Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.76 Durban 2022
http://dx.doi.org/10.17159/0379-4350/2022/v76a01
RESEARCH ARTICLE
Evaluating the influence of interactive simulations on learners' academic performance in stoichiometry
Lereko G. MohafaI; Makomosela QhobelaII, *; Mosotho J. GeorgeIII
IAbia High School
IIDepartment of Science Education
IIIDepartment of Chemistry and Chemical Technology, National University of Lesotho, Maseru, Lesotho
ABSTRACT
Traditional teaching strategies dominate science classrooms in Lesotho, resulting in poor academic performance at each level. Information and Communication Technology tools such as simulations offer potential benefits for improving science teaching. The purpose of this quasi-experimental study was to evaluate the effect of simulations on learners' performance and retention of stoichiometry concepts using Solomon's four-group design. Purposive sampling of two existing Form E (Grade 12) classes, with 81 learners, led to the creation of four groups. The study responds to the research question: How does using simulations as part of an intervention affect learners' academic performance in stoichiometry? Data were collected as learners' scores for pre-tests and post-tests, designed to assess their understanding of stoichiometry, and analysed through ANOVA and t-tests. After the intervention, the experimental group's mean score (M = 44.2, a = 18) was higher than that of control group (M = 32.6, a = 15.8), and the difference between the two mean scores was statistically significant, namely t(81) = 3.14, p = 0.002. The experimental group had a higher mean score for retention in post-test (M = 47.4, a =16.1) than the control group (M = 37.2, a = 13.7) which was statistically significant, namely t(81) = 3.10, p = 0.003. Therefore, it was concluded that simulations enhanced learners' performance and could improve the retention of stoichiometry concepts. This study recommends that simulations be used to supplement the teaching and learning of science, in particular chemistry.
Keywords: cognitive theory of multimedia learning, high school learners' performance, interactive-simulations, stoichiometry
INTRODUCTION
The teaching and learning of science and mathematics in less developed countries face significant challenges. Literature shows that many science teachers mostly adopt the chalk and talk methods characterised by several limitations and challenges.1,2 The challenges related to the chalk and talk methods include the low academic performance of learners, loss of motivation and interest in the subject, and lack of perseverance when dealing with challenging concepts.3 These challenges are brought about in part by factors such as ill-equipped laboratories and lack of laboratory consumables, making it difficult for teachers to employ experimentation.4,5 As a result, chemistry topics such as stoichiometry become very challenging to both teachers and students.6 The challenges become more pronounced when teaching and learning methods do not facilitate conceptual change.6 The teachers sometimes struggle to unpack complex stoichiometry concepts into simplified comprehensible versions to students.6,7 Learners struggle to demonstrate a high level of proficiency in the application of algebra in chemistry, to link invisible constructs with those that are visible, to use and interpret chemical symbols, to correctly apply tenets of proportion and law of conservation of mass,8 to balance and interpret chemical equations,9,10 and to identify limiting reagents.10 Meaningful understanding of stoichiometry requires that learners are able to balance equations, convert between quantities such as mole and mass, and mentally visualise processes at the sub-micro level.11 Therefore, special teaching strategies are needed for effective teaching and learning of stoichiometry.6 One suggestion involves teaching for conceptual change and incorporating 3-D representations.10 Moreover, the introduction of stoichiometry concepts has to start with sub-micro representations, to allow learners to concretise concepts.12
Attempts have been made to explore the potential benefits of Information and Communication Technology (ICT) for teaching and learning. These attempts include a focus on tools such as simulations3 and virtual laboratories.4 Simulations are 'interactive software programs that allow students to explore complex interactions among dynamic variables that model real-life situations.13 Literature documents the benefits of simulations such as responding to economic and safety challenges of real experiments and infrastructure shortage,4 assisting in the visualisation of unobservable phenomena, promotion of critical thinking,14 enhancement of motivation and improve ment of learners' performance.3 Studies on multimedia tools draw their theoretical support from theories such as Mayer's cognitive theory of multimedia learning, which proposes that meaningful learning occurs when words and pictures scaffold assimilation and accommodation.15,16
Studies reported in the literature provide insights into efforts to evaluate the effects of simulations in teaching and learning of stoichiometry concepts. For instance, undergraduate students performed better in online courses enriched with simulations than those who used only text-based material.17,18 Learners exposed to simulations demonstrated excellent performance in balancing and interpreting chemical equations,8,17,18 and determining limiting and excess reagents.19 Most of these studies were conducted with undergraduates and using an online format. Therefore, there is a need to establish the effect of simulations on learners' academic performance in stoichiometry under physical classroom settings.18
In Lesotho, available studies on science teaching focused on areas such as ICT infrastructure development20 and perceptions of the integration of ICT and its potential to improve teaching and learning of chemistry.4 Research studies on the actual use and empirical effects of simulations in classrooms appear to be limited, particularly those focusing on stoichiometry. Therefore, the purpose of this quasi-experimental study was to evaluate the effect of simulations on the effectiveness of teaching and learning stoichiometry as judged through learners' academic performance. For this study, academic performance was evaluated as '... the knowledge attained and designated by marks, assigned by teacher' .21
THEORETICAL FRAMEWORK
This study is guided by the cognitive theory of multimedia learning (CTML), which draws from several constructs that explain how learning of new concepts happens and how pedagogy can be adjusted to achieve meaningful learning.15 Proponents of CTML view meaningful learning as a 'deep understanding of the material, which includes attending to important aspects of the presented material, mentally organising it into a coherent cognitive structure, and integrating it with relevant existing knowledge.15 They argue that these aspects can be achieved through using multimedia learning which refers to learning from words and pictures, and ... multimedia instruction which refers to... presenting words and pictures that are intended to foster learning' .15 Characteristics of simulations, that make them effective learning tools, are similar to the main claims of CTML.22 Importantly, simulations use carefully balanced presentation of words and pictures to assist learners to better engage in conceptual understanding of concepts.16 Therefore, the inclusion of simulations in this study was expected to promote meaningful learning of stoichiometry concepts.
