Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.75 Durban 2021
http://dx.doi.org/10.17159/0379-4350/2021/v75a6
REVIEW ARTICLE
https://doi.org/10.17159/0379-4350/2021/v75a6
Carbon Nanodots Derived from Natural Products
Yaung KweeI; Alfinda Novi KristantiI, *; Kaido SiimonII; Nanik Siti AminahI; Mochamad Zakki FahmiI, III, *
IDepartment of Chemistry, Universitas Airlangga, Surabaya 60115, Indonesia
IIInstitute of Physics, University of Tartu, Ravila 14c, Tartu, 50411 Estonia
IIISupramodification Nano-micro Engineering Research Group, Universitas Airlangga, Surabaya 60115, Indonesia
ABSTRACT
This review highlights recent improvements, and present situations concerning the practical application of, natural products-based carbon nanodots (CNDs) in nanomedicine, sensors, drug delivery, bio-imaging, solar cells, photocatalysis and nano-encapsulation. CNDs are zero-dimensional carbonaceous nanomaterials that have recently drawn much attention because of their unique physicochemical properties, such as excellent biocompatibility and tunable photoluminescence, easy functionali-zation, solubility in water, dispersibility, and low toxicity. Additionally, they are environmentally friendly, abundant, easily accessible, and rich in multiple elements. Since CNDs derived from organic products have unique properties, we explored and found that the properties of CNDs may depend on the preparation method and the used precursors. This study also informs on the positioning of natural products in nanotechnology.
Keywords: Carbon nanodots, natural product, synthesis, applications.
1. Introduction
Carbon is one of the most plentiful elements in the earth's crust. Many applicable materials, including various nano-materials, are carbon-based materials.1,2 With all the recent nanotechnology advancements, carbon-based materials have developed from low-cost waste to high-value materials. Adding value is often achieved by using new synthesis methods, which allow controllable and straightforward preparation of nano-materials.3,4 Fluorescent carbon-based nanomaterials, like carbon nanodots (CNDs), have received interest due to their low cost and low toxicity. Therefore, researchers have put much effort into developing new synthetic approaches to prepare such materials.5,6
In 2004, CNDs were first explored during the purification of single-walled carbon nanotubes.7 Subsequently, Sun el al8 verified the properties of luminescent CNDs synthesized by surface passivation and laser ablation in 2006. Since then, research regarding the nature of CNDs has been developing fast.8 In the last few years, substantial progress has been made in synthesizing CNDs, characterizing their properties, and developing their potential applications.9-22 However, the sources of carbon and its importance have been largely neglected.23
There are abundant organic products in our environment that are good sources of carbon for the synthesis of organic products-based CNDs.24,25 These CNDs are green and eco-friendly, in contrast with other man-made carbon dots. Manmade carbon dots are prepared from precursors containing some toxic material, and oxidation of some chemical agents.26 Additionally, organic products as sources of CNDs are advantageous in many ways, for example, being inexpensive,27 easy to obtain,28 eco-friendly, relatively nontoxic29 and abundant in nature.30 Furthermore, it is often possible for CNDs to produce valuable and useful materials from low-value organic waste.31 Some of the other useful properties of CNDs are: tuneable fluorescence, nontoxicity, sustainability, small size distribution, good biocompatibility, excellent photostability, electrochemilumines-cence and multi-photon excitation (up-conversion). These properties make them potentially useful in applications for biomedicines,32 sensors,33,34 optronic35 and photo-catalysis.36 Furthermore, the as-prepared CNDs can be functionalized using biomolecules, enhancing their optical properties, optimizing and making them chemically inert to be used for some intended therapy.37 These modified CNDs are also used for sensors, optronics as catalytic materials, and effective carriers in drug delivery applications and biological imaging.25,38
Mostly, in the comparison of conventional semiconductor quantum dots, organic dyes, and up-converting nanoparticles, photoluminescent organic products derived-CNDs have several advantages which include nontoxicity, eco-friendliness, the ability to use cost-effective synthetic methods, high solubility in water, durable photostability, robust chemical inertness, and ease of modification.39 Synthesis of CNDs can be carried out either by 'top-down' approaches like laser ablation,40 electrochemical preparation methods41 and oxidation,42 or 'bottom-up' approaches like hydrothermal,43 or microwave treat-ment,44 pyrolysis,45 chemical oxidation,42 and solvothermal46 approaches. CNDs have been derived from various organic sources21 such as organic compounds,47 bamboo leaves,48 shrimp shellwaste,49 plant extracts,50 fruit shells51 and peels,52,53 fruit juice,54,55 algae56,57 and eggshells,58,59 depending on the specific applications. The produced extract is mostly carbonized to become carbon black, turning into CNDs after dispersion, separation, and purification steps.60 Many researchers have reported using CNDs derived from natural products for sensing metal ions.61 Such CNDs have been synthesized from quince fruit,62mushrooms,63 cherryblossoms,64 coffee,65 sesame66 and sago starch.67,68 Additionally, some nanoencapsulation studies of bioactive compounds are also described briefly in this review. Encapsulation can improve the water solubility of bioactive compounds, such as essential oils.69 Nanoencapsulation also improves bioavailability and health beneficial activities. Mean-while, working in the nanoscale means an increased surface area, which leads to increased activity.70 Lee et al.71have reported CNDs derived from major polysaccharides of Ocimum basilicum (basil) seeds. Fahmi el al.72emphasize the potential application of CDs prepared from waste bamboo leaves and their potential to enhance drug delivery efficacy. In particular, tumour drug doxorubicin was attached to loaded CNDs for tumour imaging and therapy.72 Tang el al.73also reported water-soluble crystalline CNDs derived from glucose, synthesized by a facile microwave-assisted hydrothermal treatment, for potential application in the field of DUV (deep ultraviolet) photonic devices. The as-prepared graphene-like CNDs have a diameter of 1.65 nm, and they are less than 5 layers thick. Konwar el al.74reported CNDs derived from tea leaves, including polyphenols such as catechins,75 tannins,76 and flavonoids,77 which showed excellent antioxidant properties. These tea carbon dots were used to prepare a chitosan-tea carbon dots nanocomposite hydrogel film with developed physico-mechanical properties. Recently, Hsu el al.78prepared CNDs from ground coffee using a simple method consisting of four stages: dehydration, polymerization, carbonization, and passivation. Such nanodots hada5±2nm average diameter, and their quantum yield was 3.8 % for cell imaging. Dong el al.79prepared fluorescent CNDs and graphene oxide (GO) by changing carbonization capability of an usual organic precursor, namely citric acid. Wang el al.80also reported using Enleromorpha prolifera (EP) as the starting material to probe the radical scavenging of natural phenolic compounds, these novel luminescent CNDs were synthesized using a facile micro-wave-assisted hydrothermal approach. Vandarkuzhali el al.63 prepared the pineapple-peel-derived CNDs for use in applications like sensors, molecular keypad lock and memory devices. Fuciftos el al.81have reported caffeine encapsulation into α-lact-albumin (α-LA) nanotubes through nanotube synthesis. α-LA nanotubes were around 100 % successful in caffeine loading.81 It could be deducted from the respective overviews above that the differently efficient CNDs done by many studies have given promising models for various applications. However, information on utilizing organic product-based material for CNDs and its application has not been clearly reported as a review. It became an essential factor in promoting other possibilities for further application. It is hoped that the potential findings of the various organic products-based CNDs would be profitable because the starting materials are sustainable, eco-friendly, and readily available. This review has collected many examples of current developments of green fluorescent CNDs with high potential. CNDs are widely prepared by various methods and from various carbon sources (Fig. 1). Hence, this review aims to extensively examine their synthesis, properties and applications in sensing, solar cells, catalysis, bioimaging and drug delivery and nanoencapsulation of bioactive compounds.

2. Synthetic Methods
The synthetic routes of CNDs were categorized as 'top-down' and 'bottom-up' methods, as shown in Fig. 2. In the case of a 'top-down' method, the method is generally used to prepare carbon nanotube, graphite nanotubes and nanofibres. Moreover, CNDs are also derived from natural products, including fruit shells and peels, plant material and animal waste. All of these sources are abundant and relatively macroscopic carbon sources.24,82,83 On the other hand, in the case of a 'bottom-up' approach, small molecules are expanded to the size of CNDs, using different kinds of starting material, such as seeds. This method can be used to prepare CNDs from small natural polymers, such as chitin and chitosan.84,85 However, to prepare a small number of CNDs from natural products, the two methods mentioned above have been brought together.86 Nowadays, various methods for preparing CNDs from organic products have been developed. Most studies aim to obtain high-quality CNDs using simple, fast, size-controllable methods or large-scale and cost-effective synthetic methods.87 These two widely used methods are discussed in detail below.

2.1. Pyrolysis
Pyrolysis is an irrecoverable thermolysis reaction that consists of chemical decomposition of organic materials in an inert atmosphere. Both physical and chemical changes occur in organic substances due to pyrolysis, resulting in solid carbonaceous residue. Generally, pyrolysis is carried out under controlled pressure and at a very high temperature above 430 °C (800 °F). Some of the organic raw material sources of carbon can be used to synthesize natural product-based CNDs.89
Sun el al.90have prepared a novel enzyme-free fluorescent sensor to probe catechins in green tea, using bifunctional graphene quantum dots (GQDs) as the starting materials. The GQDs were used as peroxidase-like catalysts and fluorescence probes. The GQDs synthesized by pyrolysis were purified, and then their solution was added into the catechin group compound, which was obtained from tea extract. It was found that an enzyme-free fluorescent nanoplatform was usable to detect catechins, and the detection limit was 5.0 nM (Fig. 3a).
In another study by Wei el al.,91fluorescent CNDs were synthesized through pyrolysis and direct calcination of gynoslemma for bioimaging applications. The as-prepared CNDs solution was pale yellow under natural light, whereas it exhibited strong blue fluorescence in 365 nm UV light (Fig. 3b). Their mean size ranged around 2.5 nm. The emitted wavelength has moved to redshift depending on excitation wavelengths (Fig. 3c). Moreover, these CNDs also demonstrated good fluorescence stability even under high ionic strength conditions. Owing to the biocompatibility and excellent fluorescence stability of CNDs, bioimaging observations could be performed in zebrafish. The CNDs can enter zebrafish embryos via chorion or mouth. Thus, the fluorescent CNDs could potentially be used in bioimaging in medicinal applications. Moreover, many published articles regarding CNDs prepared from natural carbon sources through pyrolysis for various applications. Their specific information is summarized and presented in Table 1.
2.2. Extraction
Some organic products-based CNDs can be prepared simply by extraction. Jiang el al.97prepared the extraction of CNDs directly from instant coffee. In a typically simple process, the prepared coffee powder was mixed with purified water at a heating rate of 90 °C, and the amalgam was subjected to vigorous stirring and then centrifuged. The unsinkable phase was sieved using a membrane to separate sizeable particles. Sephadex G-25 gel filtration chromatography was used to further purify the CNDs, using water as the eluent. Obtaining CNDs using this method is uncomplicated and straightforward. The optimal CNDs demonstrated good biocompatibility and were useful for bioimaging applications in cells and fish (Fig. 4).

In another study, Singh el al. 201898 prepared fluorescent CNDs from beetroot raw materials using an aqueous extraction method, for in vivo live imaging in C. elegans and BALB/c mice. The aqueous beetroot extracts displayed blue-emitting CNDs by heating temperature (160 °C) and green-emitting CNDs by oxidation with H2PO4. Their findings demonstrated that the green-emitting CNDs showed a significant optical response in the intestinal part in live BALB/c mice cleared from the body through faeces, thereby attributing that these green CNDs have potential as diagnostic models in biomedical application.
An additional study by Zhang el al.99demonstrated that fluorescent CNDs could be fabricated from a polystyrene foam waste through a facile organic solvent extraction method for biomedical application. The highly fluorescent CNDs showed very strong fluorescence because of the organic solvent's dispersible ability type. It was deducted from Zhang el al.'s findings that the strong photoluminescence, the small size, no toxicity and biocompatibility would contribute to applying these CNDS for bio-imaging and bio-labelling.
2.3. Hydrothermal Treatment
Many researchers often use this method because it is a cheap, eco-friendly, and effective technique to synthesize CNDs from diverse organic products. In this method carbon sources, like carbohydrates, were dissolved in water with or without adding some additives (acid or alkali).100 The prepared solutions were put into an autoclave, heated and kept at a constant heating rate for 7 h. The supernatant, which contains large particles, was separated by centrifugation. Natural products,101 such as starch, maltose, glucose, sucrose, xylose, amylopectin, saccharose, and cellulose, have often been used as carbon sources to develop efficient organic products-based CNDs for specific applica-tions.102
Mewada el al.103synthesized green CNDs from the Indian water plant, Trapabispinosa, peel extract in the absence of any extrinsic oxidizing agents. The size distribution ranged from 5 to 10 nm in the solution, with a leading highly green fluorescence under UV-light (λex= 365 nm). The as-prepared CNDs exhibited potential fluorescent properties and quantum yield. Thus, the as-synthesized CNDs were promising to be biocompatible in case of Madin-Darby Canine Kidney (MDCK) cells.103
Feng el al.104prepared nitrogen-doped CNDs from edible winter melon as the carbon source, using a facile one-step hydro-thermal treatment. It was found that the size distribution diameter of mono-dispersed CNDs was 4.5-5.2 nm. It had 7.51 % quantum yield (QY). These novel photoluminescent CNDs were successful as bio-imaging agents for hepG2 (liver hepatocellular carcinoma) cells. The graphical abstract of it is presented in Fig. 5a.104
Zheng el al.105published information about synthesizing pollen-CNDs through hydrothermal treatment. The as-synthesized CNDs emitted blue light fluorescence under UV light display excitation, making them potentially useful for tracking the absorption of nitrogen, potassium, and phosphorus and marking the biological carrier platform Brassica parachinensis L. They observed that Brassica parachinensis L. showed a yield of 42.90 mg during the addition of 3.5 mg L-1 pollen-CNDs in the nutrient solution. Thus, it would be possible to use the pollen-CNDs at the site of hydroponically grown vegetables. Moreover, those prepared CNDs were biocompatible for imaging of in vilro/in vivo studies. The schematic abstract diagram is shown in Fig. 5b.
Yin el al.106showed that highly efficient CNDs with down- and up-conversion fluorescence could be produced by hydrothermal treatment, using sweet pepper as the carbon source. The quantum yield of CNDs was 19.3 %. These CNDs had good photophysical properties, such as narrow and symmetric emission spectra, good photostability against photobleaching and excitation-dependent fluorescence response. The CNDs worked for fluorescent probes to detect hypochlorite (ClO-) due to the ability to perform both down- and up-conversion fluorescence. For down-conversional measurement, 0.05 μmol L-1 of ClO-concentrations were determined as a detection limit, whereas the up-conversional detection of ClO- concentrations had a small observation limit of 0.06 μmol L-1(S/N = 3). In this way, it was inferred from the above results that the CNDs prepared by this method are advantageous in anion detection applications due to being simple, sensitive, and dual-signalling models. Additionally, they could also be extensively used in biological and environmental assays (Fig. 5c).
Another report by Lu el al.4,107concerning fluorescent organic products-based CNDs, in which fluorescent CNDs were prepared from the cheap waste of pomelo peel as a raw carbon source, using hydrothermal treatment as the preparation method, was published in 2017. The quantum yield was found to be close to 6.9 %. The authors further explored that the application of such CNDs was to detect fluorescent Hg2+, which is dependent on Hg2+-induced luminescent quenching of these CNDs. High level of sensitivity and selectivity toward Hg2+ was achieved by this sensing method, and the range of detection limit was found to be as low as 0.23 nM. This study also proved that it was promising to use fluorescent CNDs to detect Hg2+ in lake water samples (Fig. 5d).
