Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.75 Durban 2021
http://dx.doi.org/10.17159/0379-4350/2021/v75a15
RESEARCH ARTICLE
Preparation and Characterisation of the Cyano-Bridged Transition Metal Complexes Using N,N-Diethyl Thiourea as a Ligand
Dursun Karaağaç *
Ulubatli Hasan Anatolian High School, 16320, Bursa, Turkey
ABSTRACT
New cyano bridged transition metal complexes, [Cu(detu)4Ni(CN)4]-2H2O (1) and [Zn(H2O)(detu)Ni(CN)4]-2H2O (2) (detu = N, N' diethyl thiourea) have been synthesised in powder form. Their structures were illuminated by using spectroscopic, thermal and elemental analysis techniques. The nickel atom exhibits square planar geometry in these complexes by coordinating with the cyano group's nitrogen atoms. The copper atom of 1 is six coordinated with two bridging cyano groups and four detu ligands. In contrast, the zinc atom of 2 is six coordinated with four bridging cyano groups, one detu ligand and one aqua ligand. In addition, the structure of 2 is formed from polymeric layers of |Zn-Ni(CN)41 „ with the detu and aqua ligands bonded to the zinc atom. Thermal stabilities and decomposition products of 1 and 2 were examined in the static air atmosphere between 30 and 900 °C.
Keywords: btetracyanonickelate(II) complex, N,N'-diethyl thiourea, cyano-bridged complex, vibration spectra, thermal analysis.
1. Introduction
Thioureas have two amino nitrogens and thiocarbonyl sulphur as potential donor atoms. They can coordinate to metal atoms using any possible donor atom but are commonly bonded to metal atoms via the sulphur atoms.1 Due to the diversity of donor atoms of thioureas, they may show different binding modes to metal atoms. Numerous studies have been performed on the biological activities and metal complexes of thiourea.2,3,4 Some thioureas exhibit antituberculosis, antitumor, antibacterial and anticonvulsant features as biological activity.5,6,7,8 Numerous studies on spectroscopic and antibacterial properties of thiourea metal complexes have been reported.2,9 A large number of structural studies of metal complexes with a thiourea ligand have been done,1,2,3,4,8,9 whereas studies on the spectroscopic and crystal structures of metal complexes containing thiourea and cyano ligands are limited.10,11,12
The cyano ligand is a multifaceted ligand that can act as σ-donor and π-acceptor. Using donor atoms, the cyano anion (CN-) can either provide monodentate (C-) or bidentate (C-, N-) coordination to metal atoms and act as a bridge ligand (M-CN-M') with the participation of both donor atoms. With this feature, the cyano ligand can produce various cyano-bridged transition metal complexes. The square-planar metal complexes with the cyano bridges are generally created by transition metal ions such as nickel, palladium and platinum, which have a d8 electronic configuration.13,14 Planar cyano metal anions are widely used to design these complexes.14,15,16,17 Especially, square-planar tetra cyanometallate(II) ions, i.e. [M(CN)4]2- (M(II) = Ni, Pd or Pt), have been widely used as building blocks of Hofmann type structures.18,19,21,22,23 The vibrational spectroscopy is extensively used in the determination of the structures of Hofmann type complexes because cyano ligand gives sharp and intense v(CN) stretching vibration between 2200 and 2100 cm-1 in the infrared (IR) and Raman spectra.18,19,20,24
This paper reported the synthesis and characterisation of the cyano-bridged transition metal complexes with N,N'-diethyl thiourea. Ligands with N-donor groups have been used extensively to obtain cyano bridged complexes, but cyano-bridged complexes with S-donor ligands are rare. In this study, two new cyano-bridged complexes given with molecular formula [Cu(detu)4Ni(CN)4]-2H2O (1) and [Zn(H2O) (detu)Ni(CN)4]-2H2O (2) (detu = N,N'-diethyl thiourea) were synthesised for the first time. In order to obtain these complexes, many experiments have been done by using metals who's the first metal are copper, zinc, and cadmium. Only the complexes with copper and zinc as the first metal have been successfully synthesised. The structures of the resulting complexes were elucidated by element analysis, IR and Raman spectra, and thermal analysis.
