Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.74 Durban 2021
http://dx.doi.org/10.17159/0379-4350/2021/v74a6
RESEARCH ARTICLE
H-FER-Catalyzed Conversion of Methanol to Ethanol and Dimethyl Ether: a First-Principles DFT Study
Cecil H. BotchwayI,*; Richard TiaI; Evans AdeiI; Nelson Y. DzadeII,*; Nora H. de LeeuwII, III,*
IDepartment of Chemistry, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
IISchool of Chemistry, Cardiff University, Main Building, Park Place, Cardiff CF10 3AT, United Kingdom
IIISchool of Chemistry, University of Leeds, Leeds LS2 9JT, United Kingdom
ABSTRACT
Methanol adsorption and dehydration reactions within zeolites represent important steps in the catalytic conversion process to form long-chain hydrocarbons. Herein, first-principles density functional theory (DFT) is employed in the determination of methanol adsorption and conversion in ferrierite (FER), where we predict the fundamental adsorption geometries and energetics of methanol adsorption. The methanol molecule is shown to physisorb at all explored binding sites, stabilized through hydrogen-bonded interactions with the acid site atOmeth-Hframbond distances ranging from 1.33-1.51 A. We demonstrate that the zeolites' adsorption capability is affected by the silicon/aluminium ratio, with stronger adsorptions predicted in the material with silicon to aluminium fractions of 5 than 8. The adsorption strength is also found to vary depending on the tetrahedral binding site, with the T1O2 site yielding the most stable methanol adsorption structure in the Si/Al ratio = 5(Eads = -22.5 kcal mol-1), whereas the T1O1 site yields the most stable adsorption geometry (Eads = -19.2 kcal mol-1) in the Si/Al ratio = 8. Upon translational and rotational motion, methanol is protonated resulting in the breaking of its C-O bond to form a methoxy species bound to the framework oxygen (O-CH3 distance of 1.37 A), whereas the water molecule is stabilized at the acid site through H-bonding (Owat-H = 2.0 A). Further reaction between the methoxy species and a second methanol molecule results in the formation of ethanol and protonated dimethyl ether, with adsorption energies of -42 and -25 kcal mol-1, respectively. The results in this study provide atomistic insight into the effect of acidity of the FER zeolite on the adsorption and conversion of methanol.
Keywords: Zeolites, ferrierite, methanol adsorption, acid sites, density functional theory (DFT).
1. Introduction
Methanol is an attractive energy carrier and an abundant resource for the synthesis of important liquid fuels and hydrocarbon products.1,2 The extensively studied methanol to hydrocarbons process (MTH) is an important step in the promising route to obtaining products that are relevant to the petrochemical industry,3-5 which is crucial for the 'Methanol Economy' concept. The olefin- and aromatic-cycles are proposed as the central mechanism of methanol conversion, which consists of two catalytic cycles6 interconverting a range of surface species (hydrocarbon pool). The hydrocarbon pool mechanism can be categorized into two main parts: the olefin cycle which involves the methylation and subsequent cracking of alkenes (both small and large) and the aromatic cycle which is governed by methylation of aromatic compounds with cracking of side chains. The local concentrations of hydrocarbon species within the zeolite dictate the contribution of each cycle.10
Earlier reports have shown that the platinum-based catalyst Pt-Re/Al2O3 shows great selectivity in the alcohol conversion process with products within the range C4-C12.1 Even with a varying yield of 20-50 wt.%, the general implementation in renewable systems is severely limited by the high cost of precious metal catalysts.7 This has caused interest in the development of more earth-abundant materials as substitutes for precious metal catalysts. Zeolites, also called molecular sieves, are attractive candidates for catalytic applications.7,8 The three-dimensional (3D) frameworks of zeolites with distinctive molecular-scale features, such as pores, channels and cavities, make them very attractive candidates for methanol conversion catalysis. The channels and cages within zeolites aid distinguishing of molecules of different geometries and sizes.9 Because of their excellent catalytic activity and high hydrothermal stability under a broad scope of environmental conditions, these aluminosilicate crystals have been utilized in the refining of petrochemical products through ion exchange and adsorption/separation processes.10-12 The reaction mechanism and product selectivity in zeolites are significantly influenced by the zeolite structure.13,14 Intermediate formation and hydrocarbon production are shown to be greatly influenced by the acidity of the zeolite.4,15 For instance, reduced selectivity for light olefin products through coking is promoted by high Bransted acid concentrations.16-18 Cleavage of the C-O bond is considered to be the rate-determining step of the overall reaction with some theoretical studies determining its activation barrier to be 72 kcal mol-1.19