Sachdev and Gopinath108 reported the preparation of CNDs from coriander leaves, through a one-step hydrothermal treatment, for potential use as antioxidants, bioimaging agents, and sensors. According to the antioxidant activity test results, raising the concentration of CNDs from 5 to 70 mL-1 subsequently increased the scavenging activity from 12 to 94 %. Moreover, 2, 2-diphenyl-1-picrylhydrazyl (DPPH) solution, which turned colourless to yellow based on an increase in the concentrations of CNDs, was studied. Additionally, CNDs was assessed for biocompatibility and bioimaging. More than 85 % of cell viability was accounted for by L-132 cells at all the tested doses (0.1 to 1 mg mL-1) of CNDs.
Meanwhile, the low cytotoxicity of CNDs under in vilro conditions strongly attributed to the favourable application of CNDs for bioimaging response. To further describe CNDs synthesized from starting materials of natural products carbon sources, more examples of published articles regarding CNDs prepared from natural carbon sources using hydrothermal method. Their specific information is summarized and presented in Table 2.
2.4. Chemical Oxidation
Hydrogen peroxide and oxidizing acids of chemical oxidants are useable as cooperative starting materials to synthesize natural products-based CNDs. Generally, natural products are first carbonized, and then chemical oxidants are added (as shown in Fig. 6). The separation and purification of the obtained CNDs were performed continually.120
Chin el al.68have reported starch nanoparticles prepared from sago starch via chemical oxidation. Raw materials of sago starch are cheap, and starch nanoparticles can be prepared in an eco-friendly way. The obtained CNDs were dehydrated, using concentrated H2SO4 and simultaneously oxidized with HNO3 for 30 min. They discovered that fluorescent CNDs have an excitation-independent, stable wavelength peak emitted at 430 nm when they get excited by distinctly powerful energy photons. Interestingly, they observed that emission wave-length peak did not shift due to particularly homogenous and mono-dispersed physiochemical characteristics. Owing to their simple preparation, shorter reaction time, and effective luminescent emission, these fluorescent CNDs are favourable candidates for diverse biomedical uses in bio-imaging and bio-sensors.68
In 2012, Suryawanshi el al.121synthesized green CNDs by oxidizing dead neem leaves with H2SO4-HNO3. The prepared CNDs contained various functional groups, for instance, -COOH and epoxy groups, in which these functional groups favour non-radiative integration of electron-hole pairs in the inherent state. The surface of the synthesized CNDs was modified with amines. Meanwhile, the intrinsic fluorescence could be fine-tuned. The -COOH and epoxy groups were substituted by the -CONH2 and -C-NH2 groups, subsequently suppressing the non-radiative track. Thus, the fluorescence intensity was stronger because the intrinsic state emission increased. This study offered new insights into CNDs from an investigational and mechanistic perspective and provided methods to prepare multicoloured CNDs by oxidation. Yan et al.122 prepared CNDs from starch and oxidized them chemically for the application of detecting the chemotherapy drug, imatinib, by a novel design depended on quenched fluorescence. The modification of surface properties and fluorescence emission of CNDs was easily achieved by chemical oxidation. However, some problems remain. As the primary reason for using CNDs prepared by chemical oxidation, the CNDs solution could increase the biological toxicity, because remaining chemical oxidation agents' can contaminate the as-prepared CNDs, which is a major draw-back this method.122
Besides, some natural products-based graphene-like nanodots have also been synthesized with a chemical oxidation/reduction method. For example, Dong et al.123synthesized CNDs with a chemical reduction pathway from humic substances (HS) without further modification and treatment. HS solid materials were dissolved in 0.1 M (pH 7) phosphate buffer solution (PBS), to measure the fluorescence. The as-prepared graphene nanodots could be promising for the clinical platform around a society in vivo or in vitro testing system. Most of these nano-sheets had the size range in lattice spacings of 0.220~0.240 nm according to HRTEM characterization, fitting well with graphene. HS samples had physical appearances similar to graphene nano-sheets.
These physical properties were modified with oxygen-containing groups. They concluded that those graphene nanoparticles are intrinsically carbon-based dots, showing excellent optical properties that included UV-vis (ultraviolet-visible), FL (fluorescence), and ECL (effective conjugated length). Natural carbon-based dots in HS could contribute to new insight into HS. These natural products derived carbon-based dots may bring about potential HS utilization in various fields, such as bio-imaging, bio-medicine, sensing and optoelectronics because of their unique properties.123 To further investigate, many CNDs examples from natural products-based carbon sources were synthesized by using chemical oxidation for bio-medical applications provided by Table 3.
2.5. Microwave-assisted Synthesis Method
The microwave-assisted process is regarded as one of the most effective and feasible methods to synthesize promising CNDs. Advantageously, this method allows CNDs to be synthesized very fast by using a proper precursor solution. Time-saving is the leading benefit of using the microwave-assisted method.127
Simsek et al.50prepared carbon nanomaterials from Nerium oleander (oleander) leaves through the microwave-assisted method. Nerium oleander contains a large number of chemical components like cardiac glycosides, triterpenes, polyphenols, flavonoid, and glycosides. The chemical composition of the natural extracts plays a significant role in synthesizing carbon-based nanodots. The Nerium oleander extracts were divided into aqueous extract and ethanol extract. The aqueous extract contains sterols, flavonoids, and terpenoids, whereas these compounds are not contained in ethanol extract. To evaluate the significant result of a surface-passivating agent in the process of CNDs formation, 1 g polyethylene glycol (PEG) 10000N was added into some of the preparations. It was found that the highest emission wavelength peaks of these CNDsE@PEG (ethanol extract-passivated PEG-CNDs) and CNDsW@PEG (water extract-passivated PEG-CNDs) were red-shifted. This red-shift of CNDs' photoluminescence (PL) emission could be corresponded to growing surface deformity with an oxygenated surface exhibiting smaller energy levels.50
Si et al.128synthesized CNDs from rice straw as a raw carbon source by treating it with an acid catalyst in a co-solvent preparation of ethanol-water, using a fast microwave-assisted one-pot method. The morphology of the as-prepared lignin-based nanoparticles observed in the sophisticated heterogeneity and exhibited crystallization defects. After lignin-based nano-particles (LNPs) were dissolved in the selected organic solvent phase, LNPs produced solution-based precipitates of CNDs, facilitating the lignin diffusion into the solution in contrast with depositing on the surface area.
The reliable zeta potential values verified the excellent stability of the lignin-based nanoparticles dispersion (Fig. 7a), which is beneficial to heated deterioration completion and good thermal durability. The sizes ranged from 2 to 3 nm of prepared lignin-based CNDs. The lignin-based CNDs were dominated by the grafting space of 0.21 nm of a crystalline structure according to the HRTEM analysis (Fig. 7b). Interestingly, the solution-based CNDs were influenced by more graphitic crystallization than the only LNPs. The wavelength peak of the UV-vis absorption of the CNDs solution displayed two shoulder peaks at 270 and 327 nm as indicated in Fig. 7d, which corresponded to the π-π* transitions of aromatic sp2 domains and the n-π* shifting of the C=O bond, respectively.
Additionally, the optical features of the CNDs solution exhibited bright purple fluorescence when irradiated. Since the increased excitation wavelength occurred from 400 nm to 500 nm, the emission wavelength shifted to irreversible lower peaks of ~380 nm. The infra-red spectroscopy results of LNPs demonstrated that the stretching absorption bands of -OH (hydroxyl groups) appeared at 3420 cm-1 and, methyl and methylene groups absorb stretching at 2927 cm-1. Subsequently, these functional groups occurred abundantly in the LNPs. Moreover, the bending vibration of the phenolic hydroxyl group appeared at 1366 cm-1. The two bands, namely the C=O stretching at 1710 cm-1 and 1655 cm-1, attributed to the presence of not only conjugated but also unconjugated C=O in the LNPs (Fig. 7e) appeared. The typical peaks in the Raman analysis centred at 1360 and 1590 cm-1 for the CNDs indicated as the D and G bands of prepared carbon nanomaterials, respectively, confirmed the intrinsic nature of nanocrystalline graphitic sp2 and sp3 carbon deformations (Fig. 7f). In this novel one-pot microwave-assisted approach, the acidolysis released the monosaccharide, and the organic acid formed and solvated into the lignin fraction. Furthermore, Fig. 8 illustrates the synthesis of CNDs from natural lignin, holocellulose and protein through dehydration, polymerization and condensation, as well as from aromatization or carbonization.128 Therefore, it was conferred from the physicochemical and optical properties of selected solution-based CNDs that these CNDs are more favourable than lignin-based nanoparticles alone.
Liu et al129conducted a rapid microwave-assisted synthesis of amphiphilic CNDs from glucose as starting material combined with distilled water and oxidized by concentrated H3PO4. The optimal CNDs solution could be dissolved and readily taken out by chloroform and other organic solvents (ethanol, THF, toluene), exhibiting their amphipathic form. When it was excited at 360 nm, the CNDs displayed two well-defined fluorescent wavelengths in the middle parts at both 447 and 560 nm. Moreover, the CNDs exhibited excitation-dependent emission feature connected with higher potency observed at 442 nm and had a quantum yield (QY) of 7.6 % in water. To further study CNDs produced from natural products-based carbon sources using chemical oxidation for biomedical applications, their specific information was summarized and presented in Table 4.
2.6. Molecular Aggregation
The molecular aggregation method is not well-known for the preparation of CNDs. Only a few reports, including that of the Niu group,133 is available. This method also offered easy preparation from organic sources of natural products derived CNDs (Fig. 9) without heating or other kinds of energy input. Owing to the presence of lignin, the formation of CNDs played an important role in forming J-type molecular clusters in the prepared solution. These aggregates optimized photoluminescence, and the newly created CNDs showed excellent results for bio-imaging applications. The CNDs emitted both up-converting and down-converting emission following excitation with ultra-violet-visible and near-infrared (NIR) radiation. It is worth noting the following two limitations of this method. Firstly, organic raw materials must have aromatic structures (as shown in Fig. 10). Secondly, the CNDs formed by aggregation do not exhibit high chemical and photostability, since the J-type bulks are easily affected by extrinsic interference.133
In 2018 Zhang el al.134prepared through a facile molecular aggregation approach luminescent CNDs from bean-shell phenolic extracts for use in the antioxidation and bioimaging. The as-prepared CNDs exhibited a favourable antioxidation efficiency with photo-bleaching resistance. Their size distribution ranged from 1 to 5 nm with pH/excitation dependent fluorescence. Their novel findings demonstrated that the bio-compatible CNDs are helpful for banana preservation and bio-imaging tumour cells in vivo.
Reckmeier el al.135prepared carbon dots using citric acid and ammonia reactants through the molecular aggregation of ammonothermal synthesis. The resulting nanoparticles showed amorphous aggregates of molecular fluorophores. Those aggregates displayed the blue-green double emission spectrum of prepared CNDs' concentration-dependent optical features and also exhibited graphitic domains to the emission behaviour.
Liu el al.136reported fluorescent CNDs from tannic acid (TA), synthesized by hydrothermal treatment, that exhibited aggregation-induced emission enhancement to visualize permissive detection. They explored organic solvents induced aggregation emission improvement of CNDs for permissive detection and demonstrated promising applications in new sensing devices.
3. Properties
3.1. Physical Properties
CNDs mostly comprise carbon, hydrogen, nitrogen and oxygen, depending on the precursor and synthetic route. Among them, carbon and oxygen are the most plentiful components with many carboxylic acids. The potential of CNDs is highly dependent on the precursor and respective synthetic methods. These moieties could be helpful for both well-dissolved water and functionalized surface with other functional groups.87 Generally, all of the organic-based CNDs are in the range of 2-100 nm-sized nanoparticles made of an sp2-hybridized graphite nucleus or core, which is modified and functionalized by a polar hydroxy group.137,138 CNDs also possess good physical and optical properties to develop their potential applications effectively. Mechanical and optical properties of the CNDs play an important role in the application of electronic devices.139,140,141 Moreover, the fluorescent features also play an important role in bio-medicinal use.142
The organic products-derived CNDs typically involve carbon, oxygen and other heteroatoms.100,143 These doped-heteroatom CNDs have been synthesized from hair and protein, respectively. Heteroatom-rich natural products also contain nitrogen and sulphur. Since lone pair electrons occupied by heteroatoms were conjugated with top orbitals in the carbon materials, the nitrogen/sulphur-doped CNDs exhibits excellent optical and electronic manners. Generally, natural products-derived CNDs showed both amorphous and crystalline features due to surface moieties on the CNDs' surface area. Generally, most of these heteroatom-doped CNDs from organic products have low crystallinity, even though many studies have reported the presence of crystalline sp2-carbon. The surface groups of the prepared organic products-based CNDs are complex, particularly oxygen-related functional groups.144
the material's properties. According to a fundamentally applicable point of view, photoluminescence (PL) has drawn many researchers' attention, and it is also one of the most promising characteristics of CNDs. PL means fluorescence that does not result from heating.145 PL emission peaks of CNDs are dependent on the excitation wavelength and are based on particle size, appearance, conformation, and intrinsic structure of CNDs. Thus, tuning of the emission peaks could be done for further real-life application. Mostly, the corresponding absorption wavelength is shorter than the wavelength of emission. That is why the energy of the absorption is higher than that of emission. Tuning the wavelength emission spectrum is an extensively considered fact of CNDs.146 CNDs can not only donate electrons, but they can also receive or accept them.147
CNDs normally show photoluminescent emission, but that fluorescence can be quenched by adding electron acceptors due to photo-induced electron transfer between the CNDs and electron acceptors. Equally, CNDs can also serve as powerful electron acceptors because the luminescence can be quenched by electron donors, for example, N, N-diethyl aniline.147 This characteristic formation is solvent-dependent and more effective in polar solvents. Photo-induced electron transfer is significant in CNDs-sensitized matters and CNDs-based photo-catalyst structuring. The photo-induced electron transfer of CNDs is influenced by electron-hole pairs, surface moieties, and adherent processes.148 Moreover, CNDs have unique optical properties. Then fluorescence absorption and emission of organic products derived-CNDs did not come initially from a bandgap but were affected by radiative recombination and p-plasmon of the surface-restricted electrons holes.139 This differs from man-made semiconductor carbon nanomaterials.