2. Experimental
2.1. Materials
Copper(II) chloride dihydrate (CuCl2-2H2O, 99%, Merck), zinc(II) chloride (ZnCl2, 96%, Merck), nickel(II) chloride hexahydrate (NiCl2-6H2O, 97%, Merck), potassium cyanide (KCN, 96%, Sigma-Aldrich) and N,N'-diethyl thiourea (C5H12N2S, 99%, Merck ) were purchased and used.
2.2. Syntheses of the complexes
Synthesis of K2[Ni(CN)i]-H2O 1 mmol of NiCl2-6H2O (0.238 g) was dissolved in 100 ml of
distilled water. 4 mmol of KCN (0.260 g) dissolved in 100 ml
of distilled water was added dropwise to this solution. The prepared solution was stirred with a magnetic stirrer for 3 hours and then allowed to stand at room temperature. Within two weeks, the K2[Ni(CN)4]-H2O complex was obtained.
Synthesis of M[Ni(CN)4]H2O [M = Cu(II) or Zn(II)]
1 mmol of K2[Ni(CN)4]-H2O (0.259 g) complex was dissolved in distilled water by mixing with a magnetic stirrer for 5 minutes. The aqueous solution of 1 mmol metal chloride (CuCl2-2H2O = 0.170 g or ZnCl2 = 0.136 g) was added drop by drop to the tetracyano nickelate solution. The solutions were stirred in the magnetic stirrer at room temperature for 3 hours, and then the M[Ni(CN)4]-H2O complex was obtained.
Synthesis of [Cu(áetu)iNi(CN)i]-2H2O and [Zn(H2O)(áetu)(CN)4]-2H2O 1 mmol of M[Ni(CN)4]-H2O (Cu[Ni(CN)4]-H2O = 0.244 g or Zn[Ni(CN)4]-H2O = 0.246 g) was dissolved in 25 ml distilled water. Into this solution, 2 mmol of the ligand dissolved in a mixture of methanol (50%), ethanol (25%) and distilled water (25%) (detu = 0.264 g) was added drop by drop. The solution obtained was stirred in a magnetic stirrer at 40 °C for 3 hours, and then the resulting complexes were filtered and washed with distilled water and ethanol, respectively, and dried in air. Analyses of these complexes were done for C, H and N: Anal. Found (Calcd.) (%) for C24H52N12O2S4CuNi (Mw = 791.25 g/mol) : C, 36.76 (36.43); H, 6.81 (6.62); N, 20.39 (21.24); for C9H18N6O3SZnNi (Mw = 414.43 g/mol) : C, 25.56 (26.08); H, 3.16
(4.38); N, 20.02 (20.28).
2.3. Measurements
The complexes obtained were analysed for C, H, and N with a LECO CHN-932 analyser at the Middle East Technical University Central Laboratory in Ankara, Turkey. The infrared spectra were recorded on a Perkin Elmer 100 infrared spectrometer using KBr pellets between 4000 and 400 cm-1 (2 cm-1 resolution), calibrated using polystyrene and CO2 bands. Raman spectrum of the obtained complexes was recorded on a Bruker Senterra Dispersive Raman apparatus between 4000 and 250 cm-1 using 785 nm laser excitation. Thermal analysis was carried out on Perkin Elmer Diamond TG/DTA thermal analyser instrument in a static air atmosphere with a heating rate of 10 K min-1 in the range of 30-900 °C.
3. Results and Discussion
3.1. Spectroscopic studies of the complexes
Vibrations of the detu ligand
The vibration spectra of the synthesised complexes are shown in Figs. 1 and 2.