CTML raises issues critical to meaningful teaching and learning in a multimedia environment.15,16 Firstly, care must be taken when presenting auditory and visual information so that the human information-processing system consisting of auditory and visual channels receives information within optimum limits. In other words, care must be taken of the limited capacity of auditory and visual channels.15 Secondly, learning is an active process where activities must be appealing to learners and be within their zone of proximal development (ZPD)23, which is the theoretical gap between learners pre-existing schemas and new ideas that a learner can learn.24 Therefore, borrowing from these tenets of CTML, this study subscribed to the following notions: 1) There is a need to optimally facilitate the acquisition of information in the form of pictures and words for meaningful learning;15 2) Lessons should be designed such that learners are active in the learning process;15 3) Learning activities should be framed within ZPD considering learners' prior knowledge and 4) Activities should also incorporate the necessary scaffolding.24
SIMULATIONS IN TEACHING AND LEARNING
Simulations have been reported to significantly promote meaningful learning by providing scaffolding effects.25 It is asserted that as applied to software, scaffolding refers to cases in which the tool changes the task in some way so that learners can accomplish tasks that would otherwise be out of their reach' .24 Simulations, therefore, have the power to bridge the gap between prior knowledge and new concepts within learners' ZPD in situations where learning requires a significant mental effort to link observable entities with unobservable entities.26 Simulations have the advantage of showing 'what is not ordinarily visible to the eyes... and how experts model their behaviour.27 As a result, simulations assist learners to build mental representations that coherently link the macro, sub-micro and symbolic levels of representations.25 Chemistry educators argue that 'when students connect their macroscopic observations of the phenomenon to the graphical representations, they are truly learning chemistry and chemical processes' .22 Therefore, simulations being similar to animations and videos, offer a key input towards meaningful learning of chemistry concepts28 taking into consideration that most concepts are abstract and not easy to understand.9
Studies reported in the literature reflect different areas in which simulations can assist the teaching and learning process. Simulations can free a learner from peripheral extraneous information processing, which has the potential to create a cognitive overload.29 Extraneous information processing may stem from multiple sources such as the complex nature of a task with many interacting entities, misalignment of pictures and words in instructional material,15 failure to recognise patterns, links, or relationships especially within interacting elements, inability to 'chunk' information into manageable units.30 Stoichiometry is one of the challenging chemistry topics31 characteristic of interacting concepts. Simulations can assist with stoichiometric quantitative calculations, the mole concept, conversion between units, balancing equations, understanding of limiting and excess reagents, and the particulate nature of matter31, which have proven challenging to learners.32
Research findings on the effectiveness of simulations towards enhancing learners' academic performance vary. Some reported improved performance, improved laboratory skills, and competencies compared to those who followed normal teaching modes.33,34,35 More specifically, there are studies that focussed on the use of simulations in teaching and learning of stoichiometry concepts that reported improved academic performance: Such studies reported that learners exposed to simulations demonstrated outstanding performance in areas such as balancing and interpretation of chemical equations.8,17,18. On the other hand, some studies found no significant differences between learners taught through traditional methods and those who received instruction through the aid of computer simulation. For instance, in one study on product creativity, there were no significant differences in product creativity scores between the group using computer simulations and the group that used hands-on practical activities.36
RESEARCH METHODOLOGY
Design
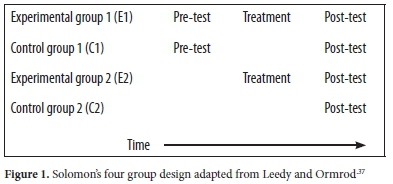
This study was quasi-experimental following Solomon's four-group pre-post-test approach37 to implement and evaluate the effects of interactive simulations on learners' academic performance and retention of concepts in stoichiometry. Solomon's four-group design allows effects of pre-test sensitisation to be traced so that degree of intervention effects can be assessed.37 The study adopted a quasi-experimental approach because participants came from two existing classes. The researcher could not change the arrangements of classes such that all participants were randomly assigned to groups. Instead, the only option available was randomly assigning intact classes to experimental and control groups. Quasi-experimental designs use naturally occurring settings like intact classrooms, which is a key factor in evaluating interventions or programs.38 As noted by Muijs, 'If we find programme effects we can at least be confident that these work in real schools and classrooms with all their complexity rather than just in the laboratory setting.38 The study design is shown in Fig. 1 below.