Methanol conversion to hydrocarbons requires the cleavage of the C-O bond and subsequent formation of C-C bonds, hence the determination of the thermodynamic stabilities of methanol and its dissociated products is of great relevance.6,20 The activation energy barrier (54 kcal mol-1) for the surface methoxy species formation in FER can be reduced by 10 kcal mol-1 when the C-O cleavage occurs near an additional methanol molecule. However, the data are limited to frameworks with a Si/Al ratio of 35 and there is barely any mention of the effect of increased acidity.21 Herein, we investigate the effects of silicon/aluminium ratios (5 and 8) on the methanol adsorption, using first-principles density functional theory (DFT) to elucidate the possible reaction pathways for the methanol C-O bond breaking and C-C bond formation proposed in previous studies. The results obtained give insights, on a molecular level, into the stable adsorption configuration with thermochemical data associated with the dehydrated process when methanol is converted in zeolite H-FER to possible precursors of short-chain hydrocarbons.
2. Computational Details
The optimized structures and energetics were determined from DFT calculations as implemented in the Quantum Espresso package.22,23 The generalized gradient approximation (GGA) with the Perdew-Burke-Ernzerhof (PBE) exchange-correlation functional was used for geometry optimizations.24 The kinetic-energy cut-off of the plane-wave was set to 40 Ry and the charge density cut-off to 480 Ry. This ensures that the convergence of the total energy is within 10-6 eV and the residual Hellmann-Feynman forces on all relaxed atoms reach 0.01 eV Ã-1.25,26 Due to the very large unit cell of FER (a = 19.0 Ã, b = 14.3 Â, c = 7.5Ã),27a1x1x1Monkhost-Packk-pointmesh was used for the integration over the Brillouin zone, which was found to be statistically adequate in describing the structural parameters of the zeolite. The lowest-energy adsorption structures and energetics of methanol were determined by adsorbing it at different sites and in different adsorption configurations. The adsorption energy (Eads), which characterized the strength and stability of the adsorbate species in the zeolite framework, was calculated using the relation:
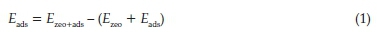
where Ezeo+ads, Ezeo, and Eads are the total adsorption energy of the zeolite with the adsorbate, isolated zeolite framework, and the free adsorbate molecule, respectively. Based on this definition, a negative or positive adsorption energy denotes an exothermic (favourable) or endothermic (unfavourable) process. The visualizations and graphical representation of all structures in this work were obtained using XCRYSDEN28 and VESTA software29.
3. Results and Discussion
3.1. Characterization of Ferrierite
All-silica FER was modelled with space group Immm, No. 71 with an orthorhombic structure.30 The initial coordinates (lattice parameters and atomic positions) obtained from the International Zeolite Association (IZA) database were subjected to full geometry optimization to attain the most stable configuration for the structure and lattice parameters such as bond length and angles based on the level of theory. Silicon atoms within the fully optimized FER framework were then substituted for aluminium atoms at the various tetrahedral sites to suit the desired Si/Al ratios of 5 and 8. The distribution of the substituted Al atoms obeyed the Löwenstein's rule,31 prohibiting Al-O-Al connections and also the Dempsey's rule permitting the maximum allocation of negative charges within the frame-work.32Figure 1 shows the fully optimized all-silica and Al-substituted H-FER. Summarized in Table 1 are the optimized structural parameters including the lattice parameters, interatomic bond distances and angles, which are all in good agreement with known experimental data33,34 and previous DFT calculations.35-37
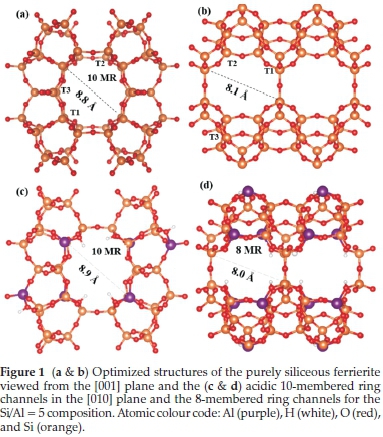
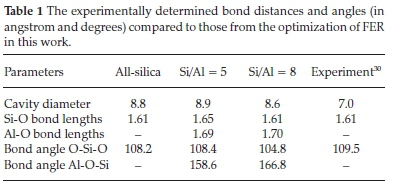
The charge deficiency created in the Al substituted framework was compensated with H protons at neighbouring oxygen atoms, thereby forming Bronsted acid sites. Two different Si/Al ratios were considered (5 and 8) and the structural parameters in each composition were determined as reported in Table 1. We observed no significant changes in the structural parameters of the Si/Al ratio composition compared to the all-silica FER.