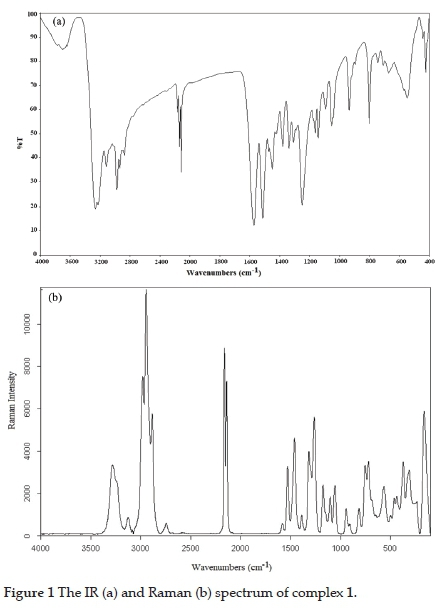
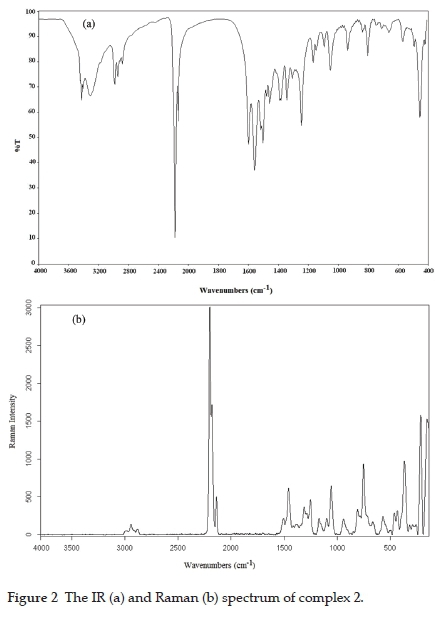
Experimentally obtained vibration frequencies of detu and the previously determined vibration assignments of the N,N'-dimethyl thiourea (dmtu) molecule are given in Table 1.25,26 The vibration assignments of the detu ligand were not available in the literature. According to our research, the dmtu and detu have very similar spectral properties. Therefore, the dmtu vibration bands in the literature were used to compare with the detu vibration bands. In addition, the vibration assignments and wavenumbers of the ethyl group were taken from N-ethyl thiourea.27
In the IR spectrum of the detu ligand, characteristic vibration bands in three frequency regions are expected. These bands are v(NH) between 3435 and 3270 cm-1, v(CN) at 1564 cm-1 and v(CS) at 756 cm-1. When the detu ligand binds to metal atoms through sulphur atoms, significant shifts occur in the characteristic bands of the ligand. These shifts generally manifest themselves in the v(CN) and v(CS) bands.28,29 Some of the v(NH) and v(OH) stretching vibration bands overlap between 3100 and 3450 cm-1 because of the expansion of the bands. This expansion may arise from the coordination or hydrogen bonds and create spectral complexity that can not be completely unregulated. In the vibration (infrared and Raman) spectra of 1 and 2, the v(NH) stretching vibrations of the ligand were observed as broad bands in strong or medium density between 3100 and 3450 cm-1. When the detu ligand is attached to the metal ion via the sulphur atom, the effect of the metal on the ligand becomes more pronounced in the N-C and C=S groups of the ligand. In this case, while the v(CN) vibration frequencies shift upwards, the v(CS) stretching vibration frequencies are expected to shift downwards.28-29 In the infrared spectrum of the detu ligand, the v(CN) frequency was observed at 1564 cm-1. As expected, the v(CN) frequency shifted to the higher frequency region as a strong and sharp band in the range of 5-30 cm-1. These apparent shifts in the spectra of 1 and 2 prove that the detu ligand is attached to the metal atoms through sulphur atoms. While v(CS) stretching vibrations have appeared as a single sharp band at 756 cm-1 in the infrared spectra of the ligand, the v(CS) stretching vibrations in the infrared spectrum of the complexes have shifted to the high-frequency region and in the Raman spectrum to the low-frequency region.
On the contrary, in some complexes, these vibrations are strongly coupled with other modes of ligand, and therefore the v(CS) stretching vibration frequencies shift upward. Similar observations were performed in ethyl thiourea complexes.1,30 As shown in Table 1, v(CH3) and v(CH2) stretching vibrations in the infrared spectrum of ligand are found in the range of 30702840 cm-1. In the complexes, these stretching vibrations were identified in the range of 3070-2874 cm-1. It was observed that these stretching vibration frequencies shifted significantly to low or high-frequency regions according to the free ligand. In addition, the ligand includes C = C stretching vibrations between 1700 and 1200 cm-1, C=N stretching vibrations between 1400 and 1300 cm-1, and C-H bond deformation vibrations between 1460 and 1400 cm-1. These vibration bands show upward or downward shifts in frequency in the complexes compared to that of the free ligand.