Sample
Two Form E (Grade 12) classes, N = 83 learners, were sampled; one class had 41 learners, and another had 42 learners. Purposive sampling was done on account of accessibility to ICT facilities, timeframe, and costs. The researchers were aware that purposive sampling addressed most needs of the study, but it is selective and does not attempt to represent wider populations.34 After randomly assigning the classes to groups, the class with 42 learners became a control group while the class with 41 learners became an experimental group.
The four groups
The final examination marks in physical science1 of the previous year were used as the criterion for placement of participants into groups, E1, C1, E2 and C2. Based on learners' marks arranged in descending order, learners in the first class (n = 42) were allocated to groups C1 and C2 while those in the second class (n = 41) were allocated to E1 and E2 following a matched pairs strategy.39 In each class, pairs of learners with similar marks were created. Each pair member could be randomly assigned to a group that received a pre-test or a group that did not receive a pre-test. This assignment was done to establish the equivalence of groups in terms of prior knowledge so that intervention results could be justified.39
Instruments
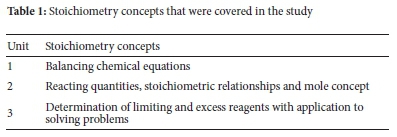
The instruments for the study were self-developed stoichiometry achievement tests (SATs) and classwork exercises (CWEs), as well as lesson plans compiled as per content and learning objectives of the syllabus. The SATs questions were in line with the specific learning outcomes for the topic of stoichiometry as outlined in the Lesotho General Certificate of Secondary Education (LGCSE) physical science syllabus. The syllabus emphasises correct balancing and use of chemical equations, the ability to calculate reacting quantities, determination of limiting reagents, knowledge and application of mole concept, stoichiometric ratios, and how they can be applied in everyday experiences.40 These concepts were grouped into broad categories referred to as units, as shown in Table 1.
A team of three teachers of mathematics and science, who were not part of the study, was engaged in evaluating content coverage, appropriateness of lesson plans, SATs, CWEs, and marking schemes before the pilot phase. The pilot phase was carried out with one Form D (Grade 11) class which was not part of the study. After the pilot phase, all instruments were evaluated, and necessary adjustments were made. For instance, questions on balancing chemical equations were reduced from 16 to 12 after colleagues pointed out that four questions were outside the scope of the syllabus. The time allocated for post-tests was increased from 1 hour 30 minutes to 1 hour 50 minutes after realising that some learners could not complete tests in 1 hour 30 minutes.
Simulation intervention
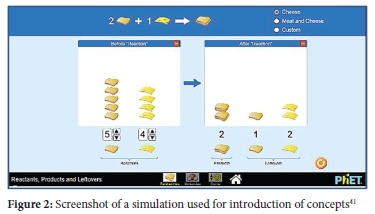
The simulations used as an intervention in this study were accessed with permission from https://phet.colorado.edu/.41 These simulations are designed such that they start with introductory activities involving common activities like sandwich making. In these introductions, starting ingredients such as bread, cheese and ham are similar to reactants, while sandwiches are similar to products. Learners could choose quantities of starting material (reactants) and observe the maximum number of sandwiches (products), leftovers, or items in excess. The use of sandwich activities as a starting point was relevant to establish stoichiometric relationships and the law of conservation of mass at a macro level. It was assumed that learners would then transfer these constructs to the next activity section involving space-filling models and symbolic representations.
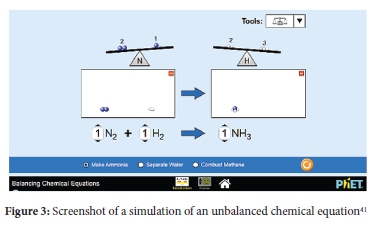
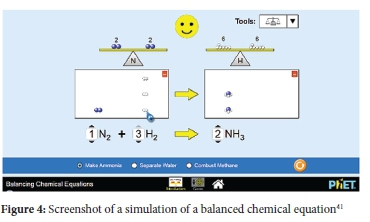
According to CTML, active learning partly involves prior knowledge which has influence on conceptual change.15 Therefore, the inclusion of simulations of making sandwiches to introduce concepts served as prior knowledge needed for active learning. Embedded within the simulations were texts descriptions and pointers to assist learners in identifying important concepts and enabling them to make meaning out of graphical representations.17 Texts descriptions and pointers were crucial as CTML asserts that meaningful learning involves learners' ability to attend to specific areas of presented material and assimilate them with reference to prior knowledge.15 Other tools incorporated within simulations included graphs and sea-saws to show two sides of chemical equations when unbalanced and balanced (see Figures 2, 3 and 4). These additional tools provided scaffolding effects so that learners could make meaning of concepts and processes.25 The simulations also had user-friendly navigation controls where learners could mute, activate, or add parameters for clarification; hence learners remained active and in control of their learning process.
Summary of lessons on the treatment of groups
Experimental groups
Both experimental and control groups had comparable learning and teaching environments except for incorporating interactive simulations for the experimental groups. Both groups were engaged in guided discovery as the main instructional method and were taught by the same teacher (first author). Students in the experimental group had access to a wireless mouse to navigate the controls of simulations projected on a large screen (2m x 2m). The teacher projected the simulated activity and then asked learners to take turns to try and formulate solutions which they shared with the rest of the class through a think-aloud technique. Problems started from common activities such as sandwich making, which were used to concretise balancing of equations as well as proportional relationships between reactants and products. When learners had acquired the concept of proportional relationships and underlying principles of the law of conservation of mass, they proceeded to simulated problems of real chemical equations and processes. Figures 2, 3 and 4 are screenshots showing some interactive simulations used by the experimental group.