3.2. Methanol Adsorption in Ferrierite
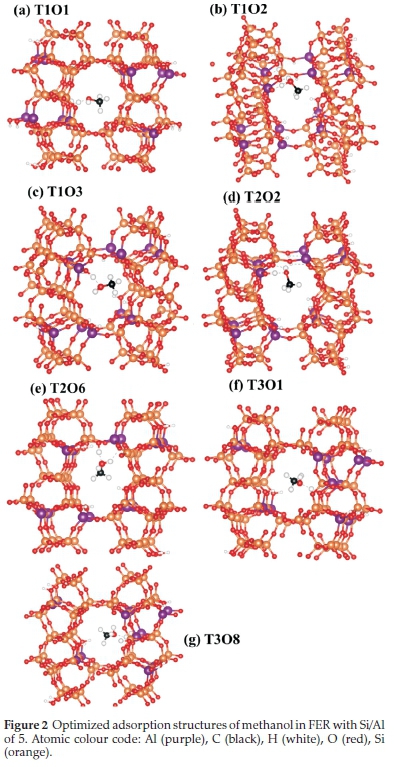
The adsorption and dehydration of a methanol molecule in the zeolite framework is an important starting step in its conversion to safer and more useful renewable fuels. We therefore first determined the lowest-energy adsorption configuration of methanol in the FER framework with Si/Al ratios of 8 and 5, and characterized the extent of C-O bond activation. The preferred methanol adsorption sites within the framework were determined by exploring the T1, T2, and T3 sites in the 10 MR channel of the H-FER zeolite (Fig. 1). The lowest-energy adsorption structures of methanol in the FER framework with ratios of 8 and 5 are shown in Figs. 2 and 3, respectively. The methanol molecule is physisorbed at all explored binding sites where it is stabilized through H-bonding with the acid site at Ometh-Hfram bond distances ranging from 1.33-1.51 Â. As shown in Table 2, the adsorption energies are found to be generally more stable in the Si/Al ratio of 5 than 8, which can be linked to the high concentration of acid sites within the zeolite ratio of 5 that permits the formation of more hydrogen-bonded interactions compared to a ratio of 8. The adsorption strength is found to vary depending on the tetrahedral binding site, with the T1O2 and T1O1 sites yielding the most stable methanol adsorption structure in ratio 5(Eads = -22.5 kcal mol-1) and 8 (Eads = -19.2 kcal mol-1), respectively. The most stable adsorption structures are characterized by shorter Ometh-Hfram bond distances as shown in Table 2. The relative energies obtained are in good agreement with previously reported values of 15-27 kcal mol-1 in the literature.38,20
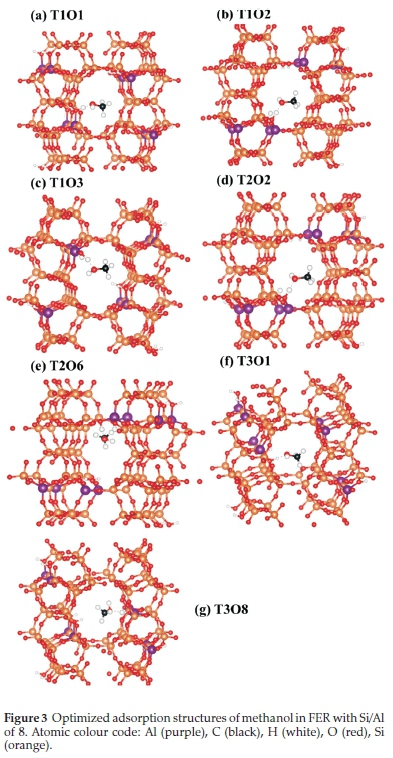
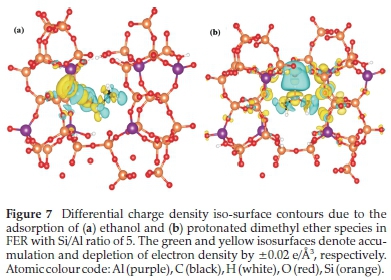
From the differential charge density isosurface analysis (Fig. 4), we observed electron density accumulation in the Ometh-Hfram regions, which is consistent with H-bonded interactions.