In relation to absorbance, the green CNDs typically demonstrated that optical emission is absorbed in the spectrum's UV region, shifting to the visible region to a small degree.10 However, the organic products derived-CNDs prepared by standard and simple methods usually showed absorption peaks in the UV range. Each UV range position of CNDs from various carbon sources (man-made carbon sources and carbon sources from organic products) is different.19 Some absorption peaks corresponded to the π-π* shifting signal of the C-C bonds, the n-π* relocating behaviour of C=O bonds and/or others. The degree of emission depends on the particle's radius, so as the particle gets smaller, both the excitation and emission spectrum shift to shorter wavelengths.149 Yoshinaga el al}50prepared CNDs from glucose solution through microwave-assisted pyrolysis. The average size distribution is of diameter 1.65 nm, and the fluorescence QY is 7-10 %.150 The clear UV absorption peaks showed two prominent bands occupied in the middle part of 228 and 282 nm. Since the length of microwave treatment increased, the intensity of both UV absorption bands changed without tuning band areas, which attributed no size-related areas of the prepared nanomaterials.150
CNDs with various sizes, which emitted different fluorescence have been synthesized by diverse methods to describe the role of the fluorescence properties. These emission spectra varied from the visible region to the near-infrared region showing blue, green, yellow, and red. Pan el al151reported the preparation of CNDs via hydrothermal method; the prepared CNDs emitted high fluorescence in alkaline solution situation, whereas this photoluminescence was entirely dim in acidic solution matter. When the experiment was repeated in an alkaline solution, the photoluminescence intensity of CNDs recovered. It confirmed that the pH of the solution does not affect the emission wave-length range, but it had a significant impact on the intensity of photoluminescence. The facile CNDs demonstrated excitation-dependent emission related to that of other CNDs derived from conventional semi-quantum dots. Liang el al.147prepared chitosan-based CNDs as a novel design for a specific study of the quenching fluorescence process. The behaviours of quenching CNDs were studied using a functional group of nitro-aromatics with various ring components, and the practical evidence showed that the preliminary procedure for CNDs-quenching is Förster resonance energy transfer (FRET) not electron transfer. However, the fluorescence emission mechanism draws little attention or interest. Owing to the fluorescence phenomenon of CNDs with plausible mechanisms regarding surface passiva-tion/deformations,152 quantum size effect,153 surface state effect,144 and edge state and carbon-core state composition might be significant to explaining the CNDs' fluorescence emission in detail.144
Some strategies were also summarized for increasing quantum yield and red-shifting absorption wavelength of CNDs. In particular, surface functional moieties,154 degree of surface functionalization,155,156 quantum size effect/conjugated sp2-domain effect155,157 and element doping141,158 are usually used to explain the characteristics of the long-wavelength and tunable emission.
In the up-conversion fluorescence phenomenon, CNDs have a significant feature of intense and tunable PL (photoluminescence). PL emission spectra are usually shoulder peaks, positioning in the transition from the visible to near-infrared (NIR) position, based on the wavelength excitation.22 The distinctive emission peaks can be obtained by evaluating excitation wave-lengths or particle sizes to provide fluorescence imaging and optical labelling. More interestingly, besides down-converted or normal PL, certain CNDs possessed incredible up-converted PL, and this up-conversion fluorescence emission is significantly agreeable for in vivo bioimaging application. A two-photon system might be performed in the up-conversion. For example, Wang el al.159prepared nitrogen-doped CNDs of 1-3 nm through a facile one-pot route from the carbon source of mela-mine and glycerol reaction. These nitrogen-doped CNDs showed multi-photon up-conversion fluorescence and had a strong two-photon absorption cross-section. That up-converting potency of CNDs is applicable for multi-photo single-molecule imaging. Similarly, the CNDs synthesized from some organic sources could offer large two-photon absorption by cross-sections. At the same time, they could absorb two long-wavelength photons upon excitation, and one short-wave-length photon is released. Besides, the CNDs with electron donors and electron acceptors could trigger triplet-triplet annihilation up-converting emission. The applications for up-converting emission have already been published in several papers, but the detailed observation of the mechanism is still challeng-ing.106 Niu el al.133tried to use up-converted emission from lignin-derived CNDs as cellular imaging agents, as shown in Fig. 11a, b. Up-converting emission can also be applied in potential catalysts, biomedicine, and energy storage application technology.

4. Applications
In contrast with green CNDs prepared from organic products, there are some drawbacks in many conventional ways of synthesizing quantum dots for semiconductor materials. For example, using toxic chemical reagents as the starting materials, requiring high temperature, tedious running, and cumbersome synthetic steps.160 Consequently, these man-made carbon quantum dots (CQDs) have more drawbacks than organic products-based CNDs. Therefore, those man-made CQDs are more limited in term of synthesis and applications.
In earlier years using simple diverse synthetic methods, green CNDs have been developed from many natural products, for instance, soy milk,161 eggshells,162 garlic,163 honey,164 ground coffee65 and prawn shells.165 These carbon source starting materials from plants, leaves, fruits, animals, microorganisms, and their excreta offered organic products derived-CNDs.21 These CNDs have several advantages, such as readily and abundantly available raw materials, low cost, convenient synthesis, acceptable renewability, multicoloured photoluminescence, and excellent biocompatibility. Researchers have been keen to synthesize these new profitable and environmental friendly nanomaterials with novel applications. In this article, the applications of organic product based-CNDs in medicinal applications, for example, bioimaging and therapy, drug delivery, sensing and detection, solar cells, and photo-catalysis, will be described.
4.1. Bioimaging and Therapy
There have been many approaches to preparing organic products-based CNDs to stain cancer cells or tumour cells to address the core for fast and accurate targeted cell detection.
Barbosa el al.125synthesized CNDs from animal excreta, like cow manure, and from glucose. The new modified CNDs showed strong fluorescence. The fluorescence means that they could be used effectively to probe living cells with magnificent sub-cellular selectivity. Also, the as-prepared CNDs can stain nucleoli in breast MCF-7 selectively. The CNDs were sensitive to four other cellular patterns and displayed the corresponding cellular selection in live-cell imaging observations.125 Wang and Zhou166 synthesized non-toxic CNDs from the milk carbon source through microwave radiation without any chemical reagents. The CNDs exhibited low-toxic behaviour in the concentrated range of 15 mg mL-1, and bright blue, green, and red colours of emission were showed under an inversely photoluminescent microscope during cellular imaging investigation.166
Song el al.167prepared CNDs from linseed. The prepared CNDs can potentially be applied in both biosensors and bioimaging. Feng el al.104synthesized green CNDs from edible winter melon via hydrothermal treatment and the resulting CNDs are successful bioimaging agents for HepG2 cells. Wei el al.91synthesized heteroatom-doped CNDs like nitrogen/ sulphur-doped CNDs from Allium fislulosum through a one-pot hydrothermal approach. The as-prepared CNDs showed 85 % of cell viability against Hela cells, even when incubated at high concentration of 20 mg mL-1. Because of their non-toxic behaviour, the as-synthesized CNDs could be selectively applied for multicolour cellular imaging in K562 and MCF-7 as excellent fluorescent probes.
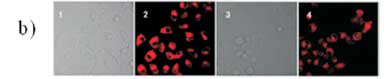
Fahmi el al.72have found that CNDs can be prepared from bamboo leaves by pyrolysis. The as-prepared CNDs were modified with CBBA (4-carboxy-benzylboronic acid) to obtain specific targeting of HeLa tumour cells. More interestingly, an anticancer agent of doxorubicin (Dox) was loaded onto the modified CBBA-CNDs to use them for drug delivery. Potential internalization of CNDs, CBBA-CNDs, and Dox/CBBA-CNDs into HeLa
tumour cells is depicted in Fig. 12. It is worth noting that significant green fluorescence was investigated from CBBA-CNDs on the cytoplasm of HeLa tumour cells after 1 h of incubation.
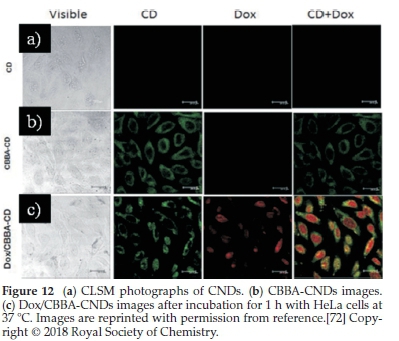
Meanwhile, green fluorescence's significant presence confirmed that CNDs were favourably absorbed by the cells via endocytosis (Fig. 9b). Moreover, Dox delivery was successfully performed using CBBA-CNDs with red fluorescence; the typical emission of Dox was explored in the nuclei of cells treated with Dox/CBBA-CNDs. Dox plays a role as a tumour drug by interfering with the DNA helix structure in the cell nucleus. The formation of red fluorescence in the cell nucleus, after incubation with Dox, confirmed that the drug had been well delivered to the target.
Moreover, Wu el al.168prepared surface passivated CNDs based on a solvent-free synthesis from commercial food-grade honey as starting material. Since intense near-infrared absorption was detected, it can be concluded that microscopic size and rapid lymphatic carrier of CNDs have dislocated from the paw to the axillary lymph node after loading. Therefore, the evidence of that lymph node is significantly improved. They were also discarded from the vessels relatively fast. This exploration can play a vital role in observing sentinel lymph nodes (SLN), where cancer cells can propagate in case of breast cancer.
Another report by Bhamore el al.169described the preparation of green CNDs from Acacia concinna seeds (a carbon source) for fluorescence imaging of fungal cells by using the microwave hydrothermal approach. To assess imaging efficiency by the as-prepared CNDs, the ultra-small CNDs-incubated Penicillium sp. cells showed the optical performance of different kinds of fluorescence such as luminous blue, green and red colours by following laser excitation wavelengths of 405, 488 and 561 nm. This optical performance means that the prepared ultra-small CNDs effectively entered (successful internalization) the cells via endocytosis. Hence, these CNDs localized in the cytoplasm and were specially wrapped in the nucleus of cells. The novel exploration clearly explained that the ultra-small CNDs synthesized from Acacia concinna seeds exhibited excellent biocom-patibility and internalization into the cells with multicoloured imaging, implying their promising use in cell imaging, as shown in Fig. 13.169

Amin el al170also reported water-dispersible nitrogen-doped CNDs (N-CNDs) from the date kernel as a starting matter through a one-pot hydrothermal synthesis without any preceding treatment. The resulting N-CNDs significantly showed 12.5 % of a quantum yield, which was relatively higher than most N-CNDs synthesized from different carbon sources. Interestingly, the as-prepared N-CNDs intrinsically have a great potential regarding optical properties and the higher QY without the addition of high cost or toxic chemical reagents. More importantly, they observed the blue excitation photographs of MG-63 cells after N-CNDs solution (20.0 mg mL-1) incubation.
They showed bright green fluorescence (Fig. 14b). Thus, several amounts of N-CNDs easily entered the cell membranes. It was suggested that they were able to keep their fluorescent behaviour in the cellular environment.170
4.2. Drug Delivery
Nanomaterial-based drug delivery system is one of the bio-medical treatments, which is not used much yet. The organic products-based CNDs have been one of the most attractive nanomedicine models. They have drawn researchers' interest and attention because of their selective targeting response, low toxic behaviour, and agreeable drug efficacy, in contrast with conventionally delivered systems.171,172 Furthermore, they can serve as multifunctional vehicles for loading and releasing drugs. That is why the most promising qualities of organic products-based CNDs and graphene nanodots are strong fluorescence, excellent biocompatibility, rapid cellular uptake, and high stability.173
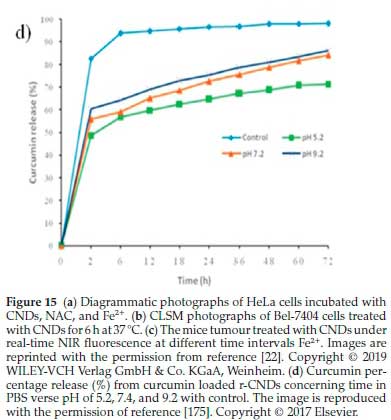
Mehta el al.174reported the facile synthesis of doped heteroatoms CNDs from pasteurized milk, using hydrothermal treatment. The synthesized CNDs have many functional groups (amino or hydroxyl groups) in their site and surface, which can subsequently be functionalized and modified. The synthesized green CNDs were loaded with the lisinopril drug. The optimal lisinopril-loaded CNDs (Lis-CNDs) showed high cell uptake in the HeLa cells. Additionally, they also have excellent physico-chemical properties, such as ultrafine size, multifunctional groups, and excellent photoluminescence. Thus, the fluorescent loaded Lis-CNDs could be used as drug carriers for drug loading and release. In addition to assessing the powerful ability of CNDs-based drug delivery methods, these lisinopril-loaded CNDs were found to have good stability when these CNDs circulated in the blood. Moreover, they released encapsulated drugs immediately after penetrating the targeted tumour cells. It was found that the release of the lisinopril drug was still stable up to 24 h. Subsequently, it was found that the release was constant over 48 h in the PBS conditions of pH 5.2, 6.2, and 7.4, respectively, as shown in Fig. 15a, b, c.174
Rai el al.175also used a microwave-assisted green and simple procedure to synthesize sulphur-doped CNDs from sulphur-containing lignosulfonate lignin as the starting raw materials. The synthesized CNDs were further modified by adding NaBH4 into the solution according to a simple chemical reduction method. Owing to the surface moieties of lignin CNDs, the reduced CNDs (r-CNDs) can offer a potential tool for drug loading and release. Moreover, curcumin was loaded on Lignin r-CNDs to reach the specifically targeted cells. It was found that the delivery of the optimal percentage of curcumin from the r-CNDs was complete, followed by supporting curcumin, as indicated by the standard calibration curve (Fig. 15d). At biological pH calibrated conditions, a maximum value of curcumin release percentage was slowly rising to 82.7 % when calibrated at pH 7.2 after 72h, but curcumin loaded r-CNDs solution at pH 5.2 offered a burst release within early 2 h. The cumulative maximum release of 69.1 % was achieved after 72 h. Significantly, at pH 9.2 calibration, 86.37 % of maximum curcumin release was observed due to the hydrophilicity development, when the pH calibration was increased specifically from 5.2 to 9.2. In conclusion, the curcumin loaded r-CNDs of fast-acting dosage can benefit the novelty of drugs for initial chemotherapy treat-ment.175
D'souza el al.176reported the preparation of nitrogen-doped fluorescent CNDs (N-CNDs) from dried shrimp as carbon precursors through a one-step hydrothermal route. They were intended to be used for potential drug delivery, transport agents and its impact on cancer cells was tested. Subsequently, boldine was loaded on the surface of the as-prepared CNDs and intracellular distribution, internalization process, and release rate were studied. Interestingly, the fabricated CNDs show diverse surface moieties (-NH2, -COOH, and -OH), which facilitates loading boldine on the surface of CNDs through hydrogen bonding. The fluorescent N-doped CNDs served as novel fluorescent probes to give simultaneous imaging/mapping of MCF-7 and SH-SY5Y (human neuroblastoma) cells and act as drug carrier agents for delivering boldine drug to specific target cells. The internalized vesicles will trigger the ability to release boldine from the CNDs, thereby applying anticancer drug delivery and cell imaging to MCF7 cells. The fluorescence of CNDs will help to monitor drug release progress in the cells. As a result, the fluorescent N-doped CNDs could sensitively probe the targeted imaging and can serve as drug carriers for selective target delivery system.176
4.3. Sensing and Detection
One of the interesting applications of CNDs lies in the field of sensing. Many organic products-based CNDs have been responsible for fluorescent directed-sensors to detect metal ions, anions, and molecules because they have unique optical and electrical properties. Several mechanisms play a significant role in the fluorescence changes of quenching or tuning of CNDs, including resonance energy transfer, photo-induced charge transfer, photo-induced electron transfer, and inner filter effect, which have been applied for sensing. The fundamental principle of the development of sensors for ions (Fe3+,Pb2+,Hg2+, CN-, and PO43-) is the quenching of the fluorescence CNDs by using some ions. Among them, Fe3+ is the most commonly detected. Biosensing application is an important application of organic products derived-CNDs, due to the highly selective and is commonly used.22
Sachdev et al.108reported coriander leaves as a carbon precursor to prepare organic products-based CNDs through a facile treatment. Black and green tea, honey, papaya, egg white, goose feathers, and other organic resources are extensively used to synthesize CNDs to detect Fe3+.
