Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.75 Durban 2021
http://dx.doi.org/10.17159/0379-4350/2021/v75a1
RESEARCH ARTICLE
https://doi.org/10.17159/0379-4350/2021/v75a1
Vortex-assisted Solid-Liquid Extraction for Rapid Screening of Oil Content in Jatropha Seed: an Alternative to the Modified Soxhlet Method
Hercílio E. ZimilaI, *; Jaime S. MandlateI, II; Enélia M. ArturI; Hermínio F. MuiamboI; Amália A. UamusseI
IDepartment of Chemistry, Eduardo Mondlane University, Main Campus, P.O. Box 257, Maputo, Mozambique
IIDepartamento de Química, Universidade Federal de Santa Maria, 97105-900, Santa Maria, RS, Brazil
ABSTRACT
In this study, an optimized vortex-assisted method for rapid screening of oil content in Jatropha curcas L. (Jatropha) seeds is described. A 24-1IV fractional factorial design was employed to study the influence of vortex stirring rate, sample:solvent ratio, extraction time, and the number of extraction cycles in the Jatropha oil extraction efficiency. Gas chromatography-mass spectrometry (GC-MS) was used for the identification of the fatty acids. The number of extraction cycles was the most important factor, and the optimum conditions were two extractions with hexane at a sample:solvent ratio of 1:5 mg:μL, under vortex-stirring at 2500 rpm for 1 min. Linoleic acid, oleic acid and palmitic acid were the major fatty acids of Jatropha oil. The method showed inherent advantages of simplicity, accuracy, short time of analysis, and low consumption of both organic solvent and sample, and may serve as a cost-effective alternative to the modified Soxhlet method in estimation of oil content and composition in Jatropha breeding programmes.
Keywords: Vortex-assisted, Jatropha oil, analysis, fractional factorial design, GC-MS.
1. Introduction
Jatropha is a perennial crop of easy cultivation and healthy life cycle that has received increasing popularity as a biodiesel feedstock, foodstuff, natural fence, and medicinal plant. The oil, the most valuable Jatropha commodity, comprises 35 % of the whole seed mass and is rich in nutritionally important fatty acids, including oleic, linoleic, palmitic and stearic acids.1 Such components also confer the Jatropha oil unique physico-chemical properties, which make it a good source of biodiesel suitable for petrodiesel engines.2 Nonetheless, the oil yield is still much lower than expected and is one of the main bottlenecks in the sustainability of Jatropha projects.3 Thus, the improvement of oilseed content is amongst the top priorities of the Jatropha breeding programmes.4
In the routine estimation of oil content for sorting, identification, and selection of elite accessions, breeders demand a rapid, efficient, non-destructive, accurate and cost-effective analytical method, applicable even when the sample amount is rather small. Liquid-solid extraction methods - such as the Soxhlet method5 and ultrasound-assisted extraction6 - are commonly employed. However, they are tedious and require long extraction times (>1 h) and consume a large amount of both samples (>2 g) and solvent (>150 mL). High-throughput methods (near-infrared reflectance spectroscopy and nuclear magnetic resonance)7,8 and supercritical CO29 were also developed as alternatives for the extraction of oil in Jatropha seeds. Although being environmentally friendly and/or fast alternatives for the estimation of oil content, these techniques are unaffordable to many laboratories, especially in developing countries.
Vortex is a low-cost mechanical stirring device that has been hugely employed in solvent extraction techniques - particularly in the determination of trace levels of various analytes by dispersive liquid-liquid microextraction - to increase the specific surface area for mass transfer, reduce the diffusion distance, hence improving the extraction rates.10 Several studies have reported that the vortex-assisted extraction methods have provided high repeatability, very low limits of detection and quantitation, good enrichment, and extraction efficiency within a short extraction time.10-13 Despite such advantages, studies focused on the optimization of vortex-assisted extraction of oil are scarce in the literature.
In this study, the vortex-assisted extraction method followed by GC-MS for the screening of oil content and composition in Jatropha seeds was optimized. Its performance was compared to the modified Soxhlet method.
2. Experimental
2.1. Chemicals and Sample Preparation
Potassium hydroxide (99 %), hexane (98 %) and methanol (99 %) were, respectively, purchased from Rochelle Chemicals, Merck and SkyLabs, all based in Johannesburg, South Africa.
Jatropha seeds were collected from 10 genotypes grown in the Jatropha germplasm in Boane District, Maputo, Mozambique (26°02'20.4" South, 32°21'06.5" East). The local climatic conditions are classified as sub-humid, with average annual rainfall, humidity and temperature of 752 mm, 80.6 %, and 23.7 °C, respectively. The samples were air-dried, without incidence of sunrays at room temperature, cracked and the kernels were finely ground using mortar and pestle and stored at -14 °C in screw-capped 15 mL polypropylene vials.
2.2. Instrumentation
The chemical composition of Jatropha oil was determined on a gas Chromatograph (Agilent GC system 7820A) equipped with mass spectrometer (Agilent Mass Spectrometry detector 5977B). ThermoScientific LP Vortex Mixer 0-3200 rpm was employed for sample stirring, whereas the centrifugation was performed on the IWAKI micro 6 CFM-100 and Thermo ALC 4206 centrifuges. The volume of extractant and extract was measured using a Gilson micropipette 200-1000 μL. Vacuum evaporation was performed on an Eyela rotary evaporator (pump EyelaA-1000S, water-bath EyelaOSB-2100, rotator EyelaN-1200A).
2.3. Optimization of Vortex-assisted Extraction Method
Vortex-assisted method was optimized based on the method described by Zhang el al.6with some modifications. Five to ten Jatropha seeds per variety were dehusked and the kernels ground using pestle and mortar. Around 300 mg of the pasty sample (obtained by quartering method) were extracted with aliquots (1500-4500 μL) of hexane, under vortex stirring (1500-2500 rpm) for 1-5 min. The extract was then centrifuged at 5000 rpm for 5 min and filtered through filter paper Advantec 5A (reduced to a diameter of ~7 cm; alternatively, a 0.45 μm membrane filter can be used). Filtration was performed rapidly at temperatures not exceeding 24 °C to avoid solvent evaporation and consequent sample concentration. Afterwards, an aliquot of 800 μL of the filtrate was quickly pipetted to a tared Petri dish and left to dry (~10 min), in a fume hood at room temperature, and then in a vacuum oven (Memmert) at 80 °C for 15 min. Then, the dish with oil was re-weighed, and the oil content was calculated using Equation 1.