Water vibrations
Water molecules have three fundamental vibrations: asymmetric and symmetric v(OH) stretching and δ(ΗΟΗ) bending. In general, v(OH) stretching and δ(ΗΟΗ) bending vibrations are found in the 3700-3200 cm-1 region and the 1700-1600 cm-1 region, respectively. In addition, lattice or coordinated water absorbs between 3550 and 3200 cm-1.31,32 It was observed from the infrared spectra of the complexes that there are water molecules in the structure of the complexes, and it was determined that the water molecules in the complexes act as crystal water (uncoordinated water molecules). The v(OH) of water molecules in the complexes were observed as broadband between 4000 and 3500 cm-1 for uncoordinated water molecules of the complexes and at 3454 cm-1 for coordinated water molecules of complex 2. In the spectrum of complex 2, stretching bands of coordinated water molecules with stretching bands of NH vibrations in the detu ligand overlap in the range of 3450-3200 cm-1. In addition, bending vibrations in the complexes overlap with other vibration bands in ligands. Elemental analysis and thermal analysis results supported the presence of water molecules in the complexes.
Vibrations of the tetracyano nickelate group
In the complexes obtained, vibration bands belonging to the tetracyano nickelate group are determined according to vibrational data of the tetracyano nickelate ion of the Na2[Ni(CN)4] salt in the solid form.33 The wavenumbers belonging to the tetracyano nickelate group in the complexes. Data for the K2[Ni(CN)4]-H2O complex are given in Table 2. The tetracyano nickelate ion has a square planar structure. The Ni atom in this ion is located at the intersection of the diagonals. The nitrogen atoms are at the corners of the squares, and the carbon atoms are located between the nickel and nitrogen atoms. Since the tetracyano nickelate ions in the salts are not bound by K+ (or Na+) cations, they can be considered as isolated units in D4h symmetry.34,35 Therefore, when the Ni-C=N-M type bridge and Ni-C=N type terminal groups occur in this salt, the changes in the cyano vibration wavenumbers in this group gain importance in evaluating the structure of the complexes. In the IR spectra of 1 and 2, the bridge-type v(CN) stretching vibrational wavenumbers is greater than those of the terminal-type. The v(CN) stretching vibrational wavenumbers of the terminal-type is close to that of K2[Ni(CN)4]-H2O. From the vibration spectra of the complexes, it can be decided that the cyano groups act as a bridge or terminal ligand by examining the shifts and splitting in the frequency values of the stretching vibration bands.
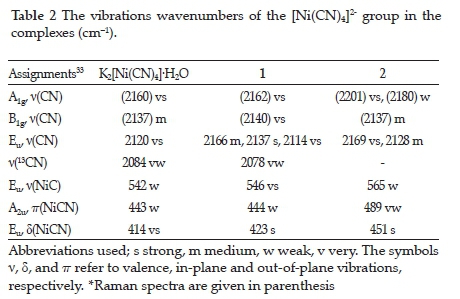
The most characteristic band in cyano complexes is the v(CN) stretching vibration band belonging to the cyano group. This band can be easily determined in the vibration spectra of 1 and 2 since it is a sharp and strong band between 2200 and 2000 cm-1.31 In the vibration spectra of 1 and 2, the v(CN) stretching vibrations have one infrared active (Eu mode) and two Raman active (A1g and B1g modes) modes. While the Eu symmetrical v(CN) stretching vibration band in the infrared spectra of K2[Ni(CN)4]-H2O is found at 2120 cm-1, the stretching vibrations with A1g and B1g symmetry resulting from Raman active v(CN) vibration are observed at 2160 cm-1 and 2137 cm-1, respectively. If there are both Ni-C=N-M type bridges and Ni-C=N terminal cyano groups in the structures of the cyano-bridged transition metal complexes, the v(CN) stretching vibration band undergoes a split in the infrared spectrum.36 In complex 1, the v(CN) stretching vibrations of the bridging cyano group are found at 2166 and 2137 cm-1, whereas the terminal cyano groups are observed at 2114 cm-1 (Table 2). According to this, the v(CN) stretching vibration frequencies in the IR spectrum of 1 are higher, around 17 and 46 cm-1, than those for K2[Ni(CN)4]-H2O salt. Shifts to high frequencies are thought to occur due to mechanical coupling between the internal vibration modes of the tetracyano nickelate group and the M-NC vibrations.18,37 In the IR spectrum of 2, the v(CN) stretching vibration frequencies are observed at 2128 and 2169 cm-1 (Table 2). These vibration frequencies are higher, around 8 and 49 cm-1, than those for K2[Ni(CN)4]-H2O salt. Therefore, all cyano groups in complex 2 act as bridged ligands. In addition, A1g and B1g modes resulting from Raman active v(CN) stretching vibration have shifted to the higher frequency region in the spectrum of the complexes. There are three basic vibration bands of the cyano ligand in the IR spectrum in the range of 400-600 cm-1: v(NiC), n(NiCN) and 5(NiCN). The most important of these bands is the in-plane bending vibration band 5(NiCN). This band is found at 414 cm-1 in the IR spectrum of K2[Ni(CN)4]-H2O complex. In the spectrum of the complexes, 5(NiCN) is observed to shift the higher frequency region. The shift seen in this band is dependent on the metal.