Xu et al}77prepared water-soluble and dispersible CNDs with bright fluorescence from potatoes through a one-step hydrothermal approach. The CNDs prepared from cheap and cost-effective carbon sources demonstrated strong blue fluorescence with QY up to 15 %. The result strongly confirmed that the synthesized CNDs could be used as a novel sensing probe for the label-free, selective, and sensitive detection of Fe3+. The detection limit was 0.025 μmol-1, corresponding to different sensitivities of 1.0-5.0 μmol-1 and 5.50 μmol-1 at least and favourable ranges of concentration. Thus, the as-prepared CNDs can detect the Fe3+ ions in real-life water samples.
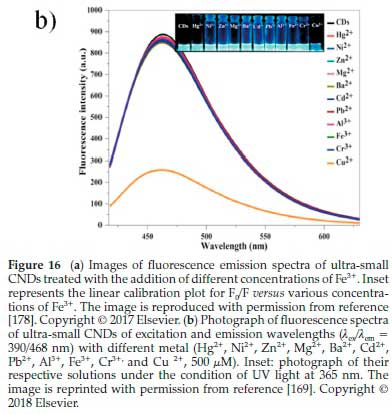
Jayaweera et al.178demonstrated the green and low-cost CNDs from durian shell waste using a microwave hydrothermal approach for label-free detection of Fe3+. The durian shell contains carboxylic acids, esters, and amino compounds that required improving the CNDs surface. The resulting quantum yield was 6.2 %. The blue-green emitting CNDs showed stability over a range of pH and chloride concentrations and indicated photobleaching resistance. There was a concentration range of 0-20 nM with the detection limit of up to 128 nM. In this way, the synthesized CNDs could be applied in sensing Fe3+ in real-life water systems, as indicated in Fig. 16a.
In addition to the detection of Fe3+, Bhamore et al169synthesized green CNDs from Acacia concinna seeds (a carbon source) for fluorescence sensing of Cu2+ ion. These CNDs comprise various phytochemicals, such as alkaloids, acacic acid, lactone, lupeol, spinasterol, ascorbic acid, citric acid, tartaric acid, succinic acid, and sugars. These phytochemicals, belonging to Acacia concinna seeds, could produce ultra-small fluorescent CNDs with intrinsically multifunctional surface groups, making them robust, selective and sensitive sensing probes. As a result, the optical properties of ultra-small CNDs (0.5 mL) exhibited fluorescence emission wavelengths with different metal ions (Hg2+,Ni2+ ,Zn2+,Mg2+,Ba2+,Cd2+,Pb2+,Al3+,Fe3+, and Cr3+). However, (0.7 mL; 500 of Cu2+) was individually analyzed by fluorescence spectrometry (Fig. 17b). The optical spectrum of ultra-small CNDs solution showed the wavelengths in the range of λex/λem = 390/468 nm, but a single fluorescence intensity of Cu2+ ion significantly quenched. Thus, the higher concentration of Cu2+ ions could remarkably quench the strong blue emission of ultra-small CNDs (Inset of Fig. 16b). These observations verified that the quenching of the significant fluorescence was connected to the Cu2+ ion addition, favourably confirming that the ultra-small CNDs are useable for the sensing of Cu2+ ions as a selective probe. Moreover, there are considerable studies of CNDs prepared from respective organic resources for the detection of Cu2+. To further describe them more, their detection limits were comparatively provided in Table 5.


Gu el al.132prepared CNDs from the raw material of lotus root as a precursor for detecting Hg2+ ions using the microwave method without any surface passivating agents. The fluorescent nitrogen-doped CNDs have a nitrogen content of 5.23 %. In particular, the synthesized lotus root-based CNDs (LR-CNDs) can remarkably serve as a selective fluorescent probe to detect Hg2+ with the linear concentration range from 0.1 to 60.0 μM. As indicated in Fig. 17, the detection limit was evaluated to be 18.7 nM.
Xu el al.179reported the flavonoid-based CNDs (Fla-CNDs) from the starting material of flavonoid extracts of Ginkgo biloba leaves to detect toxic heavy metal ion Pb2+ through a one-pot hydrothermal route. They found that Fla-CNDs can detect the quantitative range (0.1-20.0 nM) of Pb2+ following an ultrahigh sensitivity of 55 pM. The selective efficacy of Fla-CNDs for Pb2+ was almost one order of targeting extent larger than that for other appropriate metal ions. More interestingly, agarose hydrogel was loaded onto Fla-CNDs (AHG-CNDs) for better optically luminescent detection and elimination of Pb2+ from water. They concluded that the Pb2+ adsorbed AHG-CNDs could be re-established in HCl solution. This new exploration contributed to the synthesis of high-cost effective materials for targeting and detecting heavy metal ions.
In addition to metal ions, organic products-based CNDs can also be used to detect anions. Yin el al.106synthesized CNDs, using a low-temperature carbonization route, from sweet pepper. The fluorescence properties of these CND were quenched remarkably depending on the concentration of added ClO-.
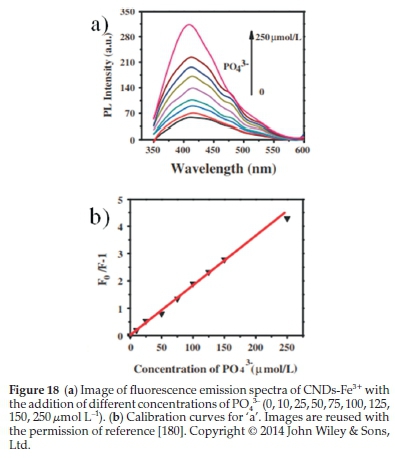
Xu el al.180synthesized blue-fluorescing CNDs from potatoes as raw materials through a hydrothermal approach. The PO43-explores the sensing performance's sensitivity- concentration followed by a detection limit of 0.8 μmol L-1 indicated in Fig. 18. They found that the photoluminescence of CNDs can be quenched successfully by Fe3+. If the PO43- concentration was increased, the fluorescence could gradually be restored. These CNDs could be a selective and sensitive probe for sensing and detecting PO43-. Besides, the obtained CNDs showed intense brightness, good biocompatibility, and well-dispersed water; that is why these promising features are effective for bioimaging applications.
4.4. Solar Cell
In the last few years, the organic products derived-CNDs have been investigated to be used in solar cells, because they exhibited excitation-independent, excitation-dependent fluorescence wavelength emissions, pH and size-dependent or independent fluorescence emissions.181 Marinovic el al.3investigated the usefulness of mesoporous TiO2-based solar cells loaded with green CNDs synthesized from organic sources, including actual food waste of lobster shells. These promising CNDs were intended to be used for optoelectronic materials in a solar cell. They were further separated for purification, subsequently performed by hydrothermal carbonization of the other starting materials. They indicated that the solar cells sensitized with lobster shells derived-CNDs exhibited a somewhat lower efficiency of 0.22 %, suggesting that optoelectronic materials for the application in renewable energy storage cell technologies generated from organic waste products were possible to some practical extent.
Marinovic and co-workers suggested that the efficiency of the solar cells was also connected to structural, chemical, and electrical properties of the synthesized CNDs.3 Moreover, there are considerable studies of respective starting materials which derived CNDs for functional optoelectronic materials to be used in solar cells. Their average internal efficiency was comparatively studied with lobster-CNDs provided in Table 6.

Zhang el al.181prepared a fluorescence dimming mechanism that remarkably developed the conversion efficiency of CNDs responsive to aqueous solar cells. Organic products-based CNDs were prepared from grass as a carbon source material. The produced CNDs were significantly reliable for fluorescence quenching development. Their findings verified the concept that was extensively applicable for increasing solar cells' efficiency using other fluorescent quantum dots or nanodots sensitizers. A schematic diagram shows that an electron acceptor or donor could make the photo-excited electron quenched (Fig. 19a). Briscoe el al.139explored CNDs synthesized from organic sources, such as chitin, chitosan, and glucose. Since the functionalization of the as-prepared CNDs was performed by the other surface functional groups in the starting materials, these loaded CNDs would potentially sensitize ZnO nanorods to sunlight, agreeing well to be applied in solid-state nano-structured energy storage cells. A single-layer CNDs and a 4-layer layer-by-layer (LbL) ZnO/CNDs/CuSCN solar cells coating to illuminated current-voltage data are shown in Fig. 19b,c, respectively. Shi el al.182prepared carbon quantum dots through bottom-up synthesis. These TiO2 photoanode loading CNDs can enhance photo-to-electrical conversion and the short-circuit current density efficiency in a solar cell device. The CNDs-sensitized solar cells prepared by HTC (high-temperature condition) of organic product sources and the applicable materials of chemically grown ZnO nanorods are displayed in Fig. 19d. The efficiency of the devices relies on which functional groups were present in the synthesized CNDs surface. This precursor-dependent solar cell efficacy also indicated that the functio-nalization signifies the binding of CNDs to the ZnO surface and the characteristic transport of the charge in the layer, which affects the solar cell efficiency.
4.5. Catalysis
In addition to their bioimaging, sensing, and solar cells applications, the organic products derived-CNDs have played significant roles in the catalysis and electrolysis fields. Photoactive CNDs have been shown to serve as both excellent electron donors and acceptors, as revealed by electron transfer with CNDs, which can be induced by photoexcitation.184 The organic products-based CNDs can enhance the photocatalytic production of hydrogen. CNDs doped with other additives, namely composited CNDs, are applicable in photocatalysis and electrocatalysis.22 Prasannan el al.185used a composite of ZnO, and CNDs synthesized from orange peel using a facile hydrothermal approach to be used as a photocatalyst for the deterioration of dyes (Fig. 20a). The ZnO/CNDs hybrid materials were synthesized through the solution-dispersible method. The naphthol blue-black azo dye was nearly degraded in 45 min by the ZnO/CNDs catalyst with UV irradiation. In contrast with using only ZnO or CNDs as the catalyst, the degradation was determined to be 84.3 % and 4.4 %, respectively, after irradiation took place for the same time. The stronger photocatalytic feature of the ZnO/CNDs composite was responsible for the electronic charge interaction. The synthetic process of ZnO/CNDs was successfully completed, which might become remarkably high on the transfer of the photogenerated matters. Although other CNDs/metal photocatalyst methods have been used in the organic compound deterioration, there might be some drawbacks with these CNDs/metal hybrid photocatalyst methods. Firstly, they are potentially toxic and expensive, which confines their future uses in industry. Secondly, it is likely to have metal-containing photocatalysts aggregation in accordance with high surface energies, thereby leading to a significant loss of catalytic activity.
Wang el al.186prepared novel non-metal photocatalysts made up of CNDs, using the hydrothermal method, from glucose inserted in a carbon matrix to overcome these issues. These photocatalysts could degrade organic dyes (methylene blue and rhodamine B) in the range of UV-vis light and NIR radiation (Fig. 20b,c). The application of solar renewable energy to generate hydrogen by decomposing water in a photocatalytic process gives a favourable model to overcome worldwide energy shortage.
Li el al.187reported the novel carbon-loaded ruthenium CNDs electrocatalysts (Ru-CNDs) for hydrogen evolution reaction (HER) using CNDs prepared from ginkgo leaves. The HER electrocatalytic efficiency of CNDs loaded with Ru (Ru-CNDs) is more profitable than of CNDs loaded with other noble metals, non-noble metals or nonmetallic materials. Ru-CNDs are cata-lytically more resistant than Pt/C in alkaline solutions. Thus, the catalytic performance of the Ru-CNDs formation was potentially explored as a new alternative design of hybridizing CNDs/metal, with remarkable potential for HER uses. HER polarization curves for Ru-CNDs and CNDs, 20 % commercial Pt/C, Ru powder, 5 % commercial Ru/C, and a stability test for Ru-CNDs in1MKOHbefore and after 10 000 cycles are shown in Fig. 21d-e. Qin el al.113also found that HTC (high-temperature condition) of cost-effective left-over of willow bark led to high photoluminescence and permeable CNDs. Such CNDs were chosen for the application as excellent photo-catalysis for the synchronous decreasing of Au complexes and graphene oxides (GOs) to produce Au nanoparticle-decorated reduced graphene oxide (AuNP/rGO) nanocomposites. Subsequently, a mixture of GO and HAuCl4 aqueous solution was irradiated by UV light to find the trace of CNDs in a mixture.
Tyagi el al.188reported green CNDs from the waste material of lemon peel through hydrothermal treatment. The photo-catalytic efficacy of immobilized CNDs on electrospun TiO2 nanofibres (TiO2-CNDs composites) was illustrated by using methylene blue (MB) dye as a model pollutant agent. The photocatalytic performance of the TiO2-CNDs composite is around 2.5 times higher than that of TiO2 nanofibres.
