Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.75 Durban 2021
http://dx.doi.org/10.17159/0379-4350/2021/v75a18
RESEARCH ARTICLE
Synthesis and Biological Evaluation of New Chromenes and Chromeno[2,3-d] pyrimidines
Fatima BelhadjI, II, *; Zahira KibouI, IV; Mohammed BenabdallahV; Mohammed AissaouiV; Mohammed Nadjib RahmounV; Didier VilleminIII; Noureddine Choukchou-BrahamI
ILaboratoire de Catalyse et Synthese en Chimie Organique, Faculté des Sciences, Université de Tlemcen, BP 119 Tlemcen, Algeria
IIUniversité d'Oran 1, Faculté de Médecine, B.P.1510 ELMENAOUAR, 31000 Oran, Algeria
IIILCMT, ENSICAEN, UMR CNRS 6507, 6 Bd du Maréchal Juin, 14050 Caen, France
IVUniversité d'Ain Témouchent, Faculté des Sciences et de la technologie, BP 284, 46000 Ain Témouchent, Algeria
VLaboratoire Antibiotiques Antifongiques : Physico-Chimie, Synthese et Activité Biologique, Faculté de SNV-STU, Université de Tlemcen, 13000 Tlemcen, Algeria
ABSTRACT
A simple and efficient approach has been developed to synthesise novel and functionalised 5H-chromeno[2,3-d] pyrimidines derivatives (4a-h). This approach entails treating 2-amino-3-cyano-4H-chromenes (3a-h) with formamidine acetate under microwave irradiations and solvent-free conditions. All structures of new compounds obtained in this study were characterised by IR, MS, 1H and 13C NMR analysis. Additionally, the synthesised compounds were investigated for their antibacterial and antioxidant potential. Compounds 3b, 3c, 3e, 4c and 4e showed significant activities.
Keywords: 5H-chromeno[2,3-d] pyrimidine; 4H-chromene; solvent-free conditions; antioxidant activity; antibacterial activity
1. Introduction
The fusion of chromene fragments with different heterocycle scaffolds gives rise to a new class of hybrid heterocycles: 5H-chromeno[2,3-d] pyrimidine. These motifs are well-established in the literature as important biologically effective heterocyclic compounds.1'2'3,4,5 These compounds are the subject of many research studies due to their large potential for pharmacological activities. These activities include anti-tumoral,6 antibacterial,7 anti-fungicidal, analgesic,8 anti-allergic,9 cardiotonic, hepatoprotective,10 antihypertensive,11 activity.
In continuation of our efforts toward constructing heterocyclic compounds,12,13,14,15 the present work reports an efficient process for the synthesis of 5H-chromeno[2,3-d] pyrimidines derivatives. The key step in our strategy is based on the condensation of 2-amino-3-cyano-4H-chromenes with formamidine acetate. The structures of these new compounds were characterised by spectroscopic analysis and evaluated for their antioxidant and antibacterial activities.
2. Results and Discussion
The synthesis of the new 5H-chromeno[2,3-d] pyrimidine derivatives was obtained through a two-step method, as shown in Scheme 1. The first step was based on the synthesis of 2-amino-3-cyano-4H-chromenes (3a-h). This step was followed by cyclisation and condensation to the 5H-chromeno[2,3-d] pyrimidines in the second step.
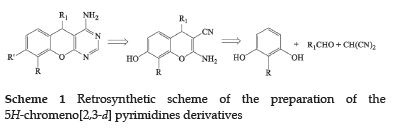
2.1. Synthesis of 2-amino-3-cyano-4H-chromenes (3a-h)
The general synthesis of 2-amino-3-cyano-4H-chromenes includes the reaction of arylidene malononitriles and yß-dicarbonyl compounds in the presence of piperidine,16 triethylamine,17 Ca(OH)218 or TFE.19 Most of these methods involve the use of volatile solvents, expensive reagents and require longer reaction times. We found that these reactions can be carried out without a base and a catalyst. Consequently, 2-amino-3-cyano-4H-chromenes (3a-h) were obtained by condensation of stoichiometric amounts of substituted resorcinol, malononitrile and aromatic aldehydes under microwave for 5 min (Scheme 2).