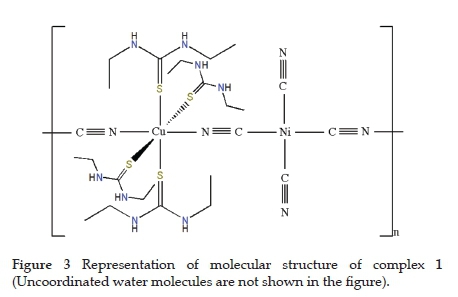
According to the obtained spectroscopic results, while the cyano ligand acts as a terminal and bridging ligand for 1, it only acts as a bridging ligand for 2. In addition, the detu (for 1) and detu and aqua (for 2) ligands bind to metal atoms (Cu(II) or Zn(II)) to form complexes. The representative illustration of the complexes is shown in Figs. 3 and 4.
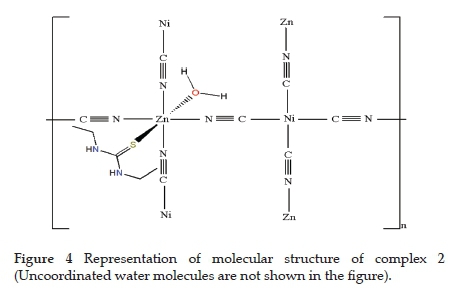
3.2. Thermal study
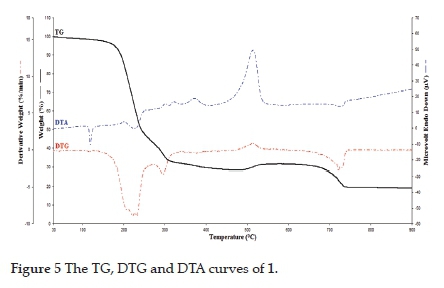
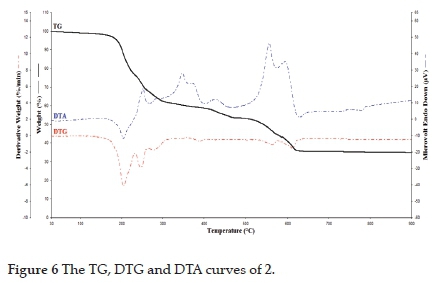
Thermal decomposition curves of 1 and 2 are obtained in a static air atmosphere between 30 and 900 °C. Thermal curves of 1 and 2 are given in Figs. 5 and 6. When these curves are examined, the thermal decomposition of 1 and 2 takes place in two stages, and these decomposition curves support the unit formulas of 1 and 2. Thermal decomposition curves of 1 and 2 are similar and stable up to 97 °C. In the first step, the detu and water molecules were endothermically separated from the structure of the complexes in the temperature range of 97359 eC (Found (Calcd.) (%) = 68.76 (71.39) for 1) and 97-417 eC (Found (Calcd.) (%) = 41.24 (44.43) for 2). In the second step, the cyano groups were between 359 and 752 °C for 1 [Found (Calcd.) (%) = 11.67 (13.15)] and between 417 and 682 eC for 2 [Found (Calcd.) (%) = 23.12 (25.11)] exothermically released from the structures of the complexes. In the complexes, the DTA curve of detu, cyano and water molecules followed a complex process, with maximum peaks at 300, 322, 372 and 514 °C for 1, and 261, 346, 426, 565 and 592 °C for 2. The peaks observed in 372 and 514 °C for 1 and 426, 565 and 592 °C for 2 belong to cyano ligands. Finally, metal oxides (CuO, ZnO and NiO) were found as final products [Found (Calcd.) (%) = 19.57 (19.49) for 1 and 35.64 (37.66) for 2]. Similar decomposition products have been observed in previously made cyano complexes.32,38
4. Conclusions
We have reported the spectroscopic and thermal analysis of two new cyano-bridged complexes as [Cu(detu)4Ni(CN)4]-2H2O (1) and [Zn(H2O)(detu)Ni(CN)4]-2H2O (2) (detu = N, N' diethyl thiourea). According to the spectroscopic analysis of these complexes, the environment of the nickel atom in the complexes is showed square planar geometry by binding four cyano ligands to the nickel atoms, while the environment of the copper and zinc atoms in the complexes is showed octahedral geometry. The environment of the copper atom in complex 1 is surrounded by two cyano and four detu ligands and forms the one-dimensional structure of the complex. The environment of the zinc atom in complex 2 is surrounded by four cyano ligands, one aqua ligand and one detu ligand in an octahedral geometry, and the cyano ligands are bonded between the zinc and nickel atoms to form |Zn-Ni(CN)41 „ polymeric layers. The detu and aqua ligands are bound from above and below to the zinc atoms in these layers. In these two complexes, the detu ligand acted only as a monodentate ligand by binding to metal atoms (Cu(II) or Zn(II)) from the S atom. The structures of the resulting complexes were also supported using thermal and elemental analysis techniques.