5. Nanoencapsulation
Recently, nanotechnology has extensively drawn interest in studying food science. The bioactive compounds encapsulated study is one of the most attractive systems for many research-ers.189 Nanoencapsulation consists of coating for bioactive compounds and can significantly affect their mechanical properties, bioavailability and stability.190,191 Since health-giving or harmless foodstuffs are demanded by consumers, scientists or researchers have paid a lot of attention to modifying or synthesizing bioactive compounds with natural ingredients. Phenolic compounds, essential oils, essential fatty acids, carotenoids and insoluble vitamins are bioactive compounds that help human well-being.192 However, these bioactive compounds, such as phenolic acid and coumarins, flavonoid derivatives are mostly hydrophobic and poorly soluble.193-195 Mostly, an unpleasant or bitter sense of taste belongs to many of those molecules, subsequently consuming limited food or oral treatment. To diminish these leading issues, a nanoencapsulation of delivery systems has been extensively attractive as an alternative food science technology method.193 Thus, the nanoencapsulated materials can deliver these defectively hydrophobic, soluble, and bio-available compounds into harmless foodstuffs. The nanoencapsulation enhances their absorption efficiency into cellular structures with agreeable particle compositions. The considerable properties of encapsulated form, size, and surface enable to improve nanoparticles' solubility, upgrading the rate of absorption and release, influencing gastrointestinal dispersion, and preventing metabolic deterioration.196 Recently, various nanoencapsulated methods and nanocarriers of bioactive compounds, for example, nanosuspensions, nanoemulsions, liposomes and nanometer phytosomes, nanostructured lipid transporters, solid lipid nanoparticles (SLNs), biopolymer SLNs, micelles made of proteins and polysaccharides, have been studied.197
Cui el al.198prepared curcumin encapsulated on lysozyme nanoparticles by the de-solvation method. The synthesized lysozyme nanoparticles (Ly-NPs) was of the average size distribution of 127.9 ± 2.12 nm. Curcumin is regarded as a physiologically and pharmacologically functional nutritional component. According to TEM analysis, curcumin (Cur) influenced the physical structure or size of Ly-NPs after curcumin encapsulated Ly-NPs. Significantly, the Cur packaged Ly-NPs displayed better dispersion in contrast with Ly-NPs alone, as shown in Fig. 21b,c. Moreover, packaged curcumin exhibited considerably enhanced survival rate after heat treatment in contrast with free curcumin. DPPH investigation also approved that packaged curcumin, indicating favourable antioxidant capability after 50 °C treatment. Thus, encapsulated curcumin in Ly-NPs is applicable for the encapsulation and protection of curcumin.198
Fuciftos el al.81have reported the caffeine-encapsulated preparation into α-lactalbumin nanotubes through nanotube synthesis. These caffeine-loaded nanotubes were synthesized by adding caffeine into the processing mixture before the nanotube became trapped for self-assembly. The results showed that α-lactalbumin (α-LA) in nanotubes prepared in the existence of Mn2+ were strongly stable while freeze-drying. The encapsulated efficacy was around 100 %, meaning caffeine loaded α-LA nanotubes were promising. Caffeine release from α-LA nano-tubes was optimal under low heating rate and strong pH.81
Basiri el al.199prepared nanoencapsulated α-tocopherol-loaded noisome through the modified heating system, using surfactants - span60 and tween60. Their findings showed that noisome played a crucial role in efficacy for encapsulated-α-TOC and combining Span 60 and Tween 60, forming a favour-able α-TOC-encapsulated nanosomia. The findings reported that α-TOC loaded niosomes had on average, a narrow size distribution (73.85 ± 0.6-186 ± 0.58 nm), and high encapsulation efficiency (61.13 ± 0.52-98.92 ± 0.92). Since the sizeable hydro-phobic moiety (HLB) decreased, the encapsulation efficiency (EE) and the niosomes durability was effectively improved.199
El-Fattah et al.200reported the phytosome nanoparticles-encapsulated quercetin by using thin-film hydration approach. They found a highly encapsulated efficacy of 98.4 %. Phyto-somenanocapsules improved the remedial advantage of quercetin in ovariectomized rats.200
Najafi et al.201prepared the achillea millefolium-encapsulated nanophytosometo to assess the post-thawing sperm quality and evaluate the oxidative signal of rooster semen. They aimed to analyze the content of antioxidants of achillea millefolium extract (AmE), and AmE loaded in nanopytosome under the freezing condition of Ross 308 rooster semen. They found that AmE-encapsulated nanophytosome at 3 mg L-1 doses can improve rooster sperm motility, feasibility and inconvenient oxidative values in the freezing.201
Arroyo-Maya et al.202have reported nanoencapsulation of anthocyanins-loaded biopolymer nanoparticles (nanospheres), through a complicated thermal and electrostatic method using a whey protein-pectin combination. The nano-loaded average sizes were as low as 200 nm in diameter. These nanospheres release 55 % of the moderate loading capacity before heating. These nanoencapsulated materials improved thermal feasibility and reduced antioxidant performance.202
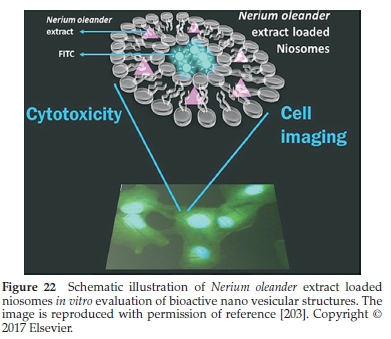
Gunes et al.203prepared niosomal formulations of methanol and hydro methanol extracts produced from N. oleander roots using a thin-film hydration technique (as shown in Fig. 22). Total phenolic capacity in the methanol oleander extract (MOE) and hydro methanol oleander extracts (MOWE) was calculated to be 64.51 ± 0.945 mg-1 and 65.05 ± 0.37μg mg- gallic acid equivalents, respectively. More interestingly, the vesicles' encapsulated efficacy was found to be 16.2 % for MON (in the presence of MOE) and 13.24 % for MWON (in the presence ofMOWE). All of the dose concentrations of methanol oleander extract (MOE), and methanol oleander niosomal formulations (MON) inhibited cell Hela and A549 cells, thereby reducing cell viabilities. Moreover, cell imaging studies indicated that the MON could readily enter the HeLa and A549 cells via endocytosis and grow around the nucleus.203
6. Conclusions and Future Perspectives
This review reveals different synthetic methods, properties, and applications of CNDs derived from organic products. Moreover, nanoencapsulation is also briefly studied. The current paper gives a systematic overview of converting natural organic products into green CNDs and describes the physicochemical properties of these CNDs. Furthermore, major applications of the organic products derived-CNDs, for example, sensing and detection, bioimaging, drug delivery, solar cells, and photo-catalysis, nanoencapsulation of some bioactive compounds are extensively reviewed. Natural products derived-CNDs have some important advantages - firstly; the organic precursors that come from diverse carbon sources of biomass are renewable, inexpensive, easy, and abundant, produced in high yield and good biocompatibility. Secondly, the organic products of carbon sources are intrinsically doped with hetero-atoms, making it easy to prepare heteroatom-doped CNDs, which exhibit excellent photoluminescence.
Additionally, they can be used in biomedical applications because excellent optical properties and red fluorescence emission of green CNDs enhanced by hetero-atom doping contents greatly attract every researcher. Lastly, the synthetic approaches used to synthesize CNDs are simple, fast, and eco-friendly to some degree. Thus, organic products are likely attractive starting carbon sources in the synthesis of green and efficient CNDs. Many studies have also paid attention to developing new natural biological wastes, new prepared approaches, new applications, and better materials and cost-effective equipment.
Despite having many advantages, there are still several challenges concerning organic products-derived CNDs, some of which are considered below.
Challenge of short-wavelength absorption and emission: currently, the organic products-based CNDs of different sizes emitting disparate fluorescence have been successfully synthesized from many organic products (foodstuffs and other valuable bio-resources), using a variety of methods. The ranges of their emission spectra of CNDs are mostly in the ultraviolet/visible region's position. Consequently, those ranges are not extended enough for use in most biomedical fields. Because the short UV light harms or destroys DNA and protein, visible light cannot go through into deep tissues for photoimaging. Near infra-red (NIR) light would be better for clinical uses since NIR light can deeply enter tissues and diminish side effects concerning biological toxicity.204 In future, much effort should be put into synthesizing CNDs with absorbance and emission in the NIR region. The short-wavelength absorbance of CNDs might adversely influence their photocatalytic performance. Light-harvesting ability is limited by the narrow absorption ranges of CNDs, which means that it would be advantageous to broaden the absorption length. More photons can be absorbed to increase their efficiency in photocatalytic materials, renewable energy storage cells.
The synthesis of CNDs with multi-photon fluorescence and up-conversion fluorescence: multiphoton photoluminescence and up-conversion fluorescence are interesting aspects, in which CNDs emit visible fluorescence emission with NIR light. However, the phenomenon of up-converting emission remains complicated, and further investigations are necessary for it to identify this in detail. The up-converting emission of CNDs is potentially applied not only for bioimaging but also for a suitable replacement for lanthanide-doped up-converting nanoparticles (UCNPs) in the manufacture of NIR-responsive matters since the organic products-based CNDs are more biocompatible than lanthanide-doped up-converting nanoparticles (UCNPs).
There are more biomedical and other applications of CNDs, such as in vivo bioimaging, photodynamic therapy, drug delivery, sensing, and nanocomposite hydrogels for tissue engineering.205 Despite many proven advantages in natural product-derived CNDs, studies of producing the great potential of CNDs are still challenging. The authors of the current paper hope that this review will inspire exciting ideas for further applications.
The synthesis of CNDs with room-temperature phosphorescence: The spin-forbidden nature of triplet exciton transitions and non-radiative decay processes hinder the phosphorescence of CNDs. In this way, the ambient temperature environment for the synthetic process of CNDs is highly recommended to attain profitable phosphorescence. Many synthetic routes have been investigated to achieve metal-free room temperature phosphorescence (RTP). More urgently and importantly, the metal-free CNDs of RTP should be maintained by other special organic groups, such as heavy halogen, aromatic carbonyl group, and a deuterated carbon atom. Thus, organic product carbon sources limit their synthesis complexity and chemical diversity. There is a significant need for new metal-free CNDs (pure organic product derived-CNDs) with long-life RTP to be synthesized using facile synthetic methods. We strongly hope that this review will offer several advantages, especially for researchers to prepare organic products-based CNDs that are more interesting and attractive with excellent properties and potential applications.
Nanoencapsulalion advanlages and disadvantages: benefits of nanoencapsulation include improved safety against pH, light, wetness, and gastric digestive system; it covers-up bitter taste or aromatic taste, enhance releasing capacity; promotes the soluble level of lipophilic compounds in aqueous condition. It supports the long-lasting absorption of nutrients and can develop loading nanomaterials with antimicrobial and antioxidant polyphenolic compounds. However, nanoencapsulation still has drawbacks concerning their toxicological effects on the safety of human consumption and the environment. Finding the best methods to obtain them further and identifying ideal nanomaterials for bioactive compounds is still challenging.
Acknowledgements
The authors thank the Ministry of Research and Technology, Republic of Indonesia, and Universitas Airlangga, for supporting this study.
Conflict of Interest
The authors claim no conflict of interest.
Abbreviations
AFM atomic absorption microscopy
BV blood vessel
CNDs CNDs
CBBA 4-carboxy-benzylboronic acid
Dox Doxorubicin
ELC effective conjugated length
FL fluorescence
FTIR Fourier-transform infrared spectroscopy
FRET Förster resonance energy
GQDs graphene quantum dots
HS humic substances
HRTEM high-resolution transmission electron microscopy
L-NPs lignin-based nanoparticles
Lis-CNDs lisinopril-loaded CNDs
LV lymph vessel
N/S-CNDs nitrogen/sulphur-doped CNDs NIR near-infrared region
OP-CNDs organic-based CNDs
PEG polyethylene glycol
PL photoluminescence
QY quantum yield
PA photoacoustic
SLN sentinel lymph node
TEM transmission electron microscopy
UV-vis ultraviolet visible
ORCID iDs
Κ. Siimon: orcid.org/0000-0001-5430-9992
Μ. Zakki Fahmi: orcid.org/0000-0001-5045-1617
References
1 S.L. Gillett, Nanolechnology: Clean Energy and Resources for lhe Fulure, White Paper for Foresight Institute, 2002. [ Links ]
2 C.T. White and T.N. Todorov, Carbon nanotubes as longballistic conductors, Nalure, 1998, 393, 240. [ Links ]
3 A. Marinovic, L.S. Kiat, S. Dunn, M.M. Titirici and J. Briscoe, Carbon-nanodot solar cells from renewable precursors, ChemSusChem, 2017, 10, 1004-1013. [ Links ]
4 W. Lu, X. Qin, S. Liu, G. Chang, Y. Zhang, Y. Luo, A.M. Asiri, A.O. Al-Youbi and X. Sun, Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury (II) ions, Anal. Chem., 2012,84,5351-5357. [ Links ]