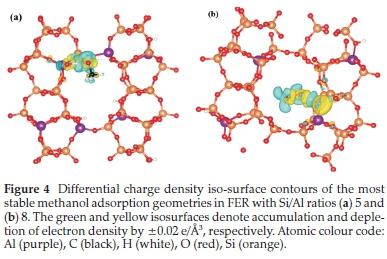
3.3. Methanol Dehydration to form Methoxy Species in FER
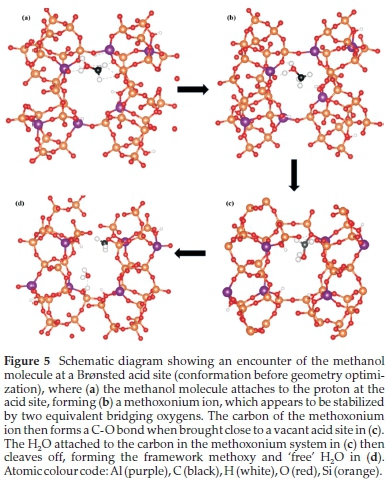
Protonation of short-chain alcohols in zeolites occurs through geometrical changes owing to a high concentration of charge. The protonation step has been described as a concerted reaction between the O-H bond of the framework and the O-H of methanol, which results in the C-O bond breaking. The protonation process is generally characterized by various translational and rotational motions39 that leads to the cleavage of the C-O in dehydration. Shown in Fig. 5 is the schematic of the dehydration process of methanol within the FER framework with Si/Al = 5, where the physisorbed methanol attracts a proton at the acid site, reorients such that the -CH3 end binds at an O-site, followed by the final spontaneous dehydration step. It is worth noting that stable protonated methanol (as observed in Fig. 5b), which was not observed in earlier studies, was obtained after geometry optimization.40,21 This structure, leading to an adsorption energy of -15 kcal mol-1, is stabilized because of the orientation of the methanol molecule between two close and electro-negatively equivalent framework O2 oxygens (O2 are 2.63 A apart). The methoxy species is bound at an O-CH3 distance of 1.37 A, whereas the water molecule is stabilized at the acid site through H-bonding (Owat-H = 2.0 A). The co-adsorption of the methoxy species and water molecule releases an energy of 11 kcal mol-1, which compared to the physisorbed methanol molecule, indicates an endothermic reaction. The understand-ingbehind the endothermicity of the methoxy species formation is that breaking a very strong methanol HO-CH3 bond requires a greater amount of energy compared to forming the weaker framework O-CH3 and H-bonded H2O to the framework.