Acknowledgements
The author would like to thank Prof. Dr Güneg Süheyla Kürkçüoglu for her support in the writing of this article.
ORCID iD
Dursun Karaagaç: https://orcid.org/0000-0003-3504-6765
References
1 G. Marcotrigiano, Preparation, infrared, Raman and NMR spectra of N,N'-Diethylthiourea complexes with zinc(II), cadmium(II) and mercury(II) halides, Z. Anorg. Allg. Chem., 1976, a422, 80-88. [ Links ]
2 S. Nadeem, M.K. Rauf, S. Ahmad, M. Ebihara, S.A. Tirmizi, S.A. Bashir and A. Badshah, Synthesis and characterisation of palladium (II) complexes of thioureas. X-ray structures of [Pd(N,N'-dnmethylthiourea)4]Cl2-2H2O and [Pd(tetramethyl thiourea)4]Cl2, Transition Met. Chem., 2009, 34, 197-202. [ Links ]
3 G. Abbati, M.C. Aragoni, M. Arca, F. Isaia and V Lippolis, X-Ray crystal structure and infrared spectrum of the monoclinic form of dichlorobis-(N, N'-Diethylthiourea) Cobalt(II), J. Coord. Chem, 1999, 47, 91-97. [ Links ]
4 H. Arslan, N. Duran, G. Borekci, C. Koray Ozer and C. Akbay, Antimicrobial activity of some thiourea derivatives and their nickel and copper complexes, Molecules, 2009, 14, 519-527. [ Links ]
5 B. Kocyigit-Kaymakcioglu, A. Celen, N. Tabanca, A. Ali, S. Khan, I. Khan and D. Wedge, Synthesis and biological activity of substituted urea and thiourea derivatives containing 1, 2, 4-triazole moieties, Molecules, 2013, 18, 3562-3576. [ Links ]
6 R.S. Upadhayaya, G.M. Kulkarni, N.R. Vasireddy, J.K. Vandavasi, S.S. Dixit, V Sharma and J. Chattopadhyaya, Design, synthesis and biological evaluation of novel triazole, urea and thiourea derivatives of quinoline against mycobacterium tuberculosis, Bioorg. Med. Chem., 2009, 17, 4681-4692. [ Links ]
7 S.A. Khan, N. Singh and K. Saleem, Synthesis, characterisation and in vitro antibacterial activity of thiourea and urea derivatives of steroids, Eur. J. Med. Chem, 2008, 43, 2272-2277. [ Links ]
8 A. Han, I. Ozturk, C.N. Banti, N. Kourkoumelis, M. Manoli, A.J. Tasiopoulos, A. Owczarzak, M. Kubicki and S.K. Hadjikakou, Antimony(III) halide compounds of thioureas: Structures and biological activity, Polyhedron, 2014, 79, 151-160. [ Links ]
9 A.A. Isab, S. Nawaz, M. Saleem, M. Altaf, M. Monim-ul-Mehboob, S. Ahmad and H. S. Evans, Synthesis, characterisation and antimicrobial studies of mixed ligand silver(I) complexes of thioureas and triphenylphosphine; crystal structure of {[Ag(PPh3) (thiourea)(NO3)]2-[Ag(PPh3)(thiourea)]2(NO3)2}, Polyhedron, 2010, 29, 1251-1256. [ Links ]
10 S. Ahmad, S. Nadeem, A. Anwar, A. Hameed, S.A. Tirmizi, W. Zierkiewicz, A. Abbas, A.A. Isab and M.A. Alotaibi, Synthesis, characterisation, DFT calculations and antibacterial activity of palladium(II) cyanide complexes with thioamides, J. Mol. Struct., 2017, 1141, 204-212. [ Links ]
11 S. Ahmad, I. Georgieva, M. Hanif, M. Monim-ul-Mehboob, S. Munir, A. Sohail and A. A. Isab, Periodic DFT modeling and vibrational analysis of silver(I) cyanide complexes of thioureas, J. Mol. Model., 2019, 25, 90-103. [ Links ]