5 V Strauss, A. Roth, M. Sekita and D.M. Guldi, Efficient energy-conversion materials for the future: understanding and tailoring charge-transfer processes in carbon nanostructures, Chem., 2016,1, 531-556. [ Links ]
6 L. Wang, S.J. Zhu, H.Y. Wang, S.N. Qu, Y.L. Zhang, J.H. Zhang, Q.D. Chen, H.L. Xu, W. Han and B. Yang, Common origin of green luminescence in carbon nanodots and graphene quantum dots, ACS Nano, 2014, 8, 2541-2547. [ Links ]
7 J. Shen, Y. Zhu, X. Yang and C. Li, Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices, Chem. Commun, 2012,48, 3686-3699. [ Links ]
8 Y.P. Sun, B. Zhou, Y. Lin, W. Wang, K.S. Fernando, P. Pathak, M.J. Meziani, B.A. Harruff, X. Wang and H. Wang, Quantum-sized carbon dots for bright and colorful photoluminescence, J. Am. Chem. Soc., 2006,128, 7756-7757. [ Links ]
9 P. Namdari, B. Negahdari and A. Eatemadi, Synthesis, properties and biomedical applications of carbon-based quantum dots: an updated review, Biomed. Pharmacolher., 2017, 87, 209-222. [ Links ]
10 Z.L. Wu, Z. X. Liu and Y.H. Yuan, Carbon dots: materials, synthesis, properties and approaches to long-wavelength and multicolor emission, J. Maler. Chem. B, 2017, 5, 3794-3809. [ Links ]
11 S. Ray, A. Saha, N.R. Jana and R. Sarkar, Fluorescent carbon nanoparticles: synthesis, characterization, andbioimagingapplication, J. Phys. Chem. C, 2009, 113, 18546-18551. [ Links ]
12 R. Wang, K.Q. Lu, Z.R. Tang and Y.J. Xu, Recent progress in carbon quantum dots: synthesis, properties and applications in photocatalysis, J. Maler. Chem. A, 2017, 5, 3717-3734. [ Links ]
13 S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang and B. Yang, The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective, Nano Res., 2015, 8, 355-381. [ Links ]
14 L. Li, G. Wu, G. Yang, J. Peng, J. Zhao and J-J. Zhu, Focusing on luminescent graphene quantum dots: current status andfuture perspectives, Nanoscale, 2013, 5, 4015-4039. [ Links ]
15 H. Li, Z. Kang, Y. Liu and S.T. Lee, Carbon nanodots: synthesis, properties and applications, J. Maler. Chem., 2012, 22, 24230-24253. [ Links ]
16 S.N. Baker and G.A. Baker, Luminescent carbon nanodots: emergent nanolights, Angew. Chem. Inl. Ed., 2010,49, 6726-6744. [ Links ]
17 S.Y. Lim, W. Shen and Z. Gao, Carbon quantum dots and their applications, Chem. Soc. Rev, 2015,44, 362-381. [ Links ]
18 K.S. Fernando, S. Sahu, Y. Liu, W.K. Lewis, E.A. Guliants, A. Jafariyan, P. Wang, C.E. Bunker and Y.P. Sun, Carbon quantum dots and applications in photocatalytic energy conversion, ACS Appl. Maler. & Inlerf., 2015, 7, 8363-8376. [ Links ]
19 P.Zuo,X. Lu,Z. Sun, Y. GuoandH. He, Areviewon syntheses,properties, characterization andbioanalytical applications of fluorescent carbon dots, Microchim. Acta, 2016,183, 519-542. [ Links ]
20 Y. Wang and A. Hu, Carbon quantum dots: synthesis, properties and applications, J. Mater. Chem. C, 2014, 2, 6921-6939. [ Links ]
21 X. Jiang, S. Zong, C. Chen, Y. Zhang, Z. Wang and Y. Cui, Goldcarbon dots for the intracellular imaging of cancer-derived exo-somes, Nanotechnol, 2018,17, 175701. [ Links ]
22 W. Meng, X. Bai, B. Wang, Z. Liu, S. Lu and B. Yang, Biomass-derived carbon dots and their applications, Energy Environ. Mater., 2019, 2, 172-192. [ Links ]
23 W. Yang, H. Zhang, J. Lai, X. Peng, Y. Hu, W. Gu and L. Ye, Carbon dots with red-shifted photoluminescence by fluorine doping for optical bio-imaging, Carbon, 2018,128, 78-85. [ Links ]
24 S.Y. Park, H.U. Lee, E.S. Park, S.C. Lee, J.W Lee, S.W. Jeong, C.H. Kim, Y-C. Lee, Y.S. Huh and J. Lee, Photoluminescent green carbon nano-dots from food-waste-derived sources: large-scale synthesis, properties, and biomedical applications, ACS Appl. Mater. & Interf., 2014, 6, 3365-3370. [ Links ]
25 W. Li, Z. Zhang, B. Kong, S. Feng, J. Wang, L. Wang, J. Yang, F. Zhang, P. Wu and D. Zhao, Simple and green synthesis of nitrogen-doped photoluminescent carbonaceous nanospheres for bioimaging, Angew. Chem. Int. Ed., 2013, 52, 8151-8155. [ Links ]
26 S. Bhandari, D. Mondal, S. Nataraj and R.G. Balakrishna, Bio-molecule-derived quantum dots for sustainable optoelectronics, Nanoscale Adv., 2019, 1, 913-936. [ Links ]
27 L. Cui, J. Wu and H. Ju, Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials, Biosens. Bioelectron., 2015, 63, 276-286. [ Links ]
28 A. Bhatnagar, S. Chinnasamy, M. Singh and K. Das, Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters, Appl. Energy, 2011, 88, 3425-3431. [ Links ]
29 S.K. Sonkar, M. Roy, D.G. Babar and S. Sarkar, Water soluble carbon nano-onions from wood wool as growth promoters for gram plants, Nanoscale, 2012,4, 7670-7675. [ Links ]
30 M. Ortega-Liebana, J. Hueso, A. Larrea, V. Sebastian and J. Santa-maria, Feroxyhyte nanoflakes coupled to up-converting carbon nanodots: a highly active, magnetically recoverable, Fenton-like photocatalyst in the visible-NIR range, Chem. Commun., 2015, 51, 16625-16628. [ Links ]
31 M. Das Purkayastha, A.K. Manhar, V.K. Das, A. Borah, M. Mandal, A.J. Thakur and C.L. Mahanta, Antioxidative, hemocompatible, fluorescent carbon nanodots from an "end-of-pipe" agricultural waste: exploring its new horizon in the food-packaging domain, J. Agric. Food Chem, 2014, 62, 4509-4520. [ Links ]
32 N.L. Teradal and R. Jelinek, Carbon nanomaterials in biological studies and biomedicine, Adv. Healthcare Mater., 2017, 6, 1700574. [ Links ]
33 W. Shi, X. Li and H. Ma, A tunable ratiometric pH sensor based on carbon nanodots for the quantitative measurement of the intra-cellularpH of whole cells, Angew. Chem. Int. Ed.,2012,51,6432-6435. [ Links ]
34 M. Lin, H.Y. Zou, T. Yang, Z.X. Liu, H. Liu and C.Z. Huang, An inner filter effect based sensor of tetracycline hydrochloride as developed by loading photoluminescent carbon nanodots in the electrospun nanofibers, Nanoscale, 2016, 8, 2999-3007. [ Links ]
35 S.S. Soley, carbon quantum dots: synthesis and optronics applications, IJAEMS, 2017,1, 121-124. [ Links ]
36 H. Ming, Z. Ma, Y. Liu, K. Pan, H. Yu, F. Wang and Z. Kang, Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property, Dalton Trans., 2012,41, 9526-9531. [ Links ]
37 S. Bayda, Synthesis of Fluorescent Carbon Nanoparticles (Cnps) and Their Applications in Drug Delivery, PhD thesis, University of Trieste, 2017. [ Links ]
38 Y. Zhao, X. Liu, Y. Yang, L. Kang, Z. Yang, W. LiuandL. Chen, Carbon dots: from intense absorption in visible range to excitation-independent and excitation-dependent photoluminescence, Fullerenes, Nanotubes Carbon Nanostruct, 2015, 23, 922-929. [ Links ]
39 H. Gao, X. Zhao and S. Chen, AIEgen-based fluorescent nano-materials: fabrication and biological applications, Molecules, 2018, 23, 419. [ Links ]
40 H.P. Castro, V.S. Souza, J.D. Scholten, J.H. Dias, J.A. Fernandes, F.S. Rodembusch, R. dos Reis, J. Dupont, S.R. Teixeira and R.R. Correia, Synthesis and characterisation of fluorescent carbon nanodots produced in ionic liquids by laser ablation, Chem. Eur. J, 2016, 22, 138-143. [ Links ]
41 J. Deng, Q. Lu, N. Mi, H. Li, M. Liu, M. Xu, L. Tan, Q. Xie, Y. Zhang and S. Yao, Electrochemical synthesis of carbon nanodots directly from alcohols, Chem. Eur. J, 2014, 20, 4993-4999. [ Links ]
42 S. Jahan, F. Mansoor, S. Naz, J. Lei and S. Kanwal, Oxidative synthesis of highly fluorescent boron/nitrogen co-doped carbon nanodots enabling detection of photosensitizer and carcinogenic dye, Anal. Chem., 2013, 85, 10232-10239. [ Links ]
43 M. Algarra, M. Perez-Martin, M. Cifuentes-Rueda, J. Jiménez-Jiménez, J.E. da Silva, T. Bandosz, E. Rodriguez-Castellon, J.L. Navarrete and J. Casado, Carbon dots obtained usinghydrothermal treatment of formaldehyde. Cell imaging in vitro, Nanoscale, 2014,6, 9071-9077. [ Links ]
44 X. Zhai, P. Zhang, C. Liu, T. Bai, W. Li, L. Dai and W. Liu, Highly luminescent carbon nanodots by microwave-assisted pyrolysis, Chem. Commun, 2012, 48, 7955-7957. [ Links ]
45 M. Ortega-Liebana, N. Chung, R. Limpens, L. Gomez, J. Hueso, J. Santamaria and T. Gregorkiewicz, Uniform luminescent carbon nanodots prepared by rapid pyrolysis of organic precursors confined within nanoporous templating structures, Carbon, 2017, 117, 437-446. [ Links ]
46 S. Qu, D. Zhou, D. Li, W. Ji, P. Jing, D. Han, L. Liu, H. Zeng and D. Shen, Toward effficient orange emissive carbon nanodots through conjugated sp2-domain controlling and surface charges engineering, Adv. Mater, 2016, 28, 3516-3521. [ Links ]
47 W. Zhang, J. Chavez, Z. Zeng, B. Bloom, A. Sheardy, Z. Ji, Z. Yin, D. H. Waldeck, Z. Jia and J. Wei, Antioxidant capacity of nitrogen and sulfur codoped carbon nanodots, ACS Appl. Nano Mater., 2018, 1, 2699-2708. [ Links ]
48 A.J. Permana, A. Haris, H. Setyawati and M.Z. Fahmi, Partial oxida-tive synthesis of fluorescent carbon derived from local bamboo leaves, J. Chem. Technol. Metall., 2017, 52, 1101-1104. [ Links ]
49 S. Wafiroh, M. Wathoniyyah, A. Abdulloh, Y. Rahardjo and M.Z. Fahmi, Application of glutaraldehyde-crosslinked chitosan membranes from shrimp shellwaste on production of biodiesel from Calophyllum inophyllum oil, Chem. Chem. Technol., 2017, 11, 65-70. [ Links ]
50 S. Simsek,M.O. Alas,B. Ozbek and R. Genc, Evaluation of the physical properties of fluorescent carbon nanodots synthesized using Nerium oleander extracts by microwave-assisted synthesis methods, J. Mater. Res. Technol., 2019, 8, 2721-2731. [ Links ]
51 H. Zhang, S. Kang, G. Wang, Y. Zhang and H. Zhao, Fluorescence determination of nitrite in water using prawn-shell derived nitrogen-doped carbon nanodots as fluorophores, ACS Sensors, 2016, 1, 875-881. [ Links ]
52 M.P. Aji and P.A. Wiguna, Facile synthesis of luminescent carbon dots from mangosteen peel by pyrolysis method, J. Theoret. Appl. Phys, 2017,11, 119-126. [ Links ]
53 D. Kukreja, J. Mathew, R. Lakshmipathy and N. Sarada, Synthesis of fluorescent carbon dots from mango peels, Int J ChemTech Res , 2015, 8, 61-64. [ Links ]
54 K.L. Penniston, S.Y. Nakada, R.P. Holmes and D.G. Assimos, Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products, J. Endourol., 2008, 22, 567-570. [ Links ]
55 VN. Mehta, S. Jha, H. Basu, R.K. Singhal and S.K. Kailasa, One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells, Sens. Actuators B: Chem., 2015, 213, 434-443. [ Links ]
56 M.T.F. Calangian, B. Abigail, K.S. Vanessa, G.J.T. Manzano, R.J.J. Ganado, A.C.C. Yago, R.M. Eduardo Jr, R.D. Vasquez and C.F. Francisco Jr, Facile synthesis of biologically derived fluorescent carbon nanoparticles (FCNPs) from an abundant marine alga and its biological activities, Orient. J. Chem, 2018, 34, 791-799. [ Links ]
57 V. Ramanan, S.K. Thiyagarajan, K. Raji, R. Suresh, R. Sekar and P. Ramamurthy, Outright green synthesis of fluorescent carbon dots from eutrophic algal blooms for in vitro imaging, ACS Sustain. Chem. Eng., 2016,4, 4724-4731. [ Links ]
58 Y. Ke, B. Garg and Y.C. Ling, Waste chicken eggshell as low-cost precursor for efficient synthesis of nitrogen-doped fluorescent carbon nanodots and their multi-functional applications, RSC Adv., 2014, 4, 58329-58336. [ Links ]
59 C.L. Wang, J.Y. Liao, S.H. Chung and A. Manthiram, Carbonized eggshell membranes as a natural and abundant counter electrode for efficient dye-sensitized solar cells, Adv. Energy Maler., 2015, 5, 1401524. [ Links ]
60 M.C. Ortega-Liebana, J.L. Hueso, S. Ferdousi, R. Arenal, S. Irusta, K.L. Yeung and J. Santamaria, Extraordinary sensitizing effect of co-doped carbon nanodots derived from mate herb: application to enhanced photocatalytic degradation of chlorinated wastewater compounds under visible light, Appl. Calal. B: Environ., 2017, 218, 68-79. [ Links ]
61 S. Qu, H. Chen, X. Zheng, J. Cao and X. Liu, Ratiometric fluorescent nanosensor based on water soluble carbon nanodots with multiple sensing capacities, Nanoscale, 2013, 5, 5514-5518. [ Links ]
62 Z. Ramezani, M. Qorbanpour and N. Rahbar, Green synthesis of carbon quantum dots using quince fruit (Cydonia oblonga) powder as carbon precursor: application in cell imaging and As3+ determination, Colloids Surf. A: Physicochem. Eng. Aspecls, 2018, 549, 58-66. [ Links ]
63 S.A.A. Vandarkuzhali, S. Natarajan, S. Jeyabalan, G. Sivaraman, S. Singaravadivel, S. Muthusubramanian and B. Viswanathan, Pineapple peel-derived carbon dots: applications as sensor, molecular keypad lock, and memory device, ACS Omega, 2018,3,12584-12592. [ Links ]
64 K. Huang, Q. He, R. Sun, L. Fang, H. Song, L. Li, Z. Li, Y. Tian, H. Cui and J. Zhang, Preparation and application of carbon dots derived from cherry blossom flowers, Chem. Phys. Lell., 2019, 731, 136586. [ Links ]
65 L. Wang, W. Li, B. Wu, Z. Li, S. Wang, Y. Liu, D. Pan and M. Wu, Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing, Chem. Eng. J., 2016,300,75-82. [ Links ]
66 C. Yu, T. Xuan, D. Yan, S. Lou, X. Hou, Y. Chen, J. Wang and H. Li, Sesame-derivedionsco-dopedfluorescent carbon nanoparticles for bio-imaging, sensing and patterning applications, Sens. Aclualors B: Chem., 2017, 253, 900-910. [ Links ]
67 X.W. Tan, A.N.B. Romainor, S.F. Chin and S.M. Ng Carbon dots production via pyrolysis of sago waste as potential probe for metal ions sensing, J. Anal. Appl. Pyrol, 2014,105, 157-165. [ Links ]
68 S.F. Chin, S.N.A.M. Yazid, S.C. Pang and S.M. Ng, Facile synthesis of fluorescent carbon nanodots from starch nanoparticles, Maler. Lell., 2012, 85, 50-52. [ Links ]
69 P.J. Rao and M.M. Naidu, Nanoencapsulation of bioactive compounds for nutraceutical food, in Nanoscience in Food and Agricullure 2, Springer, 2016, pp. 129-156. [ Links ]
70 I. Ashoush, F. Khaled and K.M. Ramadan, Nanoencapsulation and nanoemulsion of bioactive compounds to enhance their antioxidant activity in food, Inl. J. Food Sci. Technol., 2014, 4(3), 1-22. [ Links ]
71 K. Lee, E. Park, H.A. Lee, C. Sugnaux, M. Shin, C.J. Jeong, J. Lee, P.B. Messersmith, S.Y. Park and H. Lee, Phenolic condensation and facilitation of fluorescent carbon dot formation: a mechanism study, Nanoscale, 2017, 9, 16596-16601. [ Links ]