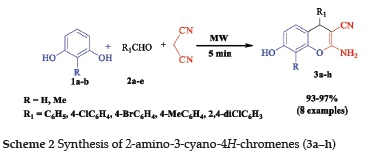
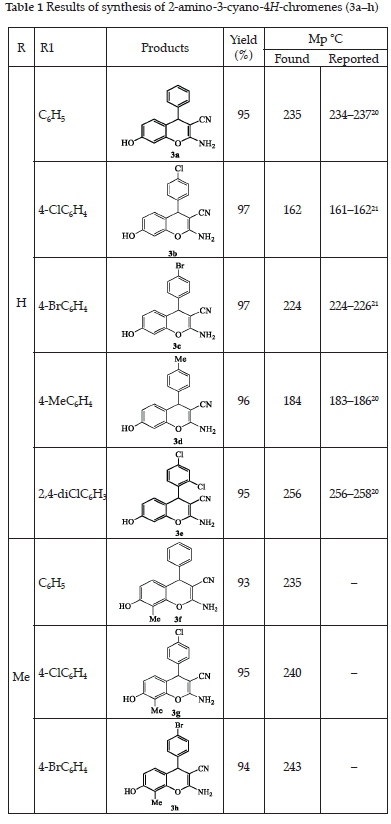
The results obtained for the preparation of compound 3a-h, with excellent yield (82-97%), are reported in Table 1. The use of microwave allowed us to reduce the reaction time from 24 h to 5 min. NMR spectra of 3a-h showed characteristic signals for 4H-chromenes: singlets at 5H 4.49-4.67 ppm in the 1HNMR and at 5C 54.87-56.97 ppm in the 13CNMR. The IR spectra showed a CN stretch at v 2197-2223 cm-1, NH2 stretch at v 3420-3362 cm-1, CO stretch at v 1670-1675 cm-1 and OH at v 3420-3446 cm-1. For all the compounds, the mass spectra gave additional evidence for the proposed structures.
The proposed mechanism for the formation of 2-amino-3-cyano-4H-chromenes (3a-h) is described in Scheme 3. First, we have the formation of alkene I by the Knoevenagel condensation of aromatic aldehyde and malononitrile. A 1,4-Michael addition of II with alkene I form intermediate III. This intermediate is converted to intermediate IV by intramolecular cyclisation.
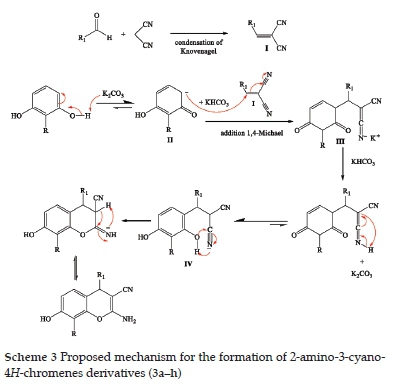
A rearrangement of intermediate IV produces the expected 2-amino-4H-chromenes.
2.2. Synthesis of 4-amino-5fZ-chromeno[2,3-d] pyrimidines (4a-h)
Different synthetic methods of 4-amino-5H-chromeno[2,3-d] pyrimidines have been reviewed.22,23,24,25 In this context and in order to obtain new bioactive heterocyclic compounds, the formamidine acetate was added to the 2-amino-3-cyano-4H-chromenes (3a-h) (Scheme 4) via microwave heating. The originality of our synthetic strategy is based on the use of formamidine acetate as a cyclisation agent. This reagent is a very useful intermediate in synthesising various heterocycles, especially pyrimidine synthesis, through its reaction with unsaturated 2-aminonitriles.
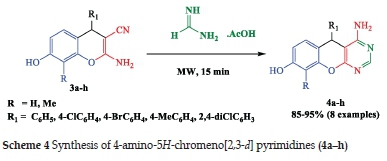
The results obtained for the synthesis of compound 4a-h with good yield (85-95%) are summarised in Table 2.
The treatment of 2-amino-3-cyano-4H-chromenes (3a-h) with formamidine acetate, without solvent and under microwave irradiation for 15 min, gave the corresponding 4-amino-5H-chromeno[2,3-d] pyrimidines (4a-h) with good yields. The IR spectra showed the absence of CN and the appearance of (C=N) at V 1645-1649 cm-1, NH2 stretch at v 3465-3402, 33563324 cm-1 and CO stretch at v 1671-1675 cm-1. The structures of the compounds 4a-h were confirmed based on 1H NMR, 13C NMR and MS data.
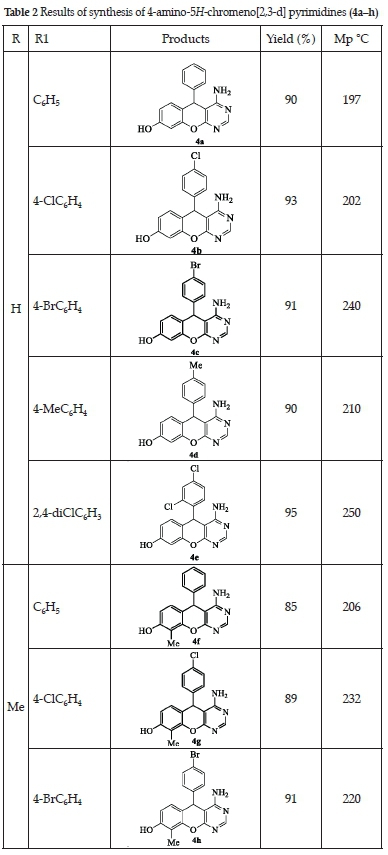
The mechanism proposed for forming 4-amino-5H-chromeno[2,3-d] pyrimidines (4a-h) was described in Scheme 5. The reaction begins with the formation of the intermediate I. The latter undergoes a nucleophilic addition of the NH2 group of 2-amino-3-cyano-4fi-chromene (II) to form intermediate III. The formation of III is followed by intramolecular cyclisation of the NH2 group with the CN group to give intermediate IV. After rearrangement and a step of aromatisation, 4-amino-5fi-chromeno[2,3-d] pyrimidine was obtained.