12 A. Isab, M. Fettouhi, M. Malik, S. Ali, A. Fazal and S. Ahmad, Mercury(II) cyanide complexes of thioureas and the crystal structure of [(N-methylthiourea)2Hg(CN)2], J. Coord. Chem, 2011, 37, 180-185. [ Links ]
13 J. Cernak, M. Orendac, I. Potocnak, J. Chomic, A. Orendacova, J. Skorsepa and A. Feher, Cyanocomplexes with one-dimensional structures: preparations, crystal structures and magnetic properties, Coord. Chem. Rev, 2002, 224, 51-66. [ Links ]
14 M. Vavra, I. Potocnák, M. Kajnaková, E. Cizmár and A. Feher, Low-dimensional compounds containing cyano groups. XVIII. Two-dimensional network made of [Cu (tmen)]2+ moieties (tmen = tetramethylethylenediamine) connected by [Pt(CN)4]2- anions with three different bridging cyano groups, Inorg. Chem. Commun., 2009, 12, 396-398. [ Links ]
15 J. Cernak and K. A. Abboud, Three different bonding modes of cyano groups in the coordination polymer [Cu(en)2(H2O)] [Cu(en)2Ni2Cu2(CN)10]-2H2O (en is 1,2-diaminoethane), Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2002, 58, m167-170. [ Links ]
16 E. Sayin, G. Süheyla Kürkçüoglu, O. Z. Yegilel and T. Hökelek, Syntheses and characterisations of tetracyanoplatinate(II) complexes with 2-pyridineethanol, J. Coord. Chem, 2015, 68, 2271-2285. [ Links ]
17 A. Karadag, Í. Önal, A. Çenocak, Í. Uçar, A. Bulut and O. Büyükgüngör, Syntheses, IR spectra, thermal properties and crystal structures of novel cyano-bridged polymeric complexes of zinc(II) and cadmium(II) with tetracyanoplatinate(II), Polyhedron, 2008, 27, 223-231. [ Links ]
18 D. Karaagaç and G.S. Kürkçüoglu, Syntheses, spectroscopic and thermal analyses of the Hofmann-type metal(II) tetra-cyanonickelate(II) pyridazine complexes: {[M(pdz)Ni(CN)4]-H2O}n (M = Zn (II) or Cd (II)), Bull. Chem. Soc. Ethiop., 2015, 29, 415-422. [ Links ]
19 G. S. Kürkçüoglu, D. Karaagaç, O. Z. Yegilel and M. Tas, Synthesis, Spectroscopic and structural properties of heteropolynuclear cyano-bridged complexes, J. Inorg. Organomet. Polym Mater., 2012, 22, 324-331. [ Links ]
20 C. Parlak, FT-IR and Raman spectroscopic analysis of some Hofmann type complexes, Spectrochim. Acta, Part A, 2012, 99, 12-17. [ Links ]
21 S. Akyüz and T. Akyüz, Raman spectroscopic study of two dimensional polymer compounds of 2-aminopyrimidine, J. Mol. Struct., 2005, 744, 277-281. [ Links ]
22 A. Ünal, S. Çentürk and M. Çenyel, Vibrational spectroscopic and thermal studies of some 3-phenylpropylamine complexes, Vib. Spectrosc, 2009, 51, 299-307. [ Links ]
23 G. S. Kürkçüglu, Z. Kantarci, R. Cogkun and M. Senyel, Infrared spectroscopic and gravimetric studies on the dicyclohexylaminecadmium(II) tetracyanopalladate(II) host-aromatic guest systems, J. Inclusion Phenom. Macrocyclic Chem., 2003, 45, 129-137. [ Links ]
24 G.S. Kürkçüoğlu, O.Z. Yeşilel, İ. Kavlak and O. Büyükgüngör, Syntheses, spectral and thermal analyses of heteronuclear aqua (2-methylpyrazine) metal(II) complexes with tetracyanonickelate ion and crystal structure of supramolecular [Cd(H2O)(2mpz)Ni(μ- CN)4]n complex, Struct. Chem, 2008, 19, 879-888. [ Links ]