Control groups
The teacher wrote problems similar to those of experimental groups on the board and then instructed learners to formulate solutions. Learners took turns to solve problems on the board and then engaged the rest of the class in live discussions through a think-aloud technique. When learners had acquired the relationships between ingredients and products, lessons proceeded to real chemical reactions and processes where they were encouraged to use the principles learned to solve problems involving balancing equations, reacting quantities, and limiting and excess reagents, respectively. During the lessons of both experimental and control groups, the teacher's main role was to pose problems, give learners time to solve problems and initiate debates on learners' solutions. During the debating process, learners in both the experimental and control groups were offered an opportunity to evaluate and justify their solutions before the class. All the topics selected for this study were taught in twelve lessons of forty minutes long.
Data collection
Data were collected employing the SAT pre-and post-tests. A pre-test was administered two days before instruction. Questions for pre and post-tests were formulated following a rubric developed for this study using the LGCSE physical science syllabus to ensure that all items were similar for both tests. Sample questions for pre and post-test on limiting reagents are shown below to illustrate the similarity of pre-and post-test questions.
Sample question from pre-test items
Soluble salts can be produced in laboratories by reacting a metal and an acid. For instance, zinc chloride (ZnCl2) can be prepared as shown by the following chemical equation.
Zn (s) + HCl(I) ZnCl2 (aq) + H2 (g)
Suppose 20 g of zinc (Zn) reacts with 10 g of hydrochloric acid (HCl). Which is the limiting reagent? Which is the excess reagent? How much mass of excess substance will be left? How much mass of hydrogen gas (H2) and zinc chloride (ZnCl2) will be produced?
Sample question from post-test items
Soluble salts can be produced in laboratories by reacting a metal and an acid. For instance, iron (II) nitrate (Fe(NO3)2) can be prepared as shown by the following chemical equation.
Fe (s) + HNO3 (I) -> Fe (NO3)2 (aq) + H2 (g)
Suppose 100 g of iron (Fe) reacts with 100 g of nitric acid (HNO3). Which is the limiting reagent? Which is the excess reagent? How much mass of excess substance will be left? How much mass of hydrogen gas (H2) and iron (II) nitrate (Fe(NO3)2) will be produced?
Learners wrote a post-test two days after completion of each unit to minimise maturation effects,43 and significant interactions between experimental and control groups. After instruction and writing posttests for all three units, scores of the three units were combined to produce an overall post-test mean score. Finally, the experimental and control groups sat for a delayed post-test after three weeks post instruction to evaluate variation in retention of concepts in stoichiometry.
Data analysis and reliability
The Statistical Package for Social Sciences (SPSS) software, in particular, ANOVA and t-tests, were used to analyse the data to establish statistical significance for any existing differences between the groups regarding academic performance and retention of concepts. The analysis of results started with the establishment of equivalence of baseline academic performance between all four groups using marks from the physical science examination of the previous year. That was followed by pre-test analysis to determine whether groups had similar prior knowledge in stoichiometry. Finally, results of the two post-tests, which evaluated academic performance immediately after instruction and three weeks after instruction, were analysed.
RESULTS
This section sequentially presents results beginning with pre-test results, which were aimed at assessing baseline knowledge of the groups in relation to selected stoichiometry topics and the determination of their equivalence of groups before intervention. After that, the results of the intervention on academic performance are presented.
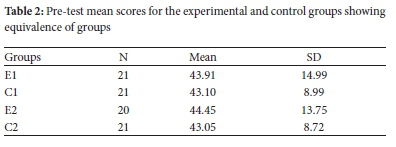
Results of the evaluation of groups' equivalence after the grouping stage show that the mean scores of four groups were comparable to one other, see Table 2. However, some variation in mean score distribution existed, as shown by varying standard deviation of means. The conclusion is that the groups had similar prior knowledge in physical science and chemistry as a separate discipline.
Assessment of performance in the pre-tests
Since the study was experimental with an intervention, it was necessary to assess participants' baseline performance before introducing the intervention. Independent samples t-test analysis was done on scores of two groups, experimental (E1) and control (C1). The results of the analysis of the pre-test are shown in Table 3.
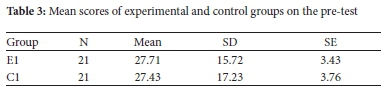
The pre-test results show that the experimental group (E1) and control group (C1) had comparable prior knowledge in the selected stoichiometry concepts. The statistical significance of the relationship between mean scores of experimental and control groups is shown in Table 4.
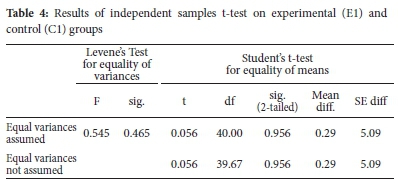
There were no statistically significant differences between mean scores of experimental (E1) and control (C1) groups on the pre-tests, t(40) = 0.056, p = 0.956. Therefore, the experimental and control groups were suitable for the experimental study as they were similar in prior knowledge in stoichiometry.