3. Biological Evaluation
3.1. Determination of antibacterial and antioxidant activity -The disc diffusion method
A panel of bacteria, namely Escherichia coli ATCC (American Type Culture Collection) 25922, Salmonella typhimurium ATCC 13311, Klesialla pneumonia ATCC 700603, Citrobacter freundi ATCC 8090, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25929, Enterococcus faecalis ATCC 49452 and Bacillus cereus ATCC 10876, were used for the screening of the antibacterial activity.
The antibacterial activity was evaluated using the broth microdilution method to estimate the Minimal Inhibitory Concentration (MIC) according to the Clinical and Laboratory Standards Institute (CLSI) recommendations (CLSI, 2012). A series of two-fold dilutions from 1024 to 2 µg ml-1 were prepared in a 96 well sterile microplate. These dilutions were inoculated with 100 µl of a solution containing 106 colony forming units (CFU).
Gentamicin and Ciprofloxacin were used as positive controls. The microplate was incubated at 37°C for 24 h. The MIC was defined as the low concentration in which there was no turbidity.
3.2. Scavenging activity
In our study, the scavenging activity of the compounds was carried out by the technique of free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging. The effect of each compound on DPPH was measured by the procedure described by Da Silva Pinto et al.26
A volume of 5 ul of various concentrations of each compound was added to 1950 µl of the methanol solution of DPPH. The negative control was prepared by mixing 50 µl of methanol with 1950 µl of the methanol solution of DPPH. The absorbance was measured at 517 nm against a blank of each concentration. These measurements were done after incubation in the dark for 30 min, using a spectrophotometer. The positive control was a solution of the synthetic antioxidant butylated hydroxyanisole (BHA) with concentrations ranging from 0.02 to 0.2 mg ml-1. The absorbance was measured in the same conditions as the samples. The results were expressed taking into account the average of three measurements obtained for each sample, and the percentage of DPPH reduction was calculated using the formula:
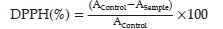
where DPPH (%): Reduction percentage of DPPH.
AControl: Absorbance of the negative control tube.
ASample: Absorbance of the sample.
The IC50 value is the concentration that was reduced to 50% of the radical DPPH. It was determined graphically for each compound from the curve of the percentage reduction depending on the concentration.
3.3. Ferric reducing antioxidant power assay (FRAP)
The reducing power was determined by the measurement of absorbance at 700 nm.27 A volume of 0.1 ml of our compounds in different concentrations (initial) was mixed with 0.25 ml of sodium phosphate buffer (0.2 M; pH = 6.6) and 0.25 ml of potassium ferricyanide 1% (w/v), and the mixture was incubated at 50°C for 20 min. After 0.25 ml of trichloroacetic acid (TCA), 10% (w/v) were added, the mixture was centrifuged at 650 g for 10 min the supernatant (0.5 ml) was mixed with 0.5 ml distilled water and 0.1 ml ferric chloride solution (FeCl3) 0.1% (w/v).28 The IC50 value (mg compound/ ml) is the effective concentration at which the absorbance was 0.5 for reducing power and was obtained by interpolation from linear regression analysis. For the same conditions, BHA and ascorbic acid were used as a positive control for comparison.
3.4 Total antioxidant capacity (TAC)
The total antioxidant capacity of our compounds was evaluated with the phosphor-molybdenum technique.29 An aliquot of 0.2 ml of different concentrations of our compounds was combined with 2 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were incubated in a thermal block at 95°C for 90 min. The absorbance of each solution was measured at 695 nm
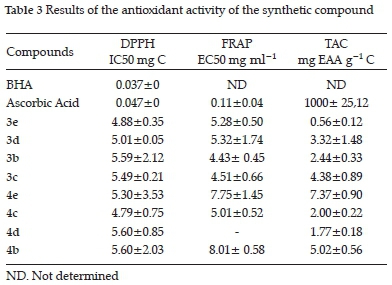
against a blank. The antioxidant capacity was expressed in milligram equivalent of ascorbic acid per gram of compound (mg EAA g-1 C). The range of the calibration curve of the ascorbic acid was 0.10 to 0.80 mg ml-1 (Table 3).
The MIC results of the synthesis products against references bacteria are shown in Table 4.
The higher activity against gram-negative bacteria was obtained toward Pseudomonas aeruginosa. The product 4e showed a MIC equal to 16 µg ml-1. The products 3e, 3b and 3c showed MICs equal to 64 µg ml-1. Compound 3d showed a MIC equal to 256 µg ml-1. However, other gram-negative bacteria exhibited resistance to products tested. For grampositive bacteria, low MICs were obtained by the products 3b and 3c and 4e against the Bacillus cereus strain; the MICs range was 32 to 128 µg ml-1. The compound 3e showed a MIC equal to 128 µg ml-1 against Bacillus cereus and Staphylococcus aureus.
