/10.1134/S1560090418010104 [ Links ]
4. Shozi ML, Zulu X, Friedrich HB. Ethylene trimerisation over supported SNS and PNP chromium catalysts. S Afr J Chem. 2021;75:32-39. [ Links ]
5. Yu K, McKittrick MW, Jones CW. Role of amine structure and site isolation on the performance ofaminosilica-immobilized zirconium CGC-inspired ethylene polymerization catalysts. Organometallics. 2004;23(17):4089-4096. https://doi.org/10.1021/om049765w [ Links ]
6. McKittrick MW, Jones CW. Effect of site isolation on the preparation and performance of silica-immobilized Ti CGC-inspired ethylene polymerization catalysts. J Catal. 2004;227(1):186-201. https://doi.org/10.1016/j.jcat.2004.06.024 [ Links ]
7. Coates GW. Precise control of polyolefin stereochemistry using single-site metal catalysts. Chem Rev. 2000 Apr 12;100(4):1223-1252. https://doi.org/10.1021/cr990286u [ Links ]
8. Basset JM, Choplin A. Surface organometallic chemistry: A new approach to heterogeneous Catalysis. J Mol Catal. 1983;21(1-3):95-108. https://doi.org/10.1016/0304-5102(93)80113-9 [ Links ]
9. McKittrick MW, Yu K, Jones CW. Effect of metallation protocol on the preparation and performance ofsilica-immobilized Ti CGC-inspired ethylene polymerization catalysts. J Mol Catal Chem. 2005;237(1-2):26-35. https://doi.org/10.1016/j.molcata.2005.04.037 [ Links ]
10. McKittrick MW, Jones CW. Toward single-site functional materials: preparation of amine-functionalized surfaces exhibiting site-isolated behavior. Chem Mater. 2003;15(5):1132-1139. https://doi.org/10.1021/cm020952z [ Links ]
11. McKittrick MW, Jones CW. Modulating the reactivity of an organometallic catalyst via immobilization on a spatially patterned silica surface. Chem Mater. 2005;17(19):4758-4761. https://doi.org/10.1021/cm050925j [ Links ]
12. Müller CA, Schneider M, Mallat T, Baiker A. Amine-modified titania-silica hybrid gels as epoxidation catalysts. Appl Catal A Gen. 2000;201(2):253-261. https://doi.org/10.1016/S0926-860X(00)00446-4 [ Links ]
13. Fey T, Fischer H, Bachmann S, Albert K, Bolm C. Silica-supported TEMPO catalysts: synthesis and application in the Anelli oxidation of alcohols. J Org Chem. 2001 Nov 30;66(24):8154-8159. https://doi.org/10.1021/jo010535q [ Links ]
14. Kramer J, Garcia AR, Driessen WL, Reedijk J. Tuning the recovery of an Rh-containing catalyst with silica-based (poly) amine ion exchangers. Chem Commun (Camb). 2001 Dec 7;2420-2421(23):2420-2421. https://doi.org/10.1039/b108457g [ Links ]
15. Walcarius A, Etienne M, Bessière J; Amorphous silica gels grafted with amine or thiol groups. rate of access to the binding sites in organically modified silicates. 1. Amorphous silica gels grafted with amine or thiol groups. Chem Mater. 2002;14(6):2757-2766. https://doi.org/10.1021/cm021117k [ Links ]
16. Evans J, Zaki AB, El-Sheikh MY, El-Safty SA. Incorporation of transition- metal complexes in functionalized mesoporous silica and their activity toward the oxidation of aromatic amines. J Phys Chem B. 2000;104(44):10271-10281. https://doi.org/10.1021/jp000564p [ Links ]
17. Maumela MC, Blann K, de Bod H, Dixon JT, Gabrielli WF, Williams DBG. Efficient synthesis of novel n-substituted bulky diphosphinoamines. Synthesis. 2007;2007(24):3863-3867. https://doi.org/10.1055/s-2007-990868 [ Links ]
18. McKittrick MW, Jones CW. Toward single-site, immobilized molecular catalysts: site-isolated Ti ethylene polymerization catalysts supported on porous silica. J Am Chem Soc. 2004 Mar 17;126(10):3052-3053. https://doi.org/10.1021/ja031725g [ Links ]
19. Pintér A, Haberhauer G, Hyla-Kryspin I, Grimme S. Configurationally stable propeller-like triarylphosphine and triarylphosphine oxide. Chem Commun (Camb). 2007 Sep 28;3711-3713(36):3711-3713. https://doi.org/10.1039/b709655k [ Links ]
20. Huo Q, Margolese DI, Ciesla U, Feng P, Gier TE, Sieger P, Leon R, Petroff PM, Schüth F, Stucky GD. Generalized synthesis of periodic surfactant/ inorganic composite materials. Nature. 1994;368(6469):317-321. https://doi.org/10.1038/368317a0 [ Links ]
21. Hicks JC, Jones CW. Controlling the density of amine sites on silica surfaces using benzyl spacers. Langmuir. 2006 Mar 14;22(6):2676-2681. https://doi.org/10.1021/la053024y [ Links ]
22. Maciel GE, Haw JF, Chuang IS, Hawkins BL, Early TA, McKay DR, Petrakis L. NMR studies ofpyridine on silica-alumina. J Am Chem Soc. 1983;105(17):5529-5535. https://doi.org/10.1021/ja00355a001 [ Links ]
23. Bollmann A, Blann K, Dixon JT, Hess FM, Killian E, Maumela H, McGuinness DS, Morgan DH, Neveling A, Otto S, et al. Ethylene tetramerization: a new route to produce 1-octene in exceptionally high selectivities. J Am Chem Soc. 2004 Nov 17;126(45):14712-14713. https://doi.org/10.1021/ja045602n [ Links ]
24. Killian E, Blann K, Bollmann A, Dixon JT, Kuhlmann S, Maumela MC, Maumela H, Morgan DH, Nongodlwana P, Overett MJ, et al. The use of bis(diphenylphosphino)amines with N-aryl functionalities in selective ethylene tri- and tetramerisation. J Mol Catal Chem. 2007;270(1-2):214-218. https://doi.org/10.1016/j.molcata.2007.01.046 [ Links ]
25. Kuhlmann S, Blann K, Bollmann A, Dixon JT, Killian E, Maumela MC, Maumela H, Morgan DH, Prétorius M, Taccardi N. N-substituted diphosphinoamines: toward rational ligand design for the efficient tetramerization of ethylene. J Catal. 2007;245(2):279-284. https://doi.org/10.1016/j.jcat.2006.10.020 [ Links ]
26. Blann K, Bollmann A, de Bod H, Dixon JT, Killian E, Nongodlwana P, Maumela MC, Maumela H, McConnell AE, Morgan DH. Ethylene tetramerisation: subtle effects exhibited by N- substituteddiphosphinoamine ligands. J Catal. 2007;249(2):244-249. https://doi.org/10.1016/j.jcat.2007.04.009 [ Links ]
Received 08 March 2021
Revised 27 October 2021
Accepted 27 October 2021
* To whom correspondence should be addressed: Email: shozim2@ukzn.acza
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]





![C-H activation: a Critical Evaluation of a Published Method and its Application Towards Inherently Chiral Calix[4]arenes](/img/en/next.gif)