Assessment effects of interactive simulations on the performance of learners
Scores on post-tests were analysed through ANOVA to determine the differences in mean scores of experimental and control groups. Descriptive statistics of mean scores is shown in Table 5, followed by the analysis of statistical significance for any existing differences in mean scores.
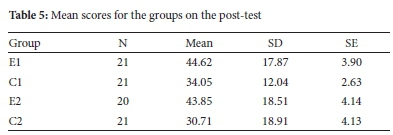
From Table 5, both experimental groups' mean scores are higher than the control groups' mean scores. Both experimental and control groups exhibited large distribution of scores among the subjects, as shown by large standard deviations. However, the distributions were comparable among groups. In the case of control groups, standard deviation was much higher for C2 than C1, showing a larger distribution of scores. ANOVA was performed to assess statistical significance in the mean score differences, and results are shown in Table 6.
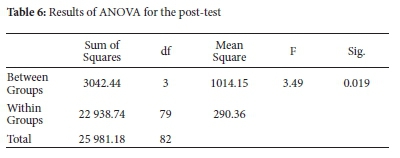
Results in Table 6 show that the differences in mean scores between and within groups are statistically significant, F (3, 79) = 3.493, p = 0.019. Tukey's post hoc test on post-test mean scores was performed at p < 0.05 to establish where the statistically significant differences occurred. The results of the analysis are shown in Table 7.
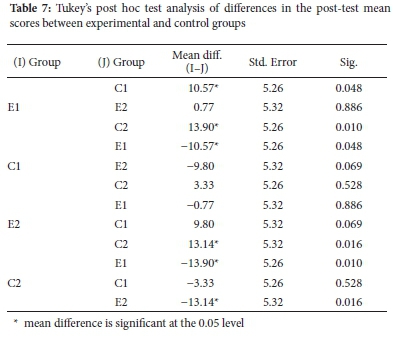
Tukey's post hoc analysis results in Table 7 show that the differences between post-test mean scores of E1, C1, and C2 are statistically significant with p<0.05. Another statistically significant difference is observed between E2 and C2 but not between E2 and C1. This absence of a statistically significant difference between mean scores of E2 and C1 may be attributed to the fact that C1 was offered a pre-test which may have primed them to work harder or anticipate what type of questions to expect, consequently narrowing the gap between them and E2. However, the mean score of the E2 group is 9.80 points higher than that of C1. There is no statistically significant difference between post-test mean scores of the two experimental groups, E1 and E2, perhaps due to similar effects of the intervention on their learning process. Similarly, there is no statistically significant difference between post-test mean scores of the two control groups, C1 and C2. These comparable post-test mean scores of control groups can also be associated with a similar effect of teaching and learning without simulations.
Post hoc results also show that the pre-test did not affect the mean scores for the post-test as there are no statistically significant differences between those groups that wrote the pre-test and those which did not (see Table 7). Therefore, any differences observed could be attributed to the mode of teaching. As a result, the posttest mean scores of experimental groups (E1 and E2) and control groups (C1 and C2) were combined. Two groups resulted, combined experimental group and combined control group, which were used in the subsequent analysis of overall performance in the post-tests. An independent samples t-test was carried out on the mean scores of combined experimental and combined control groups (see Table 8).
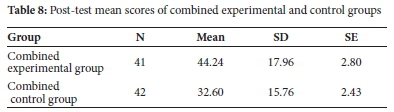
The results in Table 8 show that the mean scores of combined groups were different. On average, the combined experimental group's mean score (M=44.24, 0=17.96) is higher than the combined control group (M=32.60, 0=15.79). The independent samples t-test analysis of statistical significance for the observed difference is shown in Table 9.
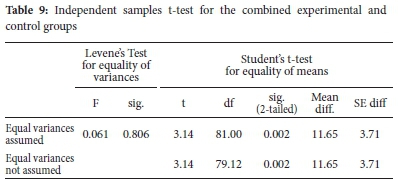
From Table 9, it can be seen that the two groups have statistically significant differences in terms of post-test mean scores, t(81) = 3.14, p = 0.002.
Assessment of performance on retention of concepts
Learners in both experimental and control groups sat for delayed post-test, three weeks post-instruction, to evaluate performance on retention of concepts. Results of groups' performance are shown in Table 10.
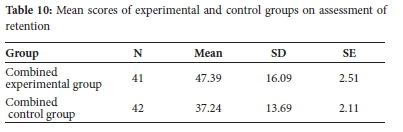
Table 10 shows that the combined experimental group had a higher retention mean score (M = 47.39, a = 16.09) than the combined control group (M = 37.24, a = 13.69). Independent samples t-test was done to establish whether the observed differences in mean scores were statistically significant or not. The results are shown in Table 11.
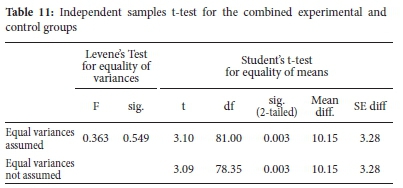
The independent samples t-test in Table 11 shows that the difference in experimental and control groups' retention mean scores was statistically significant, t(81)=3.10, p=.003. Learners in the experimental group had a statistically higher retention mean score than the control group.