The products 3b and 3c have Cl or Br halogen groups respectively at the 4-position, while the 3e has two Cl located in the 2- and 4-positions on the aromatic (phenyl) ring. The products with the electron-withdrawing groups in position 4 showed antibacterial activity with respect to gram-positive and -negative bacteria. These results are in accordance with the work of Nirav et al.,30 which focused on the synthesis and evaluation of the antimicrobial activity of a new series of 4H-chromenes against Staphylococcus aureus (S. aureus), and Bacillus subtilis (B. subtilis). The best result has been observed with chromene, which has a chlorine group. In the study of Sudhan and Mansoor,31 the synthesis and evaluation of the antimicrobial activity of a new series of 7,8-dihydro-2-(2-oxo-2H-chromen-3-yl)-5-aryl-cyclopenta[b]pyranopyrimidine-4,6-5H-dione were investigated; the results suggest that aromatic substituents strongly influence antibacterial and antifungal activities.
In the chromeno[2,3-d] pyrimidines, the products 4e, 4c, 4d and 4b showed interesting activities against Bacillus cereus (B. cereus) and Pseudomonas aeruginosa (P. aeruginosa). The best results were obtained with compound 4e, where the structure of the chromeno pyrimidine is composed of two fused heterocyclic units, namely, the substituted 4H-chromene and the pyrimidine. The pyrimidine has an amine in the 4-position of the ring. Chlorine is well known to have good bacterial activities, as shown in the case of 4e.
These results agree with the work of Agrod,32 who found that the chlorine group linked to chromenopyrimidine heterocycles has good antibacterial activities compared to their analogues. The modification of the 4H-chromene structure through the chromenopyrimidine products allowed the improvement of the antibacterial activity.
In the study of Sankappa et al.,33the new series of synthesised chromeno[2,3-d] pyrimidine compounds did not exhibit activity against: Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa.
In the antioxidant study, two products (3e and 3d) of the 4H-chromenes family showed good DPPH scavenging activity. The products 3b and 3c have Cl or Br respectively at the 4-position, while the 3e has two Cl located in the 2- and 4-positions of the aromatic (phenyl) ring. The grafting of electron-withdrawing halide substituents in position 4 improve the DPPH trapping activity. In iron reduction and antioxidant capacity, the situation is reversed; products 3b and 3c showed the most interesting reduction.
Concerning chromenopyrimidines, the 4c product showed the most interesting DPPH and iron reduction trapping activity. The grafting of electron-withdrawing halide substituents on the phenyl nucleus seems to improve this activity. The most important antioxidant capacity was obtained with 4e. It seems that the grafting of the electron-donating groups improves this activity.
Finally, we can say that the importance of this research work lies in the possibility that an in-depth investigation of the structure-activity relationship, toxicity and biological effects of these compounds may be useful for designing more potent oxidising agents at higher concentrations for therapeutic purposes.
4. Experimental
The melting points were measured using a Bank Kofler HEIZBANK apparatus standard WME 50-260°C and were uncorrected. IR spectra were obtained with solids with a Fourier transform Perkin Elmer Spectrum One with ATR accessory. Only significant absorptions are listed in the results. The 1H NMR spectra were recorded at 400 MHz on a Brüker AC 400 spectrometer, and 13C NMR spectra were recorded on the same spectrometers at 100.6 MHz. Samples were dissolved in DMSO-d6, values for 5 are in parts per million relative to tetramethyl silane (TMS) as an internal standard. Mass spectra were recorded on a QTOF Micro (Waters). Microwave irradiation experiments use a microwave reactor (Biotage). The multiplicities are reported as s (singlet), d (doublet), t (triplet), q (quartet) and m (multiplet).
4.1. Synthesis
4.1.1. General procedure 1 for the synthesis of 2-amino-3-cyano-4H-chromenes (3a-h)
A mixture of the aromatic aldehyde (2a-e) (10 mmol), substituted resorcinol (1a-b) (10 mmol), malononitrile (10 mmol), H2O (2 ml) and carbonate of ammonium (0.5 g) was irradiated in a single-mode microwave at 300 W for 5 min under 12 bar pressure. The completion of the reaction was determined by thin-layer chromatography (TLC). After the completion of the reaction, the residue that formed was diluted with 30 ml of CH2Cl2. The organic layer obtained was washed with water (3 X 20 ml), then with a solution of saturated NaCl (10 ml), dried over MgSO4, filtered and evaporated under vacuum. The compounds 3a-h were obtained as white solids.