25 R. Gosavi, U. Agarwala and C. Rao, Infrared spectra and configuration of alkylthiourea derivatives. Normal vibrations of N, N'-Dimethyl-and tetramethylthiourea, J. Am. Chem. Soc., 1967, 89,235-239. [ Links ]
26 K.R.G. Devi and D.N. Sathyanarayana, Assignment of fundamental vibrations of N, N'-dimethylthiourea, Bull. Chem. Soc. Jpn., 1980, 53, 2990-2994. [ Links ]
27 D. Gambino, E. Kremer and E.J. Baran, Infrared spectra of new Re(III) complexes with thiourea derivatives, Spectrochim. Acta, Part A, 2002, 58, 3085-3092. [ Links ]
28 PA. Ajibade, N.H. Zulu and A.O. Oyedeji, Synthesis, characterisation, and antibacterial studies of some metal complexes of dialkyl thiourea: The X-ray single crystal structure of [CoCl2(detu)2], Synth. React. Inorg. M., 2013, 43, 524-531. [ Links ]
29 M. Şenyel and G. Kürkçüoğlu, Infrared spectroscopic study of N,N'dimethylthioformamide complexes of the Hofmann type, J. Appl. Spectrosc, 2001, 68, 862-866. [ Links ]
30 D. Karaağaç, Spectroscopic and thermal studies of cyano bridged hetero-metallic polymeric complexes derived from ligands containing N and S donor atoms, Bull. Chem. Soc. Ethiop., 2020, 34, 365-376. [ Links ]
31 K. Nakamoto, Infrared and Raman spectra of inorganic and coordination compounds, applications in coordination, organometallic, and bioinorganic chemistry, Wiley-Interscience, 2009. [ Links ]
32 D. Karaagaç and G.S. Kürkçüoglu, Syntheses and characterisations of the cyanide-bridged heteronuclear polymeric complexes with 2-ethylimidazole, Bull. Chem. Soc. Ethiop, 2016, 30, 263-272. [ Links ]
33 R. McCullough, L. Jones and G. Crosby, An analysis of the vibrational spectrum of the tetracyanonickelate(II) ion in a crystal lattice, Spectrochim. Acta, 1960, 16, 929-944. [ Links ]
34 Z. Kartal and E. Sayin, FTIR spectroscopic and thermal study of M(Cyclohexanethiol)2Ni(CN)4-(1,4-dioxane) clathrate (M = Mn, Co, Ni and Cd), J. Mol. Struct., 2011, 994, 170-178. [ Links ]
35 G.S. Kürkçüoglu, F.Ç. Kiraz and E. Sayn, Vibrational spectra, powder X-ray diffractions and physical properties of cyanide complexes with 1-ethylimidazole, Spectrochim. Acta, Part A, 2015, 149, 8-16. [ Links ]
36 D. Karaagaç, G.S. Kürkçüoglu and O.Z. Yegilel, Two dimensional heteronuclear complexes with cyanide and 4-aminomethylpyridine ligands, J. Mol. Struct., 2014, 1074, 339-348. [ Links ]
37 T. izgi, C. Parlak and M. Çenyel, Vibrational spectroscopic study on some Hofmann type clathrates: M(2-(1-cyclohexenyl) ethylamine)2Ni(CN)4-2benzene (M = Ni and Cd), Spectrochim. Acta, Part A, 2011, 79, 308-311. [ Links ]
38 G.S. Kürkçüoglu, O.Z. Yegilel, I. Kavlak and O. Büyükgüngör, Hetero-Metallic Coordination Polymers: Syntheses, vibrational spectra, thermal analyses and crystal structures of trans-[M(N-Meim)2Ni(u-CN)4]n (M = Cu(II), Zn(II) and Cd(II)), J. Inorg. Organomet. Polym, 2009, 19, 539-548. [ Links ]
Received 12 April 2020
Revised 31 December 2020
Accepted 20 January 2021
* To whom correspondence should be addressed Email: ddkaraagac@hotmail.com