3.4. Post Dehydration Reactions (Ethanol Formation)
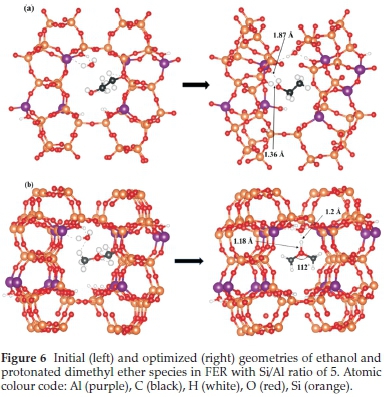
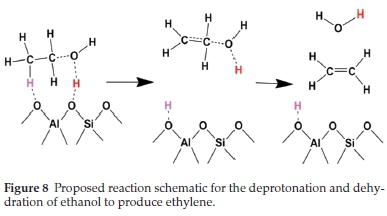
The thermodynamic stability of the products formed when a second methanol molecule reacts with the framework methoxy species was also investigated. The incoming methanol molecule can attach to the methoxy species via two possible modes: either through its carbon end (forming a C-C bond) or the hydroxyl oxygen (forming an O-C bond). The C-C mode of attachment resulted in the formation of ethanol, which released an energy of 42 kcal mol-1, as clearly illustrated in Fig. 6a. The O-C mode of attachment, on the other hand, resulted in the formation of a stable protonated dimethyl ether species (Fig. 6b), which released an energy of 25 kcal mol-1. The increased stability of the ethanol molecule is a direct result of strong H-bond interaction between the O-H end of the ethanol and the proton of the Br0nsted acid sites (O-H = 1.36 A), which is consistent with the observed electron density accumulation in the interaction regions, as revealed by the differential charge densityisosurface contour plot shown in Fig. 7a. A similar observation was made in a previous study, where the product was referred to as an ethoxonium ion.41 The formation of the ethanol molecule is made possible by hydrogen transfer from the carbon end of the incoming methanol molecule to the hydroxyl oxygen end, which finally detaches from the framework O site. Although the protonated dimethyl ether species is lower in thermodynamic stability compared to the formed ethanol, it is known to be the predominant species that is formed in the methanol to gasoline (MTG) process.42,43 The protonated dimethyl ether is observed in some experiments to form at lower temperatures, but at higher temperatures, it degenerates back to the methoxy species.33 H-bonding between the proton attached to the dimethyl ether and the water molecule in the channel at 1.2 A enhances the stabilization of the ion formed (Fig. 7b). An O-H bond distance of 1.18 A and C-O-C bond angle of 112 ° for dimethyl ether was observed, as canbe seen in the optimized structure (Fig. 6b). This result can be attributed to the highly acidic nature of the zeolite and the existence of a water molecule as a product of dehydration in the zeolite. Water molecules are not only speculated to pose as delocalizing agents of protons in the framework, but also facilitate the thermodynamic stability of the products that are formed through H-bonding. It was also observed that the FER pore undergoes a distortion where the T-O-Si bond angle changes by 28 ° with a corresponding reduction in the pore diameter from 8.9 to 8.3 A. Since the pore is charge-saturated, the framework oxygens are more likely to cause an elliptical distortion of the channel to better accommodate the ethoxonium, which has been reported previously.44-46 The adsorbed ethanol molecule could undergo deprotonation and dehydration reactions to produce ethylene, based on the proposed scheme shown in Fig. 8.
4. Conclusion
The adsorption and conversion reactions of methanol in the FER framework with different Si/Al ratios of 5 and 8, have been studied employing first-principles DFT calculations. Based on predicted adsorption geometries and energetics, it was demonstrated that the methanol molecule is physisorbed at all explored binding sites, where it is stabilized through hydrogen-bonded interactions with the acid site at Ometh-Hfram bond distances ranging from 1.33-1.51 A. Stronger adsorption energies were predicted for the FER with Si/Al ratio of 5 than 8, with the most stable adsorption geometries releasing energies of -22.5 and -19.2 kcal mol-1, which suggests that the adsorption strength of methanol is affected by the Si/Al ratio. Protonation of the adsorbed methanol molecule results in translational and rotational motions leading to the breaking of the C-O bond to form methoxy species bound to the framework oxygen (O-CH3 distance of 1.37 A), whereas a water molecule is stabilized at the acid site through hydrogen-bonded interactions (Owat-H = 2.0 A). The formation of stable physisorbed ethanol and protonated dimethyl ether species is demonstrated from further reaction with a second methanol molecule. These results provide atomistic insight into the adsorption geometries and energetics of methanol and its reaction products in the FER zeolite.