4.1.2. General procedure 2 for the synthesis of-amino-5H-chrom-eno[2,3-d] pyrimidines (4a-h)
A mixture of 3a-h (5 mmol) and formamidine acetate (15 mmol, 1,56 g) was irradiated in a single-mode microwave at 300 W for 15 min and under 12 bar pressure. TLC determined the completion of the reaction. After the completion of the reaction, the residue that formed was diluted with 30 ml of CH2Cl2. The organic layer obtained was washed with water (3 X 20 ml), then with a solution of saturated NaCl (10 ml), dried over MgSO4, filtered and evaporated under vacuum to afford desired compounds 4a-h.
2-amino-4-(bromophenyl)-3-cyano-7-hydroxy-4H-chromene (3c) was obtained, according to procedure 1 using 4-bromobenzaldéhyde (10 mmol; 1.83 g), resorcinol (10 mmol; 1.10 g) and malononitrile (10 mmol; 0.66 g), as white solid; 89%a, 93%b, or 97%c; mp 251°C.
1H NMR (DMSO)6ppm: 9.80 (1H, s, OH); 7.49 (2H, d, Jh-h= 8.40 Hz, Harom); 7.13 (2H, d, Jh-h= 8.40 Hz, Harom); 6.91 (2H, s, NH2); 6.78 (1H, d, Jh-h = 8.40 Hz, Harom); 6.49 (1H, dd, Jh-h = 2.40 Hz, Jh-h = 2.40 Hz Harom); 6.41 (1H, d, Jh-h = 2 Hz, Harom);4.64 (1H, s, Hpyran).
13C NMR (DMSO)6ppm: 160.72 (C-OH); 157.60 (C-O-CNH2); 149.26 (C-NH2) ; 146.18-120.22 (9 X Carom); 113.57 (CN); 112.96 (C=C-O); 102.72 ( C=C-CN); 56.52 (CH-Ph).
IR (neat cm-1): 3457; 3340; 2220; 1645; 1597.
Results: C16H11BrN2O2 M+H 343.1703. Found 343.1704.
2-amino-3-cyano-7-hydroxy-4-p-tolyl-4H-chromène (3d) was obtained according to general procedure 1, using 4-methylbenzaldehyde (10 mmol; 1.20 g), resorcinol (10 mmol; 1.10 g) and malononitrile (10 mmol; 0.66 g), as white solid; 85%a, 90%b or 96%c; mp 184°C.
1H NMR (DMSO)6ppm: 8.39 (1H, s, OH); 7.11 (2H, d, Jh-h= 8.40 Hz, Harom); 7.07 (2H, d, Jh-h = 8.40 Hz, Harom); 6.81 (2H, s, NH2); 6.78 (1H, d, Jh-h = 8.40 Hz, Harom); 6.52 (1H, d, Jh-h = 8.40 Hz, Harom); 6.47 (1H, dd, Jh-h= 2.40 Hz, Jh-h= 2.40 Hz Harom); 4.49 (1H, s, Hpyran); 2.26 (3H, s, CH3).
13C NMR (DMSO)6ppm: 160.20 (C-OH); 157.53 (C-O-CNH2); 149.88 (C-NH2); 143.36-120.96 (9 X Carom); 113.87 (CN); 112.81 (C=C-O); 103.00 ( C=C-CN); 56.94 (CH-Ph); 21.06 (CH3).
IR (neat cm-1): 3420; 3416; 2217; 1649; 1622.
Results: C17H14N2O2 M+H 278.3099. Found 278.3101.
2-amino-4-(2,4-dichlorophenyl)-3-cyano-6-hydroxy-4H-chromene (3e) was obtained, according to general procedure 1 using 2,4-dichlorobenzaldehyde (10 mmol; 1.75 g), resorcinol (10 mmol; 1.10 g) and malononitrile (10 mmol; 0.66 g), as white solid; 88%A, 90%B, 95%C; mp 256°C.
1H NMR (DMSO)6ppm: 9.80 (1H, s, OH); 7.58 (1H, d, Jh-h= 2.10 Hz, Harom); 7.40 (1H, dd, Jh-h= 2.10 Hz, Jh-h= 2 Hz, Harom); 7.21 (1H, d, Jh-H = 12 Hz, Harom); 6.98 (2H, s, NH2); 6.72 (1H, d, Jh-h = 8.40 Hz, Harom); 6.49 (1H, dd, Jh-h = 2.40 Hz, Jh-h = 2.40 Hz Harom); 6.42 (1H, d, Jh-h = 2.00 Hz, Harom); 5.13 (1H, s, Hpyran).
13C NMR (DMSO)δppm: 160.95 (C-OH); 157.95 (C-O-CNH2); 149.49 (C-NH2); 142.37-120.63(9 X Carom); 113.5(CN); 112.33 (C=C-O); 102.74 (C=C-CN); 54.87 (CH-Ph).
IR (neat cm-1): 3417; 3342; 2198; 1647; 1595.
Results: C16H10Cl2N2O2 M+H 333.1760. Found 333.1762.
4-amino-5-(4-bromophenyl)-8-hydroxy-5H-chromeno[2,3-d] pyrimidine (4c) was obtained, according to general procedure 2 using 3c (5 mmol, 1.71 g), as white solid; 91%; mp 240°C.