DISCUSSION
This study was quasi-experimental, aiming to evaluate the influence of interactive simulations on learners' academic performance in stoichiometry. Therefore, the equivalence of groups and prior knowledge in stoichiometry were assessed before introducing the intervention. Both equivalence and pre-tests results of experimental and control groups were not statistically different in any significant way. The conclusion was that experimental and control groups were comparable in terms of prior knowledge in stoichiometry and, therefore, suitable for the study. These results formed a crucial baseline for assessing intervention effects with reasonable internal validity, enabling justified conclusions concerning intervention effects.39 Concerning the intervention, the post-test results of this study indicate that the experimental group have significantly higher mean scores than the control group. This result emerged from the post-test administered two days after instruction and the delayed post-test administered three weeks later. These observed higher mean scores of the experimental group could be attributed to the positive influence of simulations.
Therefore, the results of this study imply that simulations have a great potential to enhance learners' academic performance and retention of concepts in stoichiometry. It has to be noted that this study adopted a definition of academic performance asserting that 'academic performance refers to the knowledge attained and designated by marks, assigned by teacher'.21 Students in both experimental and control groups were awarded marks for every correct concept and step in solving problems as well as for correct final answer. Thus, high mean scores imply high marks and hence more 'knowledge attained' in each concept. Therefore, a high mean score is linked to enhanced academic performance in this study.
The higher performance of the experimental group can be attributed to the construct of CTML, which proposes that 'students are better able to build mental connections between corresponding words and pictures when both are presented (i.e. animation and narration) than when only one is presented (i.e., narration) and the learner must mentally create the other'.16 Simulations in this regard are viewed as tools that provide scaffolding effects by showing entities which are not visible to the naked eye both in form and how they interact.22 When lessons are enriched with simulations, learners can observe patterns and links of concepts in a way similar to experts.22 Experimental groups had the advantage of visualising equations and interactions in three dimensions and had a chance to make better sense of coefficients and subscripts,10 than those in control groups. Simulations help students deal with the problem of 'unfamiliarity with scientific language'32 to make meanings by manipulating the simulation parameters.17 Language has a direct link to thinking, and hence barriers imposed by language contribute to cognitive overload.30 A study on balancing chemical equations conducted with undergraduates reported that students could make meanings of stoichiometry concepts with the help of simulations;17 in this case, the language barrier was bridged with simulations leading to improved performance.
In the same dimension, high mean scores were observed for the experimental group in the delayed post-test to evaluate the retention of concepts. It could be argued that information has to be processed and stored15 for it to be retrieved later. 'According to the temporal and spatial principles, learners are more likely to retain information in their working memory when texts and images are simultaneously presented'.28 Retention of information in working memory is crucial as it creates room for assimilation and accommodation of information into the old schema.15 When information has been assimilated and/or accommodated, it forms part of the new knowledge bank stored in the long-term memory.15 The information stored in long-term memory is stored for an unlimited time15 and could potentially be retrieved when needed. However, learners in the control groups were exposed to text-based materials, not pictorial representations, which could have hindered them from experiencing deep conceptual understanding comparable to the experimental group.
The disparities in academic performance between experimental and control groups are consistent with the premise of CTML that multimedia instruction promotes meaningful learning when words and pictures are presented to supplement each other.15 Optimal stimulation of auditory and visual senses significantly facilitate conceptual understanding, leading to the storage of crucial concepts in long-term memory.15
The findings of this study corroborate similar studies that evaluated learners' performance in stoichiometry. Those studies show that learners demonstrated improved academic performance in areas such as solving conceptual problems in online stoichiometry courses,8 balancing chemical equations and deducing meanings of chemistry concepts,17,19 determining limiting and excess reagents as well as performing stoichiometry calculations.19 Improved performance was also recorded in an online-based laboratory exercise involving problem-solving.18 Other studies, though not focussing specifically on stoichiometry, reported similar findings. For instance, one study evaluated how computer simulations enhanced with conceptual change texts would affect first-year university students' understanding of concepts in chemical equilibrium. The findings revealed that the experimental group had higher mean scores than the control group in a post-test immediately after instruction and a delayed post-test eight weeks post-instruction.42 Since the experimental groups only had simulations as additional learning tools; the differences could be attributed in part to the scaffolding effects associated with simulations.25
Even though there are similarities in the findings between the current study and cited studies, there are important insights that this study highlights. First, the study reports on results from a physical face-to-face classroom encounter. Second, it unveils learners' performance from a context reportedly dominated by chalk and talk6 and lack of resources.4,5 Third and lastly, the findings are from a local context and therefore offer insights into practice for alternative ways to mitigate teaching and learning challenges such as the prevailing lack of resources for physical experimentation.