72 M.Z. Fahmi, A. Haris, A.J. Permana, D.L.N. Wibowo, B. Purwanto, Y.L. Nikmah and A. Idris, Bamboo leaf-based carbon dots for efficient tumor imaging and therapy, RSC Adv., 2018, 8, 38376-38383. [ Links ]
73 L. Tang, R. Ji, X. Cao, J. Lin, H. Jiang, X. Li, K.S. Teng, C.M. Luk, S. Zeng and J. Hao, Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots, ACS Nano, 2012, 6, 5102-5110. [ Links ]
74 A. Konwar, N. Gogoi, G. Majumdar and D. Chowdhury, Green chitosan-carbon dots nanocomposite hydrogel film with superior properties, Carbohydr. Polym., 2015,115, 238-245. [ Links ]
75 D.A. Balentine, M.E. Harbowy and H.N. Graham, Tea: the plant and its manufacture; chemistry and consumption of the beverage, Caffeine, 1998,35-72. [ Links ]
76 A. Crozier, I.B. Jaganath and M.N. Clifford, Dietary phenolics: chemistry,bioavailability and effects on health, Nal. Prod. Rep., 2009, 26, 1001-1043. [ Links ]
77 J. Peterson, J. Dwyer, S. Bhagwat, D. Haytowitz, J. Holden, A. Eldridge, G. Beecher and J. Aladesanmi, Major flavonoids in dry tea, J. Food Compos. Anal., 2005,18, 487-501. [ Links ]
78 P.C. Hsu, Z.Y. Shih, C.H. Lee and H.T. Chang, Synthesis and analytical applications of photoluminescent carbon nanodots, Green Chem., 2012, 14, 917-920. [ Links ]
79 Y. Dong, J. Shao, C. Chen, H. Li, R. Wang, Y. Chi, X. Lin and G. Chen, Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid, Carbon, 2012, 50, 4738-4743. [ Links ]
80 X. Wang, T. Gao, M. Yang, J. Zhao, F.L. Jiang and Y. Liu, Micro-wave-assisted synthesis, characterization, cell imaging of fluorescent carbon dots using l-asparagine as precursor, New J. Chem., 2019, 43, 3323-3331. [ Links ]
81 C. Fucinos, M. Míguez, P. Fucinos, L. M. Pastrana, M. L. Rúa and A. A. Vicente, Creating functional nanostructures: encapsulation of caffeine into α-lactalbumin nanotubes, Innov. Food Sci. Emerg. Technol., 2017, 40, 10-17. [ Links ]
82 B. Thangaraj, P.R. Solomon and S. Ranganathan, Synthesis of carbon quantum dots with special reference to biomass as a source - A review, Curr. Pharmaceul. Design, 2019, 25, 1455-1476. [ Links ]
83 A.A. Mohammed, C. Chen and Z. Zhu, Low-cost, high-performance supercapacitor based on activated carbon electrode materials derived from baobab fruit shells, J. Colloid Inlerf. Sci., 2019, 538, 308-319. [ Links ]
84 T. Jain, S. Kumar and P. Dutta, Chitosan in the light of nanobiotech-nology: a mini review, J. Biomed. Technol. Res., 2015,1, 101-107. [ Links ]
85 R. Devi and R. Dhamodharan, Sustainable process for separating chitin and simultaneous synthesis of carbon nanodots from shellfish waste using2% aqueous urea solution, ACS Suslain. Chem. Eng., 2018, 6, 11313-11325. [ Links ]
86 K.K. Chan, S.H.K. Yap and K.T. Yong, Biogreen synthesis of carbon dots for biotechnology and nanomedicine applications, Nano-micro Lell., 2018,10, 72. [ Links ]
87 P. Miao, K. Han, Y. Tang, B. Wang, T. Lin and W. Cheng, Recent advances in carbon nanodots: synthesis, properties and biomedical applications, Nanoscale, 2015, 7, 1586-1595. [ Links ]
88 X. Zhang, M. Jiang, N. Niu, Z. Chen, S. Li, S. Liu and J. Li, Natural-product-derived carbon dots: from natural products to functional materials, ChemSusChem, 2018,11, 11-24. [ Links ]
89 A. Bridgwater and G. Peacocke, Fast pyrolysis processes for biomass, Renew. Suslain. Energy Rev., 2000,4, 1-73. [ Links ]
90 J. Sun, Y. He and L. Wang, Enzyme-free fluorescence sensing of catechins in green tea using bifunctional graphene quantum dots, Anal. Melhods, 2017, 9, 3525-3530. [ Links ]
91 X. Wei, L. Li, J. Liu, L. Yu, H. Li, F. Cheng, X. Yi, J.Heand B. Li, Green synthesis of fluorescent carbon dots from Gynoslemma for bio-imaging and antioxidant in zebrafish, ACS Appl. Maler. & Inlerf., 2019, 11, 9832-9840. [ Links ]
92 W. Wang, J.C. Martin, X. Fan, A. Han, Z. Luo and L. Sun, Silica nanoparticles and frameworks from rice husk biomass, ACS Appl. Maler. & Inlerf, 2012, 4, 977-981. [ Links ]
93 M. Xue, M. Zou, J. Zhao, Z. Zhan and S. Zhao, Green preparation of fluorescent carbon dots from lychee seeds and their application for the selective detection of methylene blue and imaging in living cells, J. Maler. Chem. B, 2015, 3, 6783-6789. [ Links ]
94 L. Zhu, Y. Yin, C.F. Wang and S. Chen, Plant leaf-derived fluorescent carbon dots for sensing, patterning and coding, J. Maler. Chem. C, 2013, 1, 4925-4932. [ Links ]
95 M. Xue, Z. Zhan, M. Zou, L. Zhang and S. Zhao, Green synthesis of stable and biocompatible fluorescent carbon dots from peanut shells for multicolor living cell imaging, New J. Chem., 2016, 40, 1698-1703. [ Links ]
96 M. Saxena and S. Sarkar, Synthesis of carbogenic nanosphere from peanut skin, Diamond Relaled Maler., 2012, 24, 11-14. [ Links ]
97 C. Jiang, H. Wu, X. Song, X. Ma, J. Wang and M. Tan, Presence of photoluminescent carbon dots in Nescafe(®) original instant coffee: applications to bioimaging, Talanla, 2014,127, 68-74. [ Links ]
98 V. Singh, K.S. Rawat, S. Mishra, T. Baghel, S. Fatima, A.A. John, N. Kalleti, D. Singh, A. Nazir and S.K. Rath, Biocompatible fluorescent carbon quantum dots prepared from beetroot extract for in vivo live imaging in C. elegans and BALB/c mice, J. Maler. Chem. B, 2018, 6, 3366-3371. [ Links ]
99 R. Zhang, Y. Liu, L. Yu, Z. Li and S. Sun, Preparation of high-quality biocompatible carbon dots by extraction, with new thoughts on the luminescence mechanisms, Nanolechnol, 2013, 24, 225601. [ Links ]
100 B. Hu, K. Wang, L. Wu, S.H. Yu, M. Antonietti and M.M. Titirici, Engineering carbon materials from the hydrothermal carbonization process of biomass, Adv. Maler, 2010, 22, 813-828. [ Links ]
101 X. He, H. Li, Y. Liu, H. Huang, Z. Kang and S.T. Lee, Water soluble carbon nanoparticles: hydrothermal synthesis and excellent photo-luminescence properties, Colloids Surf. B: Bioinlerf, 2011,87,326-332. [ Links ]
102 M.M. Titirici, M. Antonietti and N. Baccile, Hydrothermal carbon from biomass: a comparison of the local structure from poly- to monosaccharides and pentoses/hexoses, Green Chem., 2008, 10, 1204-1212. [ Links ]
103 A. Mewada, S. Pandey, S. Shinde, N. Mishra, G. Oza, M. Thakur, M. Sharon and M. Sharon, Green synthesis of biocompatible carbon dots using aqueous extract of Trapa bispinosa peel, Maler. Sci. Eng.: C, 2013, 33, 2914-2917. [ Links ]
104 X. Feng, Y. Jiang, J. Zhao, M. Miao, S. Cao, J. Fang and L. Shi, Easy synthesis of photoluminescent N-doped carbon dots from winter melon for bio-imaging, RSC Adv., 2015, 5, 31250-31254. [ Links ]
105 Y. Zheng, H. Zhang, W. Li, Y. Liu, X. Zhang, H. Liu and B. Lei, Pollen derived blue fluorescent carbon dots for bioimaging and monitoring of nitrogen, phosphorus and potassium uptake in Brassica para-chinensis L, RSC Adv., 2017, 7, 33459-33465. [ Links ]
106 B. Yin, J. Deng, X. Peng, Q. Long, J. Zhao, Q. Lu, Q. Chen, H. Li, H. Tang and Y. Zhang, Green synthesis of carbon dots with down- and up-conversion fluorescent properties for sensitive detection of hypochlorite with a dual-readout assay, Analysl, 2013, 138, 6551-6557. [ Links ]
107 J. Li, W. Liu, D. Xiao and X. Wang, Oxygen-rich hierarchical porous carbon made from pomelo peel fiber as electrode material for super-capacitor, Appl. Surf. Sci., 2017, 416, 918-924. [ Links ]
108 A. Sachdev and P. Gopinath, Green synthesis of multifunctional carbon dots from coriander leaves and their potential application as antioxidants, Analysl, 2015,140, 4260-4269. [ Links ]
109 X. Feng, Y. Jiang, J. Zhao, M. Miao, S. Cao, J. Fang and L. Shi, Easy synthesis of photoluminescent N-doped carbon dots from winter melon for bio-imaging, RSC Adv., 2015, 5, 31250-31254. [ Links ]
110 J. Zhang, Y. Yuan, G. Liang and S.H. Yu, Scale-up synthesis of fragrant nitrogen-doped carbon dots from bee pollens for bio-imaging and catalysis, Adv. Sci., 2015, 2, 1500002. [ Links ]
111 A. Himaja, P.S. Karthik, B. Sreedhar and S.P. Singh, Synthesis of carbon dots from kitchen waste: conversion of waste to value added product, J. Fluores., 2014, 24, 1767-1773. [ Links ]
112 Z. Ding, F. Li, J. Wen, X. Wang and R. Sun, Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass, Green Chem, 2018, 20, 1383-1390. [ Links ]
113 X. Qin, W. Lu, A.M. Asiri, A.O. Al-Youbi and X. Sun, Green, low-cost synthesis of photoluminescent carbon dots by hydrothermal treatment of willow bark and their application as an effective photo-catalyst for fabricating Au nanoparticles - reduced graphene oxide nanocomposites for glucose detection, Calal. Sci. Technol., 2013, 3, 1027-1035. [ Links ]
114 Q. Wang, X. Liu, L. Zhang and Y. Lv, Microwave-assisted synthesis of carbon nanodots through an eggshell membrane and their fluorescent application, Analysl, 2012, 137, 5392-5397. [ Links ]
115 W. Shi, H. Fan, S. Ai and L. Zhu, Preparation of fluorescent graphene quantum dots from humic acid for bioimaging application, New J. Chem., 2015, 39, 7054-7059. [ Links ]
116 Y. Liu, Y. Zhao and Y. Zhang, One-step green synthesized fluorescent carbon nanodots from bamboo leaves for copper (II) ion detection, Sens. Aclualors B: Chem, 2014,196, 647-652. [ Links ]
117 R. Liu, J. Zhang, M. Gao, Z. Li, J. Chen, D. Wu and P. Liu, A facile microwave-hydrothermal approach towards highly photoluminescent carbon dots from goose feathers, RSC Adv., 2015,5,4428-4433. [ Links ]
118 R. Purbia and S. Paria, A simple turn on fluorescent sensor for the selective detection of thiamine using coconut water derived luminescent carbon dots, Biosens. Bioeleclron., 2016, 79, 467-475. [ Links ]
119 X. Yang, Y. Zhuo, S. Zhu, Y. Luo, Y. Feng and Y. Dou, Novel and green synthesis of high-fluorescent carbon dots originated from honey for sensing and imaging, Biosens. Bioeleclron., 2014, 60, 292-298. [ Links ]
120 S.K. Das, Y. Liu, S. Yeom, D.Y. Kim and C.I. Richards, Single-particle fluorescence intensity fluctuations of carbon nanodots, Nano Lell., 2014, 14, 620-625. [ Links ]
121 A. Suryawanshi, M. Biswal, D. Mhamane, R. Gokhale, S. Patil, D. Guin and S. Ogale, Large scale synthesis of graphene quantum dots (GQDs) from waste biomass and their use as an efficient and selective photoluminescence on-off-on probe for Ag+ ions, Nanoscale, 2014, 6, 11664-11670. [ Links ]
122 Z. Yan, Z. Zhang and J. Chen, Biomass-based carbon dots: synthesis and application in imatinib determination, Sens. Aclualors B: Chem., 2016, 225, 469-473. [ Links ]
123 Y. Dong, L. Wan, J. Cai, Q. Fang, Y. Chi and G. Chen, Natural carbon-based dots from humic substances, Sci. Rep., 2015, 5, 10037. [ Links ]
124 Z.A. Qiao, Y. Wang, Y. Gao, H. Li, T. Dai, Y. Liu and Q. Huo, Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation, Chem. Commun, 2010,46, 8812-8814. [ Links ]
125 C. D'Angelis do ES Barbosa, J.R. Corrêa, G.A. Medeiros, G. Barreto, K.G. Magalhäes, A.L. de Oliveira, J. Spencer, M.O. Rodrigues and B.A. Neto, Carbon dots (C-dots) from cow manure with impressive subcellular selectivity tuned by simple chemical modification, Chem. Eur. J., 2015, 21, 5055-5060. [ Links ]
126 H. Liu, T. Ye and C. Mao, Fluorescent carbon nanoparticles derived from candle soot, Angew. Chem. Inl. Ed., 2007, 46, 6473-6475. [ Links ]
127 E.C. Gaudino, G. Cravotto, M. Manzoli and S. Tabasso, From waste biomass to chemicals and energy via microwave-assisted processes, Green Chem., 2019, 21, 1202-1235. [ Links ]
128 M. Si, J. Zhang, Y. He, Z. Yang, X. Yan, M. Liu, S. Zhuo, S. Wang, X. Min and C. Gao, Synchronous and rapid preparation of lignin nanoparticles and carbon quantum dots from natural lignocellu-lose, Green Chem., 2018, 20, 3414-3419. [ Links ]
129 X. Liu, T. Li, Y. Hou, Q. Wu, J. Yi and G. Zhang, Microwave synthesis of carbon dots with multi-response using denatured proteins as carbon source, RSC Adv., 2016, 6, 11711-11718. [ Links ]
130 S. Chandra, P. Das, S. Bag, D. Laha and P. Pramanik, Synthesis, functionalization and bioimaging applications of highly fluorescent carbon nanoparticles, Nanoscale, 2011, 3, 1533-1540. [ Links ]
131 S. Rodrigues, M. Dionísio, C.R. López and A. Grenha, Biocompat-ibility of chitosan carriers with application in drug delivery, J. Func-lional Biomaler., 2012, 3, 615-641. [ Links ]
132 D. Gu, S. Shang, Q. Yu and J. Shen, Green synthesis of nitrogen-doped carbon dots from lotus root for Hg (II) ions detection and cell imaging, Appl. Surf. Sci., 2016, 390, 38-42. [ Links ]
133 N. Niu, Z. Ma, F. He, S. Li, J. Li, S. Liu and P. Yang, Preparation of carbon dots for cellular imaging by the molecular aggregation of cellulolytic enzyme lignin, Langmuir, 2017, 33, 5786-5795. [ Links ]
134 X. Zhang, H. Wang, C. Ma, N. Niu, Z. Chen, S. Liu, J. Li and S. Li, Seeking value from biomass materials: preparation of coffee bean shell-derived fluorescent carbon dots via molecular aggregation for antioxidation and bioimaging applications, Maler. Chem. Fronl., 2018, 2, 1269-1275. [ Links ]
135 C.J. Reckmeier, J. Schneider, Y. Xiong, J. Häusler, P. Kasák, W Schnick and A.L. Rogach, Aggregated molecular fluorophores in the ammonothermal synthesis of carbon dots, Chem. Maler., 2017,29, 10352-10361. [ Links ]
136 Z.X. Liu, Z.L. Wu, M.X. Gao, H. Liu and C.Z. Huang, Carbon dots with aggregation induced emission enhancement for visual permittivity detection, Chem. Commun, 2016, 52, 2063-2066. [ Links ]
137 K. Hola, Y. Zhang, Y. Wang, E.P. Giannelis, R. Zboril andA.L. Rogach, Carbon dots - Emerging light emitters for bioimaging, cancer therapy and optoelectronics, Nano Today, 2014, 9, 590-603. [ Links ]
138 P.C. Hsu and H.T. Chang, Synthesis of high-quality carbon nanodots from hydrophilic compounds: role of functional groups, Chem. Commun., 2012, 48, 3984-3986. [ Links ]
139 J. Briscoe, A. Marinovic, M. Sevilla, S. Dunn and M. Titirici, Bio-mass-derived carbon quantum dot sensitizers for solid-state nano-structured solar cells, Angew. Chem. Inl. Ed., 2015, 54, 4463-4468. [ Links ]