1H NMR (DMSO)6ppm: 9.98 (1H, s, OH); 9.80 (1H, s, Hpyrimidine); 7.49 (2H, d, Jh-h= 8.40 Hz, Harom); 7.13 (2H, d, Jh-h= 8.40 Hz, Harom); 6.91 (2H, s, NH2); 6.78 (1H, d, Jh-h = 8.40 Hz, Harom); 6.54 (1H, dd, Jh-h= 2.40 Hz, Jh-h= 2.40 Hz Harom); 6.42 (1H, d, Jh-h =2.40 Hz, Harom); 4.64 (1H, s, Hpyrane).
13C NMR (DMSO)6ppm: 162.84 (C-O); 160.72 (C-NH2); 157.66 (C-OH); 149,26 (-N-C=N-); 146,18 (C=C-N); 132.77-120.22 (9 X Carom); 113.57 (C=C-Ph); 112.96 (C=C-N); 102.72 (CH-Ph).
IR (neat cm-1): 3439; 3440; 1669; 1569.
Results: C17H12BrN3O2 M+H 370.0191. Found 370.0203.
4-amino-8-hydroxy-5-p-tolyl-5H-chromeno[2,3-d] pyrimidine (4d) was obtained, according to general procedure 2 using 3d (5 mmol, 1.39), as white solid; 91%; mp 210°C.
1H NMR (DMSO)6ppm: 9.68 ( 1H, s, OH); 8.08 ( 1H, s, Hpyrimidine); 7.14 (2H, d, Jh-h= 8.00 Hz, Harom); 7.04 (2H, d, Jh-h= 8.00 Hz, Harom); 6.95 (1H, d, Jh-H = 8.40 Hz, Harom); 6,72 (2H, s, NH2); 6.53 (1H, dd, Jh-h= 2.40 Hz, Jh-h= 2.40 Hz Harom); 6.50 (1H, d, Jh-h =2.40 Hz, Harom); 5.09 (1H, s, Hpyran); 3.19 (3H, s, CH3).
13C NMR (DMSO)6ppm: 163.08 (C-O); 162.81(C-NH2); 157.57 (C-OH); 156.86 (-N-C=N-); 150.40 (C=C-N); 142.54-113.13(9 X Carom); 112.73 (C=C-O); 103.40 (c=C-N); 96.66 (CH-Ph); 20.99 (CH3).
IR (neat cm-1): 3444; 3413; 1647; 1595.
Results: C18H15N3O2 M+H 305. 3334. Found 305.333
4-amino-5-(2,4-dichlorophényl)-8-hydroxy-5H -chromeno[2,3-d] pyrimidine (4e) was obtained, according to general procedure 2 using 3e (5 mmol, 1.66 g), as white solid; 95%; mp 250°C.
1H NMR (DMSO)6δppm: 9.78 ( 1H, s, OH); 8.12( 1H, s, Hpyrimidine); 7,52 (2H, d, Jh-h = 8 Hz, Harom); 7.39 (1H, d, Jh-h = 8 Hz, Harom); 6.89 (1H, d, Jh-h= 8 Hz, Harom); 6.53 (1H, dd, Jh-h= 2.40 Hz, Jh-h = 2.40 Hz Harom); 6.48 (1H, d, JH.H=2.40 Hz, H™); 6.33 (2H, s, NH2); 5.51 (1H, s, Hpyra„).
13C NMR (DMSO)δppm: 163.11(C-O); 163.02 (C-NH2); 158.08 (C-OH); 157.30 (-N-C=N-); 150.50( C=C-N); 140.41-112.84(9 X Carom); 112.84 (C=C-O); 103.27 (C=C-N); 94.60 (CH-Ph).
IR (neat cm-1): 3379; 3427; 1647; 1585.
Results: C17H11Cl2N3O2 M+H 360.0307 Found 360.0309
5. Conclusions
In conclusion, we have successfully developed a novel and efficient approach for synthesising new chromenes and 5H-chromeno[2,3-d] pyrimidines derivatives under solvent-free conditions and microwave irradiations with high yields. The originality of our synthetic strategy is based on the use of formamidine acetate as cyclisation agents. This easy to execute methodology with rapid access and good yields open a new route for synthesising various substituted nitrogen heterocycles of biological and pharmaceutical. Evaluating the antioxidant and antibacterial activity of the various compounds tested against three bacterial strains showed good activity.
Acknowledgements
The authors wish to thank Karine Jarsalé for the mass spectroscopy spectra. We gratefully acknowledge financial support from the 'Ministère de la Recherche et des Nouvelles Technologies', CNRS (Centre National de la Recherche Scientifique), the 'Region Basse-Normandie' and the European Union (FEDER funding). The authors acknowledge the financial support of the French Agence Nationale de la Recherche (ANR) through the program 'Investissements d'Avenir' (ANR-10-LABX-09-01) LabEx EMC. We thank DGRSDST and the University of Tlemcen- Algeria for funding this work.