CONCLUSIONS, LIMITATIONS AND RECOMMENDATIONS
The mean scores of learners in the experimental group in post-test administered two days after instruction and post-test administered three weeks after instruction are significantly higher than those of the control groups. Therefore, these findings provide evidence for the argument that simulations can enhance academic performance and improve better retention of concepts in stoichiometry. The study's findings could address the study's objective though some limitations were identified. The first limitation concerns sample selection; all participants were from the same school and possibly had similar science learning characteristics. Therefore, findings may not be generalised to other schools such as private and rural-based schools. However, the study's findings still offer insights into simulations as important tools for teaching complex topics like stoichiometry. The second limitation is one mode of assessment (written post-tests) used to assess the effect of simulations on learners' performance. It is acknowledged that some information may have been missed, which could have assisted in deeper assessment. Therefore, in the future, similar studies involving a mixed-methods approach and several schools from different backgrounds and geographical locations could be conducted to explore further the effects of interactive simulations on learners' academic performance in chemistry or science in general. Thirdly, this study analysed quantitative data. Therefore, by its very nature, the determination of explicit considerations of how deep learning or retention of concepts happened was not possible due to the absence of qualitative data. Finally, this study suggests that science teachers should be innovative and use interactive simulations to increase their repertoire of teaching and learning strategies.
ORCID IDs
Lereko G. Mohafa - https://orcid.org/0000-0001-6057-8750
Makomosela Qhobela - https://orcid.org/0000-0001-9071-6843
Mosotho J. George - https://orcid.org/0000-0003-3217-5641
REFERENCES
1. Qhobela M, Moru EK. Understanding challenges physics teachers come across as they implement learner-centred approaches in Lesotho. Afr. J. Res. Math. Sci. Technol. Educ. 2014;18(1):67-73. https://doi.org/10.1080/10288457.2014.884351. [ Links ]
2. McFarlane DA. Understanding the challenges of science education in the 21st century: New opportunities for scientific literacy. Int. Lett. Soc. Humanist. Sci. 2013;4:35-44. [ Links ]
3. Gambari IA, Gbodi BE, Olakanmi EU, Abalaka EN. Promoting intrinsic and extrinsic motivation among chemistry students using computerassisted instruction. Contemp Educ Technol. 2016;7(1):25-46. https://doi.org/10.30935/cedtech/6161. [ Links ]
4. George MJ, Kolobe M. Exploration of the potential of using a virtual laboratory for chemistry teaching at secondary school level in Lesotho. S Afr J Chem. 2014;67:113-117. [ Links ]
5. George MJ. Assessing the level of laboratory resources for teaching and learning of chemistry at advanced level in Lesotho secondary schools. S Afr J Chem. 2017;70:154-162. https://doi.org/10.17159/0379-4350/2017/v70a22. [ Links ]
6. Makhechane M, Qhobela M. Understanding how chemistry teachers transform stoichiometry concepts at secondary level in Lesotho. S Afr J Chem. 2019;72:59-66. https://doi.org/10.17159/0379-4350/2019/v72a9. [ Links ]
7. Kanime MK. An Investigation into how Grade 11 Physical Science teachers mediate learning of the topic stoichiometry: a case study. [Masters dissertation]. ,Grahamstown, South Africa: Rhodes University; 2015. [ Links ]
8. Evans KL. Learning ltoichiometry: a comparison of text and multimedia instructional formats [PhD thesis]. Pittsburgh, USA: University of Pittsburgh; 2007. [ Links ]
9. Marais F, Combrinck S. An approach to dealing with the difficulties undergraduate chemistry students experience with stoichiometry. S Afr J Chem. 2009;62:88-96. [ Links ]
10. Taha H, Hashim R, Ismail Z, Jusoff K, Yin KY. The influence of students' concept of Mole, problem representation ability and mathematical ability on stoichiometry problem solving. Scottish. J. A. Soc. Sci. & Sci. Stud. 2014;21(1):3-21. [ Links ]
11. Sostarecz MC, Sostarecz AG. A Conceptual approach to limiting-reagent problems. J Chem Educ. 2012;89(9):1148-1151. https://doi.org/10.1021/ed200420h. [ Links ]
12. Davidowitz B, Chittleborough G, Murray E. Student-generated submicro diagrams: a useful tool for teaching and learning chemical equations and stoichiometry. Chem Educ Res Pract. 2010;11(3):154-164. https://doi.org/10.1039/C005464J. [ Links ]
13. Park SI, Lee G, Kim M. Do students benefit equally from interactive computer simulations regardless of prior knowledge levels? Comput Educ. 2009;52(3):649-655. https://doi.org/10.1016/j.compedu.2008.11.014. [ Links ]
14. Gilbert GK. The role of visual representations in the learning and teaching of science: an introduction. Asia.-Pac. Forum. Sci. Learn. & Teach. 2010;11(1):1-19. [ Links ]
15. Mayer RE, Moreno R. Nine ways to reduce cognitive load in multimedia learning. Educ Psychol. 2003;38(1):43-52. https://doi.org/10.1207/S15326985EP3801_6. [ Links ]