Acknowledgements
The authors acknowledge the Royal Society and the UK Department for International Development, for funding under the Africa Capacity Building Initiative (ACBI), which has supported this research, and we thank Prof. Richard Catlow for valuable consultations. We also acknowledge funding from the UK Engineering and Physical Sciences Research Council (NHdL: grant no. EP/K009567; NYD: grant no. EP/S001395/1) and UK Natural Environment Research Council (NH.d.L.: grant no. NE/R009376). Computing resources of the Centre for High-Performance Computing (CHPC), South Africa, and the Advanced Research Computing ©Cardiff (ARCCA) Division were used for the calculations in this work.
Supplementary Material
The supplementary information contains the optimized atomic coordinates for acidic FER with and without adsorbing species.
ORCID iDs
C.H. Botchway: orcid.org/0000-0001-7733-9473
N.Y.Dzade: orcid.org/0000-0003-1583-588X
N.H. de Leeuw: orcid.org/0000-0002-8271-0545
References
1 M.V. Tsodikov, V.Y. Murzin, A.V. Chistyakov, F.A. Yandieva, M.A. Gubanov, P.A. Zharova, S.S. Shapovalov, O.G. Tikhonova, A.A. Pasynskii, S. Paul and F. Dumeignil, The direct ethanol conversion to hydrocarbons over Pt-containing catalysts, Chem. Engin. Trans., 2014, 37, 583-588. [ Links ]
2 U. Olsbye, S. Svelle, M. Bjorgen, P. Beato, T.V.W. Janssens, F. Joensen, S. Bordiga and K.P. Lillerud, Conversion of methanol to hydrocarbons: how zeolite cavity and pore size control product selectivity, Angew. Chemie - Int. Edn., 2012,1, 5810-5831. [ Links ]
3 S. Ilias and A. Bhan, Mechanism of the catalytic conversion of methanol to hydrocarbons, ACS Catal., 2013, 3, 18-31. [ Links ]
4 M. Westgârd Erichsen, S. Svelle and U. Olsbye, The influence of catalyst acid strength on the methanol to hydrocarbons (MTH) reaction, Catal. Today, 2013, 215, 216-223. [ Links ]
5 I. Yarulina, A.D. Chowdhury, F. Meirer, B.M. Weckhuysen and J. Gascon, Recent trends and fundamental insights in the methanol-to-hydrocarbons process, Nat. Catal., 2018, 1, 398-411. [ Links ]
6 I.M. Dahl and S. Kolboe, On the reaction mechanism for hydrocarbon formation from methanol over SAPO-34. I. Isotopic labeling studies of the co-reaction of ethene and methanol, J. Catal., 1994, 149, 458-464. [ Links ]
7 C.D. Chang and A.J. Silvestri, The conversion of methanol and other O-Compounds to hydrocarbons over zeolite catalysts, J. Catal., 1977, 47, 249-259. [ Links ]
8 C.T.-W. Chu, G.H. Kuehl, R.M. Lago and C.D. Chang, Isomorphous substitution in zeolite frameworks: catalytic properties of [B]ZSM-5, J. Catal., 1985, 93, 451-458. [ Links ]
9 C. Marcilly, Zeolites and Mesoporous materials at the dawn of the 21st century, Proceedings of the 13th International Zeolite Conference, Stud. Surf. Sci. Catal., 2001, 135, 37-60. [ Links ]
10 A. Corma, M. J. Diaz-Cabañas, J. Martínez-Triguero, F. Rey and J.A. Rius, Large-cavity zeolite with wide pore windows and potential as an oil refining catalyst, Nature, 2002, 418, 514-517. [ Links ]
11 W. Vermeiren and J.P. Gilson, Impact ofzeolites on the petroleum and petrochemical industry, Top. Catal., 2009, 52, 1131-1161. [ Links ]
12 J.E. Naber, K.P. de Jong, W.H.J. Stork, H.P.C.E. Kuipers and M.F.M. Post, Industrial applications of zeolite catalysis, Stud. Surf. Sci. Catal., 1994, 84, 2197-2219. [ Links ]