ORCID iDs
Fatima Belhadj: https://orcid.org/0000-0001-6238-0430
References
1 L. Moafi, S. Ahadi, A. Bazgir. New HA 14-1 analogues: synthesis of 2-amino-4-cyano-4H-chromenes. Tetrahedron Lett. 2010, 51, 6270-6274. [ Links ]
2 A.H. Abd El-Wahab. Synthesis, reactions and evaluation of the antimicrobial activity of some 4-(p-Halophenyl)-4H-naphthopyran, pyranopyrimidine and pyranotriazolopyrimidine derivatives. Pharmaceuticals (Basel), 2012, 5, 745-757. [ Links ]
3 N. Karimi, A. Davoodnia, M. Pordel. Synthesis of new 3H-chromeno[2,3-d] pyrimidine-4,6(5H,7H) diones via the tandem intramolecular Pinner/Dimroth rearrangement. Heterocycl. Commun., 2018, 24, 31-35. [ Links ]
4 R.A. Haggam, G. Mohamed, M.G. Assy, K.M. Enaiat, S.M. Abdussattar. Synthesis of pyrano[2,3-d]pyrimidine-2,4-diones and pyridino[2,3-d]pyrimidine-2,4,6,8-tetraones: Evaluation antitumor activity. J. Heterocycl. Chem, 2019, 57, 842-850. [ Links ]
5 R. Ghahremanzadeh, F. Fereshtehnejad, A. Bazgir. Chromeno[2,3-d] pyrimidine-triones synthesis by a three-component coupling reaction. Cheem. Pharm. Bull. (Tokyo), 2010, 58, 516-520. [ Links ]
6 F. Eiden, F. Denk. Synthesis of CNS-activity of pyran derivatives: 6, 8-dioxabicyclo (3, 2, 1) octane]. Arch. Pharm. (Weinheim), 1999, 324, 353-354. [ Links ]
7 A. El-Wahab. AHF Synthesis of some new pyrano [2, 3-d][1, 2, 4] triazolo [1, 5-c] pyrimidine and pyrimido [1, 6-b] triazine derivatives. Acta Pharm, 2003, 58, 701-720. [ Links ]
8 K.M. Meepagala, K.K. Schrader, C.L. Burandt, D.E. Wedge, S.O. Duke. New class of algicidal compounds and fungicidal activities derived from a chromene amide of Amyris texana. J. Agric. Food Chem, 2010, 58, 9476-9482. [ Links ]
9 Kitamura N, Onishi A. In: European Patent 163599,1984, Chem. Abstr, 1984, p 186439. [ Links ]
10 G.H. Churchill, S.A. Raw, L. Powell. Improved synthesis of substituted pyrido[2,3-d]pyrimidinediones. Tetrahedron Lett., 2011, 52, 3657-3661. [ Links ]
11 C.W. Johannes, M.S. Visser, G.S. Weatherhead, A.H. Hoveyda. Zr-catalyzed kinetic resolution of allylic ethers and Mo-catalysed chromene formation in synthesis. Enantioselective total synthesis of the antihypertensive agent (S, R, R, R)-Nebivolol. J. Am. Chem. Soc., 1998, 120, 8340-8347. [ Links ]
12 F. Belhadj, Z. Kibou, N. Cheikh, N. Choukchou-Braham, D. Vllemin. Convenient access to new 4-substituted aminopyrido [2, 3-d] pyrimidine derivatives. Tetrahedron Lett., 2015, 56, 5999-6002. [ Links ]
13 M. Benabdallah, O. Talhi, F. Nouali, N. Choukchou-Braham, Kh. Bachari, A.M.S. Silva. Advances in spirocyclic hybrids: chemistry and Medicinal actions. Curr. Med. Chem, 2018, 25, 3748-3767. [ Links ]
14 Z. Kibou, D. Vllemin, J.-F. Lohier, N. Cheikh, N. Bar, N. Choukchou-Braham. Easy solventless synthesis of new mono and bis amino-5H-chromeno [3, 4-c] pyridin-5-one derivatives. Tetrahedron, 2016, 72, 1653-1661. [ Links ]
15 D. Villemin, Z. Belhadj, N. Cheikh, N. Choukchou-Braham, N. Bar, J-F. Lohier. Solventless convenient synthesis of new cyano-2-aminopyridine derivatives from enaminonitriles. Tetrahedron Lett., 2013, 54, 1664-1668. [ Links ]
16 S-P. Eszter, S-F. Rita. Application of ionic liquids in synthetic procedures leading to pharmaceutically active organic compounds. Curr. Green Chem., 2018, 5, 4-21. [ Links ]
17 N. Martin, C. Pascual, C. Seoane, J. Soto. The use of some activated nitriles in heterocyclic syntheses. Heterocycles, 1987, 26, 2811-2816. [ Links ]
18 S. RaoKolla, Y.R. Lee. Ca(OH)2-mediated efficient synthesis of 2-amino-5-hydroxy-4H-chromene derivatives with various substituents. Tetrahedron, 2011, 67, 8271-8275. [ Links ]