140 S.Y. Park, H.U. Lee, Y.C. Lee, S. Choi, D.H. Cho, H.S. Kim, S. Bang, S. Seo, S.C. Lee and J. Won, Eco-friendly carbon-nanodot-based fluorescent paints for advancedphotocatalytic systems, Sci. Rep., 2015,5, 12420. [ Links ]
141 M.L. Liu, B.B. Chen, C.M. Li and C.Z. Huang, Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications, Green Chem, 2019, 21, 449-471. [ Links ]
142 T. Ji, P. Fan, X. Li, Z. Mei, Y. Mao and Y. Tian, EDTA-bonded multi-connected carbon-dots and their Eu 3+ complex: preparation and optical properties, RSC Adv., 2019, 9, 10645-10650. [ Links ]
143 Y. Choi, B. Kang J. Lee, S. Kim, G.T. Kim, H. Kang B.R. Lee, H. Kim, S.H. Shim and G. Lee, Integrative approach toward uncovering the origin of photoluminescence in dual heteroatom-doped carbon nanodots, Chem. Maler., 2016, 28, 6840-6847. [ Links ]
144 C. Sun, Y. Zhang, P. Wang, Y. Yang, Y. Wang, J. Xu, Y. Wang and W. Y William, Synthesis of nitrogen and sulfur co-doped carbon dots from garlic for selective detection of Fe3+, Nanoscale Res. Lell., 2016, 11, 110. [ Links ]
145 N. Greenham, I. Samuel, G. Hayes, R. Phillips, Y. Kessener, S. Moratti, A. Holmes and R. Friend, Measurement of absolute photoluminescence quantum efficiencies in conjugated polymers, Chem. Phys. Lell., 1995, 241, 89-96. [ Links ]
146 A. Sharma and J. Das, Small molecules derived carbon dots: synthesis and applications in sensing, catalysis, imaging, andbiomedicine, J. Nanobiolechnol, 2019,17, 92. [ Links ]
147 Z. Liang, M. Kang, G.F. Payne, X. Wang and R. Sun, Probing energy and electron transfer mechanisms in fluorescence quenching of biomass carbon quantum dots, ACS Appl. Maler. & Inlerf., 2016, 8, 17478-17488. [ Links ]
148 X. Wang, L. Cao, F. Lu, M.J. Meziani, H. Li, G. Qi, B. Zhou, B.A. Harruff, F. Kermarrec and Y.P. Sun, Photoinduced electron transfers with carbon dots, Chem. Commun., 2009, 3774-3776. [ Links ]
149 F. Yan, Z. Sun, H. Zhang, X. Sun, Y. Jiang and Z. Bai, The fluorescence mechanism of carbon dots, and methods for tuning their emission color: a review, Microchim. Acla, 2019, 186, 1-37. [ Links ]
150 T. Yoshinaga, Y. Iso and T. Isobe, Particulate, structural, and optical properties of D-glucose-derived carbon dots synthesized by micro-wave-assisted hydrothermal treatment, ECS J. Solid Slale Sci. Technol., 2017, 7, R3034. [ Links ]
151 D. Pan, J. Zhang, Z. Li and M. Wu, Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots, Adv. Maler., 2010, 22, 734-738. [ Links ]
152 A. Cayuela, M.L. Soriano and M. Valcárcel, Strong luminescence of carbon dots induced by acetone passivation: efficient sensor for a rapid analysis of two different pollutants, Anal. Chim. Acla, 2013,804, 246-251. [ Links ]
153 L. Zheng, Y. Chi, Y. Dong, J. Lin and B. Wang, Electrochemilumines-cence of water-soluble carbon nanocrystals released electrochemi-cally from graphite, J. Am. Chem. Soc., 2009,131, 4564-4565. [ Links ]
154 H. Nie, M. Li, Q. Li, S. Liang, Y. Tan, L. Sheng, W. Shi and S.X.A. Zhang, Carbon dots with continuously tunable full-color emission and their application in ratiometric pH sensing, Chem. Maler., 2014, 26, 3104-3112. [ Links ]
155 H. Ding, S.B. Yu, J.S. Wei and H.M. Xiong, Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism, ACS Nano, 2015, 10, 484-491. [ Links ]
156 L. Bao, Z.L. Zhang, Z.Q. Tian, L. Zhang, C. Liu, Y. Lin, B. Qi and D.W Pang, Electrochemical tuning of luminescent carbon nanodots: from preparation to luminescence mechanism, Adv. Maler., 2011,23, 5801-5806. [ Links ]
157 X. Tan, Y. Li, X. Li, S. Zhou, L. Fan and S. Yang, Electrochemical synthesis of small-sized red fluorescent graphene quantum dots as a bioimaging platform, Chem. Commun., 2015, 51, 2544-2546. [ Links ]
158 S. Yang, J. Sun, P. He, X. Deng, Z. Wang, C. Hu, G. Ding and X. Xie, Selenium doped graphene quantum dots as an ultrasensitive redox fluorescent switch, Chem. Maler., 2015, 27, 2004-2011. [ Links ]
159 C. Wang, X. Wu, X. Li, W. Wang, L. Wang, M. Gu and Q. Li, Up-conversion fluorescent carbon nanodots enriched with nitrogen for light harvesting, J. Maler. Chem, 2012, 22, 15522-15525. [ Links ]
160 Y. Pu, F. Cai, D. Wang, J.X. Wang and J.F. Chen, Colloidal synthesis of semiconductor quantum dots toward large-scale production: a review, Induslr. Eng. Chem. Res, 2018, 57, 1790-1802. [ Links ]
161 C. Zhu, J. Zhai and S. Dong, Bifunctional fluorescent carbon nanodots: green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction, Chem. Commun., 2012, 48, 9367-9369. [ Links ]
162 M. Baláz, Eggshell membrane biomaterial as a platform for applications in materials science, Acta biomaterialia, Acla Biomaler., 2014,10, 3827-3843. [ Links ]
163 S. Zhao, M. Lan, X. Zhu, H. Xue, T.W. Ng X. Meng, C.S. Lee, P. Wang and W. Zhang, Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging, ACS Appl. Maler. & Inlerf., 2015, 7, 17054-17060. [ Links ]
164 S. Mandani, D. Dey, B. Sharma and T.K. Sarma, Natural occurrence of fluorescent carbon dots in honey, Carbon, 2017,119, 569-572. [ Links ]
165 G. Gedda, C.Y. Lee, Y.C. Lin and H.F. Wu, Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions, Sens. Aclualors B: Chem., 2016, 224, 396-403. [ Links ]
166 L. Wang and H. S. Zhou, Green synthesis of luminescent nitrogen-doped carbon dots from milk and its imaging application, Anal. Chem., 2014, 86, 8902-8905. [ Links ]
167 Y. Song, X. Yan, Z. Li, L. Qu, C. Zhu, R. Ye, S. Li, D. Du and Y. Lin, Highly photoluminescent carbon dots derived from linseed and their applications in cellular imaging and sensing, J. Maler. Chem. B, 2018, 6, 3181-3187. [ Links ]
168 L. Wu, X. Cai, K. Nelson, W. Xing, J. Xia, R. Zhang, A.J. Stacy, M. Luderer, G.M. Lanza and L.V. Wang, A green synthesis of carbon nanoparticles from honey and their use in real-time photoacoustic imaging, Nano Res., 2013, 6, 312-325. [ Links ]
169 J.R. Bhamore, S. Jha, T.J. Park and S.K. Kailasa, Fluorescence sensing of Cu2+ ion and imaging of fungal cell by ultra-small fluorescent carbon dots derived from Acacia concinna seeds, Sens. Aclualors B: Chem., 2018, 277, 47-54. [ Links ]
170 N. Amin, A. Afkhami, L. Hosseinzadeh and T. Madrakian, Green and cost-effective synthesis of carbon dots from date kernel and their application as a novel switchable fluorescence probe for sensitive assay of zoledronic acid drug in human serum and cellular imaging, Anal. Chim. Acla, 2018, 1030, 183-193. [ Links ]
171 M.Z. Fahmi, J.K. Chen, C.C. Huang, Y.C. Ling and J.Y. Chang, Phenylboronic acid-modified magnetic nanoparticles as a platform for carbon dot onjugation and doxorubicin delivery, J. Maler. Chem. B, 2015, 3, 5532-5543. [ Links ]
172 L. Thoo, M.Z. Fahmi, I.N. Zulkipli, N. Keasberry and A. Idris, Interaction and cellular uptake of surface-modified carbon dot nano-particles by J774. 1 macrophages, Cenlral-Eur. J. Immunol., 2017, 42, 324. [ Links ]
173 Q. Wang, X. Huang, Y. Long, X. Wang, H. Zhang, R. Zhu, L. Liang, P Teng and H. Zheng, Hollow luminescent carbon dots for drug delivery, Carbon, 2013, 59, 192-199. [ Links ]
174 VN. Mehta, S.S. Chettiar, J.R. Bhamore, S.K. Kailasa and R.M. Patel, Green synthetic approach for synthesis of fluorescent carbon dots for lisinopril drug delivery system and their confirmations in the cells, J. Fluores., 2017, 27, 111-124. [ Links ]
175 S. Rai, B.K. Singh, P. Bhartiya, A. Singh, H. Kumar, P. Dutta and G. Mehrotra, Lignin derived reduced fluorescence carbon dots with theranostic approaches: nano-drug-carrier and bioimaging, J. Lumin, 2017,190, 492-503. [ Links ]
176 S.L. D'souza, B. Deshmukh, J.R. Bhamore, K.A. Rawat, N. Lenka and S.K. Kailasa, Synthesis of fluorescent nitrogen-doped carbon dots from dried shrimps for cell imaging and boldine drug delivery system, RSC Adv., 2016, 6, 12169-12179. [ Links ]
177 J. Xu, Y. Zhou, S. Liu, M. Dong and C. Huang, Low-cost synthesis of carbon nanodots from natural products used as a fluorescent probe for the detection of ferrum (III) ions in lake water, Anal. Melhods, 2014, 6, 2086-2090. [ Links ]
178 S. Jayaweera, K. Yin, X. Hu and W.J. Ng, Facile preparation of fluorescent carbon dots for label-free detection of Fe3+, J. Pholochem. Pholobiol. A: Chem, 2019, 370, 156-163. [ Links ]
179 J. Xu, X. Jie, F. Xie, H. Yang, W. Wei and Z. Xia, Flavonoid moiety-incorporated carbon dots for ultrasensitive and highly selective fluorescence detection and removal of Pb2+, Nano Res., 2018, 11, 3648-3657. [ Links ]
180 J. Xu, Y. Zhou, G. Cheng, M. Dong, S. Liu and C. Huang, Carbon dots as a luminescence sensor for ultrasensitive detection of phosphate and their bioimaging properties, Luminescence, 2015, 30, 411-415. [ Links ]
181 J. Zhang, Y. Ma, Y. Du, H. Jiang, D. Zhou and S. Dong, Carbon nanodots/WO3 nanorods Z-scheme composites: remarkably enhanced photocatalytic performance under broad spectrum, Appl. Calal. B: Environ, 2017, 209, 253-264. [ Links ]
182 Y. Shi, Y. Na, T. Su, L. Li, J. Yu, R. Fan and Y. Yang, Fluorescent carbon quantum dots incorporated into dye-sensitized TiO2 photoanodes with dual contributions, ChemSusChem, 2016, 9, 1498-1503. [ Links ]
183 H. Zhang, Y. Wang, P. Liu, Y. Li, H.G. Yang, T. An, P.K. Wong, D. Wang, Z. Tang and H. Zhao, A fluorescent quenching performance enhancing principle for carbon nanodot-sensitized aqueous solar cells, Nano Energy, 2015, 13, 124-130. [ Links ]
184 P. Bhartiya, A. Singh, H. Kumar, T. Jain, B.K. Singh and P. Dutta, Carbon dots: chemistry, properties and applications, J. Indian Chem. Soc., 2016, 93, 759-766. [ Links ]
185 A. Prasannan and T. Imae, One-pot synthesis of fluorescent carbon dots from orange waste peels, Indusl. & Eng. Chem. Res., 2013, 52, 15673-15678. [ Links ]
186 H. Wang, J. Zhuang, D. Velado, Z. Wei, H. Matsui and S. Zhou, Near-infrared- and visible-light-enhanced metal-free catalytic degradation of organic pollutants over carbon-dot-based carbocata-lysts synthesized from biomass, ACS Appl. Maler. & Inlerf., 2015, 7, 27703-27712. [ Links ]
187 W. Li, Y. Liu, M. Wu, X. Feng, S.A. Redfern, Y. Shang, X. Yong, T. Feng, K. Wu and Z. Liu, Carbon-quantum-dots-loaded ruthenium nano-particles as an efficient electrocatalyst for hydrogen production in alkaline media, Adv. Maler., 2018, 30, 1800676. [ Links ]
188 A. Tyagi, K. Tripathi, N. Singh, S. Choudhary and R. Gupta, Green synthesis of carbon quantum dots from lemon peel waste: applications in sensing and photocatalysis, RSC Adv., 2016, 6, 72423-72432. [ Links ]
189 M.T. Bazana, C.F. Codevilla and C.R. de Menezes, Nanoencapsu-lation of bioactive compounds: challenges and perspectives, Curr. Opin. Food Sci., 2019, 26, 47-56. [ Links ]
190 M.Z. Fahmi, D.L.N. Wibowo, S.C.W. Sakti and H.V. Lee, Human serum albumin capsulated hydrophobic carbon nanodots as stain-ingagent on HeLa tumor cell, Maler. Chem. Phys., 2020,239,122266. [ Links ]
191 K.F. Mahmoud, H.S. Ali and A.A. Amin, Nanoencapsulation of bioactive compounds extracted from Egyptian prickly pears peel fruit: antioxidant and their application in guava juice, Asian J. Sci. Res., 2018, 11, 574-586. [ Links ]
192 A. Rezaei, M. Fathi and S.M. Jafari, Nanoencapsulation of hydro-phobic and low-soluble food bioactive compounds within different nanocarriers, Food Hydrocol., 2019, 88, 146-162. [ Links ]
193 A. Munin and F. Edwards-Lévy, Encapsulation of natural poly-phenolic compounds; a review, Pharmaceulics, 2011, 3, 793-829. [ Links ]
194 E. Assadpour and S. Mahdi Jafari, A systematic review on nano-encapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers, Cril. Rev. Food Sci. Nulrilion, 2018, 1-23. [ Links ]
195 A. Rezaei, J. Varshosaz, M. Fesharaki, A. Farhang and S.M. Jafari, Improving the solubility and in vitro cytotoxicity (anticancer activity) of ferulic acid by loading it into cyclodextrin nanosponges, Inl. J. Nanomed., 2019, 14, 4589. [ Links ]
196 A. Di Costanzo and R. Angelico, Formulation strategies for enhancing the bioavailability of Silymarin: the state of the art, Molecules, 2019, 24, 2155. [ Links ]
197 P. Ezhilarasi, P. Karthik, N. Chhanwal and C. Anandharama-krishnan, Nanoencapsulation techniques for food bioactive components: a review, Food Bioprocess Technol., 2013, 6, 628-647. [ Links ]
198 J. Cui, J. Zhou, L. Huang, J. Jing, N. Wang and L. Wang, Curcumin encapsulation and protection based on lysozyme nanoparticles, Food Sci. Nulrilion, 2019, 7, 2702-2707. [ Links ]
199 L. Basiri, G. Rajabzadeh and A. Bostan, α-Tocopherol-loaded niosome prepared by heating method and its release behavior, Food Chem,, 2017, 221, 620-628. [ Links ]
200 A.I.A. El-Fattah, M.M. Fathy, Z.Y. Ali, A.E.R.A. El-Garawany and E.K. Mohamed, Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats, Chem.-biol. Inler-acl., 2017, 271, 30-38. [ Links ]
201 D. Najafi, R.A. Taheri, A. Najafi, A.A. Rouhollahi and M. Alvarez-Rodriguez, Effect of Achillea millefolium-loaded nanophytosome in the post-thawing sperm quality and oxidative status of rooster semen, Cryobiology, 2018, 82, 37-42. [ Links ]
202 I.J. Arroyo-Maya and D.J. McClements, Biopolymer nanoparticles as potential delivery systems for anthocyanins: fabrication and properties, Food Res. Inl., 2015, 69, 1-8. [ Links ]
203 A. Gunes, E. Guler, R.N. Un, B. Demir, F.B. Barlas, M. Yavuz, H. Coskunol and S. Timur, Niosomes of Nerium oleander extracts: in vitro assessment of bioactive nanovesicular structures, J. DrugDeliv. Sci. Technol., 2017, 37, 158-165. [ Links ]
204 D.K. Chatterjee, L.S. Fong and Y. Zhang, Nanoparticles in photo-dynamic therapy: an emerging paradigm, Adv. Drug Deliv. Rev., 2008, 60, 1627-1637. [ Links ]
205 S.T. Yang, L. Cao, P.G. Luo, F. Lu, X. Wang, H. Wang, M.J. Meziani, Y Liu, G. Qi and Y.P. Sun, Carbon dots for optical imaging in vivo, J. Am. Chem. Soc, 2009, 131, 11308-11309. [ Links ]
Received 17 February 2020
Revised 29 June 2020
Accepted 19 August 2020
* To whom correspondence should be addressed. E-mail: A.N.K., alfinda-n-k@fst.unair.ac.id / M.Z.F., m.zakki.fahmi@fst.unair.ac.id