16. Mayer RE, Moreno R. Animation as an aid to multimedia learning. Educ Psychol Rev. 2002;14(1):87-99. https://doi.org/10.1023/A:1013184611077. [ Links ]
17. Carpenter Y, Moore EB, Perkins K. Representations and Equations in an Interactive Simulation that Support Student Development in Balancing Chemical Equations. ConfChem: Interactive Visualisations for Chemistry Teaching and Learning. University of Colorado, Boulder; 2015. [ Links ]
18. Gupta T. Promoting mathematical reasoning and problem solving through inquiry-based relevance focused computer simulations: a stoichiometry lab. Chem. Teach. Int., 2019. https://doi.org/10.1515/cti-2018-0008 [ Links ]
19. Figueiredo M, Rafael C, Neves J, Vicente H. Assessing the Role of Computer Simulation in Chemistry Learning. In P. Vittorini et al. (Eds.) Methodologies and Intelligent Systems for Technology Enhanced Learning.Adv. Intell. Syst. Comput. 2017,617, 47-56. https://doi.org/10.1007/978-3-319-60819-8_6 [ Links ]
20. Kalanda K. An investigation of ICT integration in the Lesotho secondary and high school science classroom [PhD Thesis]. Pretoria: University of South Africa; 2012. [ Links ]
21. Narad A, Abdullah B. Academic Performance of Senior Secondary School Students: Influence of Parental Encouragement and School Environment. Rupkatha J. Interdiscip. Stud. Humanit. 2016;8(2):12-19. https://doi.org/10.21659/rupkatha.v8n2.02 [ Links ]
22. Falvo DA. Animations and simulations for teaching and learning molecular chemistry. Int J Technol Teach Learn. 2008;4(1):68-77. [ Links ]
23. Vygotsky LS. Mind in Society: the Development of Higher Psychological Processes. Massachusetts, USA: Harvard University Press; 1978. [ Links ]
24. Reiser BJ. Scaffolding complex learning: the mechanisms of structuring and problematising student work. J Learn Sci. 2004;13(3):273-304. https://doi.org/10.1207/s15327809jls1303_2 [ Links ]
25. Laxman K, Chin YK. Impact of simulations on the mental models of students in the Online learning of science concepts. i-manager's J Sci Educ Technol. 2011;7(2):1-12. [ Links ]
26. Kozma R, Chin E, Russell J, Marx N. The roles of representations and tools in the chemistry laboratory and their implications for chemistry learning. J Learn Sci. 2000;9(2):105-143. https://doi.org/10.1207/s15327809jls0902_1 [ Links ]
27. Wieman CE, Perkins KK, Adams WK. Oersted Medal Lecture 2007: Interactive simulations for teaching physics: What works, what doesn't, and why. Am J Phys. 2008;76(4):393-399. https://doi.org/10.1119/1.2815365 [ Links ]
28. Lou SJ, Shih HC, Tseng KH. Improving the effectiveness of organic chemistry experiments through multimedia teaching materials for junior high school students. TurkishJ. Educ. Technol. 2012;11(2):135-141. [ Links ]
29. Sawyer RK. The Cambridge handbook of the learning sciences. Cambridge, United Kingdom: Cambridge University Press; 2006. [ Links ]
30. Sirhan G. Learning difficulties in chemistry: an overview. J. Turkish Sci. Educ. 2007;4(2):2-20. [ Links ]
31. Gulacar O, Overton TL, Bowman CR. A Closer Look at the relationships between college students' cognitive abilities and pProblem solving in stoichiometry. Eurasian J. Phys. & Chem. Educ. 2013;5(2):144-163. [ Links ]
32. Hanson R. Ghanaian Teacher Trainees' Conceptual understanding of stoichiometry. J. Educ. e-Learn. Res. 2016;3(1):1-8. [ Links ]
33. Rutten N, Van Joolingen WR, van der Veen JT. The learning effects of computer simulations in science education. Comput Educ. 2012;58(1):136-153. https://doi.org/10.1016/jxompedu.2011.07.017 [ Links ]
34. Tüysüz C. The Effect of the virtual laboratory on students' achievement and attitude in chemistry. Int Online J Educ Sci. 2010;2(1):37-53. [ Links ]
35. Pyatt K. Use of chemistry software to teach and assess model-based reaction and equation knowledge. J. Technol. & Sci. Educ. 2014;4(4):215-227. https://doi.org/10.3926/jotse.110 [ Links ]
36. Michael KY. The effect of a computer activity versus a hands on activity on product creativity in technology education. J Technol Educ. 2001;13(1):31-43. [ Links ]
37. Leedy PD, Ormrod JE. Practical Research: Planning and Design. New Jersey, USA: Pearson Educational Inc; 2005. [ Links ]
38. Muijs D. Doing Quantitative Research in Education with SPSS. London, UK: SAGE Publications Ltd; 2004. https://doi.org/10.4135/9781849209014 [ Links ]
39. Cohen L, Manion L, Morrison K. Research Methods in Education. 5th ed. New York: Routledge Falmer; 2005. [ Links ]
40. Examinations Council of Lesotho. Lesotho General Certificate of secondary Education: Physical Science Syllabus. Maseru: Lesotho; 2019. [ Links ]
41. PhET™ interactive simulations; Boulder: University of Colorado; 2020. [accessed 27 August 2020]. https://phet.colorado.edu/en/simulations/filter?subjects=chemistry&type=html&sort=alpha&view=grid [ Links ]
42. Özmen H, Naseriazar A. Effect of simulations enhanced with conceptual change texts on university students' understanding of chemical equilibrium. J Serb Chem Soc. 2018;83(1):121-137. https://doi.org/10.2298/JSC161222065O [ Links ]
Received 28 January 2021
Revised 16 August 2021
Accepted 17 August 2021
* To whom correspondence should be addressed: Email: qhobela@yahoo.com