19 S. Khaksar, A. Rouhollahpour, S.M. Talesh. A facile and efficient synthesis of 2-amino-3-cyano-4H-chromenes and tetrahydrobenzo[b] pyrans using 2,2,2-trifluoroethanol as a metal-free and reusable medium. J. Fluor. Chem., 2012, 141, 11-15. [ Links ]
20 J. Safari, Z. Zarnegar, M. Heydarian. Practical, ecofriendly, and highly efficient synthesis of 2-amino-4H-chromenes using nanocrystalline MgO as a reusable heterogeneous catalyst in aqueous media. J. Taibah Univ. Sci., 2013, 7, 17-25. [ Links ]
21 J. Albadi, A. Razeghi, A. Mansournezhad, Z. Azarian. CuO-CeO2 nanocomposite catalysed efficient synthesis of aminochromenes. J. Nanostructure Chem., 2013, 3, 85. [ Links ]
22 K.R. Desale, K.P Nandre, S.L. Patil. p-Dimethylaminopyridine (DMAP): A highly efficient catalyst for one pot, solvent free synthesis of substituted 2-amino-2-chromenes under microwave irradiation. Org. Comm., 2012, 5. [ Links ]
23 J. Malviya, P. Rana Krishna. One-pot three-component synthesis of chromeno [2,3-d] pyrimidine derivatives: Novel, simple, and efficient electrochemical approach. J. Heterocycl. Chem., 2019, 57, 39-49. [ Links ]
24 A. Mobinikhaledi, N. Foroughifar, T. Mosleh, A. Hamta. Synthesis of some novel chromenopyrimidine derivatives and evaluation of their biological activities. Iranian J. Pharm. Res., 2014, 13, 873. [ Links ]
25 Z. Ebrahimi, A. Davoodnia, A. Motavalizadehkakhky, J. Mehrzad. Synthesis of benzo[f]chromeno[2,3-d]pyrimidines via the tandem intramolecular Pinner/Dimroth rearrangement and their antibacterial and antioxidant evaluation. Org. Prep. Proced. Int., 2019, 51, 357-367. [ Links ]
26 A.K. Gupta, K. Kumari, N. Singh, D.S. Raghuvanshi, K.N. Singh. An eco-safe approach to benzopyranopyrimidines and 4H-chromenes in ionic liquid at room temperature. Tetrahedron Lett., 2012, 53, 650-653. [ Links ]
27 M. Da Silva Pinto, F.M. Lajolo, M.I. Genovese. Bioactive compounds and quantification of total ellagic acid in strawberries (Fragaria x ananassa Duch.). Food Chem, 2008, 107, 1629-1635. [ Links ]
28 M. Oyaizu. Antioxidative activities of products of browning reaction prepared from glucosamine. Jap. J. Nutr. Diet., 1986, 44, 307-315. [ Links ]
29 A.A. Karagozler, B.B. Erdag, Y. Emek, D.A. Uygun. Antioxidant activity and proline content of leaf extracts from Dorystoechas hastata. Food Chem., 2008, 111, 400-407. [ Links ]
30 S. Barek, N.M. Rahmoun, M. Aissaoui, I.A. El Haci, C. Bensouici, N. Choukchou-Braham. Phenolic contents, antioxidant, and antibacterial activities of the Algerian Genista saharae solvent extracts. J. Herbs Spices Med. Plants, 2019, 26, 1-13. [ Links ]
31 N.K. Shah, N.M. Shah, M.P. Patel, R.G. Patel. Synthesis of 2-amino-4H-chromene derivatives under microwave irradiation and their antimicrobial activity. J. Chem. Sci., 2013, 125, 525-530. [ Links ]
32 P.N. Sudhan, S.S. Mansoor. Facile synthesis and antimicrobial activity of a novel series of 7,8-dihydro-2-(2-oxo-2H-chromen-3-yl)-5-aryl-cyclopenta[b]pyrano-pyrimidine-4,6-5H-dione derivatives catalysed by reusable silica-bonded N-propyl diethylenetriamine sulfamic acid. J. Assoc. Arab. Univ. Basic. App. Sc., 2016, 21, 1-9. [ Links ]
33 A.M. El-Agrody, N.M. Sabry, S.S. Motlaq. Synthesis and antimicrobial activities of 2-substituted 12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c] pyrimidines, 3-ethoxycarbonyl-12H-chromeno[3,2-e][1,2,4] triazolo [1,5-c] pyrimidine-2-one and ethyl 2-formylamino- and 2-acetylamino-4H-chromene-3-carboxylates. J. Chem. Res., 2011, 35, 77-83. [ Links ]
Received 27 September 2020
Revised 28 January 2021
Accepted 01 February 2021
* To whom correspondence should be addressed. Email: fbelhadj88@yahoo.fr