13 T. Frising and P. Leflaive, Extraframework cation distributions in X and Y faujasite zeolites: a review, Microp. Mesop. Mater., 2008, 114, 27-63. [ Links ]
14 B. Yilmaz and U. Müller, Catalytic applications of zeolites in chemical industry, Top. Catal., 2009, 52, 888-895. [ Links ]
15 F. Bleken, M. Bj0rgen, L. Palumbo, S. Bordiga, S. Svelle, K.P. Lillerud and U. Olsbye, The effect of acid strength on the conversion of methanol to olefins over acidic microporous catalysts with the CHA topology, Top. Catal., 2009, 52, 218-228. [ Links ]
16 J. Chen, J. Li, C. Yuan, S. Xu, Y. Wei, Q. Wang, Y. Zhou, J. Wang, M. Zhang, Y. He, S. Xu and Z. Liu, Elucidating the olefin formation mechanism in the methanol to olefin reaction over AlPO-18 and SAPO-18, Catal. Sci. Technol., 2014,4, 3268-3277. [ Links ]
17 J.F. Haw, W. Song, D.M. Marcus and J.B. Nicholas, The mechanism of methanol to hydrocarbon catalysis, Acc. Chem. Res., 2003, 36, 317-326. [ Links ]
18 W. Dai, N. Li, L. Li, N. Guan and M. Hunger, Unexpected methanol-to-olefin conversion activity of low-silica aluminophosphate molecular sieves, Catal. Commun., 2011, 16, 124-127. [ Links ]
19 S. Kim, D.J. Robichaud, G.T. Beckham, R.S. Paton and M.R. Nimlos, Ethanol dehydration in HZSM-5 studied by density functional theory: evidence for a concerted process, J. Phys. Chem. A, 2015,119, 3604-3614. [ Links ]
20 F. Haase and J. Sauer, Interaction of methanol with Bransted acid sites of zeolite catalysts: an ab initio study, J. Am. Chem. Soc., 1995, 117, 3780-3789. [ Links ]
21 J. Andzelm, N. Govind, G. Fitzgerald and A. Maiti, DFT study of methanol conversion to hydrocarbons in a zeolite catalyst, Int. J. Quantum Chem., 2003, 91, 467-473. [ Links ]
22 D. Vanderbilt, Soft self-consistent pseudopotentials in a generalized eigenvalue formalism, Phys. Rev. B, 1990, 41, 7892-7895. [ Links ]
23 P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G.L. Chiarotti, M. Cococcioni, I. Dabo, A. Dal Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A.P. Seitsonen, A. Smogunov, P. Umari and R.M. Wentzcovitch, QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials, J. Phys. Condens. Matter, 2009, 21, 395502. [ Links ]
24 J.P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865-3868. [ Links ]
25 J. Fletcher, A new approach to variable metric algorithms, Comput. J., 1970,13, 1689-1699. [ Links ]
26 C.G. Broyden, The convergence of a class of double-rank minimization algorithms 1. General considerations, IMA J. Appl. Math., Institute Math. Appl., 1970, 6, 76-90. [ Links ]
27 C. ColellaandW.S.Wise,The IZA Handbook of natural zeolites:a tool of knowledge on the most important family of porous minerals, Microp. Mesop. Mater., 2014, 189, 4-10. [ Links ]
28 A. Kokalj, XCrySDen - a new program for displaying crystalline structures and electron densities, J. Mol. Graph. Model., 1999, 17, 176-179. [ Links ]
29 K. Momma and F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data, J. Appl. Crystallogr., 2011,44, 1272-1276. [ Links ]
30 P.A. Vaughan, The crystal structure of the zeolite ferrierite, Acta Crystallogr., 1966, 21, 983-990. [ Links ]
31 W. Loewenstein, The distribution of aluminum in the tetrahedra of silicates and aluminates, Am. Mineral., 1954, 39, 92-96. [ Links ]
32 E. Dempsey, G.H. Kühl and D.H. Olson, Variation of the lattice parameter with aluminum content in synthetic sodium faujasites. Evidencefor ordering of the framework ions, J. Phys. Chem., 1969, 73, 387-390. [ Links ]
33 T.R. Forester and R.F. Howe, In situ FTIR studies of methanol and dimethyl ether in ZSM-5, J. Am. Chem. Soc., 1987,109, 5076-5082. [ Links ]
34 K. Koyama and Y. Takéuchi, Clinoptilolite: the distribution of potassium atoms and its role in thermal stability, Z. Krist. - New Cryst. Struct., 1977, 145, 216-239. [ Links ]
35 E. Martínez-Morales and C.M. Zicovich-Wilson, Adjusting framework ionicity to favour crystallisation of zeolites with strained structural units. Periodic quantum chemical studies, Catal. Sci. Technol., 2011,1, 868-878. [ Links ]
36 C.M. Zicovich-Wilson, M.L. San Román and A. Ramírez-Solís, Mechanism of F-elimination from zeolitic D4R units: a periodic B3LYP study on the octadecasil zeolite, J. Phys. Chem. C, 2010,114,2989-2995. [ Links ]
37 K. Valdiviés-Cruz, A. Lam and C.M. Zicovich-Wilson, Full mechanism of zeolite dealumination in aqueous strong acid medium: ab initio periodic study on H-clinoptilolite, J. Phys. Chem. C, 2017, 121, 2652-2660. [ Links ]
38 P.E. Sinclair and C.R.A. Catlow, Computational studies of the reaction of methanol at aluminosilicate Bransted acid sites, J. Chem. Soc. - Faraday Trans., 1996, 92, 2099-2105. [ Links ]
39 A.J. O'Malley, S.F. Parker, A. Chutia, M.R. Farrow, I.P. Silverwood, V García-Sakai and C.R.A. Catlow, Room temperature methoxylation in zeolites: insight into a key step of the methanol-to-hydrocarbons process, Chem. Commun., 2016, 52, 2897-2900. [ Links ]
40 F. Haase and J. Sauer, Ab initio molecular dynamics simulation of methanol interacting with acidic zeolites of different framework structure, Microp. Mesop. Mater., 2000, 35-36, 379-385. [ Links ]
41 K. Alexopoulos, M.S. Lee, Y. Liu, Y. Zhi, Y. Liu, M.F. Reyniers, G.B. Marin, V.A. Glezakou, R. Rousseau and J.A. Lercher, Anharmonicity and confinement in zeolites: structure, spectroscopy, and adsorption free energy of ethanol in H-ZSM-5, J. Phys. Chem. C, 2016, 120, 7172-7182. [ Links ]
42 Z. Li, J. Martinez-Triguero, J. Yu and A. Corma, Conversion of methanol to olefins: stabilization of nanosized SAPO-34 by hydrothermal treatment, J. Catal., 2015, 329, 379-388. [ Links ]
43 A.T. Aguayo, A.G. Gayubo, A.M. Tarrio, A. Atutxa and J. Bilbao, Study of operating variables in the transformation of aqueous ethanol into hydrocarbons on an HZSM-5 zeolite, J. Chem. Technol. Biotechnol., 2002, 77, 211-216. [ Links ]
44 D.R. Corbin, L. Abrams, G.A. Jones, R.L. Harlow and P.J. Dunn, Flexibility of the zeolite RHO framework: effect of dehydration on the crystal structure of the beryllophosphate mineral, pahasapaite, Zeolites, 1991, 11, 364-367. [ Links ]
45 A. Nearchou, M.U. Cornelius, Z.L. Jones, I.E. Collings, S.A. Wells, P.R. Raithby and A. Sartbaeva, Pressure-induced symmetry changes in body-centred cubic zeolites, R. Soc. Open Sci., 2019, 6, 182158. [ Links ]
46 I. Stich, J.D. Gale, K. Terakura and M.C. Payne, Role of the zeolitic environment in catalytic activation of methanol, J. Am. Chem. Soc., 1999,121, 3292-3302. [ Links ]
Received 10 February 2020
Revised 6 August 2020
Accepted 9 September 2020
* To whom correspondence should be addressed. E-mail: C.H.B., cecllhbotchway@outlook.com / N.Y.D., dzadeny@cardlff.ac.uk / N.H.d.L., n.h.deleeuw@leeds.ac.uk
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