where Wdish+oil and Wdish are Petri dish weights (mg) with and without oil, respectively; V is the total volume of hexane used for extraction (mL); Va is the volume of the aliquot (mL) (in this case 0.8 mL); and Wsis the sample weight (mg)
To study the influence of the vortex stirring rate (1500,2000 and 2500 rpm), sample:solvent ratio (1:5, 1:10 and 1:15 mg:μL), extraction time (1, 3 and 5 min) and the number of extraction cycles (1,2 and 3) on oil extraction yield, a resolution IV fractional factorial design (24-1IV), nine randomized runs and two replicates were chosen.
2.4. Soxhlet Extraction
The Soxhlet method according to Shivani el al ,14 with slight modifications, was performed. Almost 3 g of ground kernels (in triplicate) were placed in the thimble, plugged with cotton, and placed in the Soxhlet apparatus. Subsequently, hexane was added through the siphon until two consecutive solvent discharges occurred (volume corresponding to more than 150 mL in a 250 mL flask). The sample was extracted for 8 h at 64-66 °C (mild boiling). The solvent was removed by vacuum evaporation at 40 °C and then dried in a vacuum oven at 80 °C for 15 min. The oil content was calculated as a percentage of total oil present in seed kernels.
2.5. GC-MS Analysis
For GC-MS analysis, the triacylglycerides were transesterified with methanol, according to Senou el al.,15 with modifications. Two drops of oil from the Soxhlet method were dissolved in 1200 μL of hexane ina2mL centrifuge microtube. In the case of vortex-assisted method extracts, an aliquot of 1200 μL of hexane extract of seeds was placed in the microtube. In both tubes, 500 μL of 2 mol L-1 potassium hydroxide in methanol was added. Then, the mixture was shaken in a vortex for 2 min at 2500 rpm and centrifuged for 2 min. One microlitre of the upper layer was injected in the GC-MS system in the split mode at 1:30. The fatty acids methyl esters were separated on an HP-5MS 5 % phenyl methyl siloxane capillary column (30 m x 250 μm x 0.25 μm). Helium was used as carrier gas at a flow rate of 1 mL min-1. The oven temperature was initially set to 150 °C, increased to 190 °C by 8 °C min-1, and ramped at 10 °C min-1 to 230 °C, which was held for 18 min. The injector and detector temperatures were 280 °C and 300 °C, respectively. The components were identified by matching their recorded mass spectra with those of NIST14.LIB library data.
2.6. Statistical Analysis
The analysis of variance (ANOVA) was performed to study the significance of the factors and their interaction in the experimental design. While statistical significance between oil contents obtained by two methods was assessed by paired t-test, one-way ANOVA followed by Fisher's least significant difference (LSD) method was used to assess the significance of differences between mean oil contents in at least three samples. All statistical analyses were performed at the 95 % confidence interval, and the results were considered significant at P < 0.05. The Minitab 17.0 software package was used for data analysis.
3. Results and Discussion
3.1. Optimization of the Vortex-assisted Method
Previous studies have shown that hexane is the most efficient oil extractant, and the so-obtained oil is less turbid.16-20 Oil extraction yield is influenced by kernel particle size and experimental conditions, including solvent-to-sample ratio, stirring rate, as well as extraction temperature and time.17,18,20 In the current study four continuous variables were evaluated, namely: vortex stirring rate (1500, 2000 and 2500 rpm), sample:solvent ratio (1:5,1:10 and 1:15 mg: μL), extraction time (1, 3 and 5 min) and number of extraction cycles (1, 2 and 3).
A resolution IV screening design (24-1 fractional factorial, four replications) was chosen, to determine the most important factors affecting the oil extraction process. The design matrix with oil recovery (average ± S.D.) is summarized in Table 1. A centre point (trial # 9) was added to investigate a possible non-linear relationship between the factors and extraction yield.

Table 1 shows that the best yield (57.9 ± 3.6 %) was achieved by three extraction cycles with hexane under stirring at 2500 rpm, at a sample:solvent ratio of 1 mg:5 μL for 1 min (run 6). The lowest recovery was registered in run 5 whose analytical conditions are quite similar to the run 6 except for the extraction time and number of extractions.
The significance of the studied factors in the oil yield is shown in the Pareto chart (Fig. 1), in which the effects are represented by bars in descending order of their standardized effects 21.

Significant factors at the 95 % confidence interval are those whose bars extend beyond the vertical line, i.e. effects with t value higher than ±2.26.21 Therefore, the number of extraction cycles (D) is the unique main effect that significantly affects (P < 0.05) the oil extraction and has a positive effect on the extraction yield. Likewise, the interaction stirring rate x extraction time (AC) is the only statistically significant interaction, and its behaviour is portrayed in Fig. 2.

According to Fig. 2, if the mixture is shaken at high frequencies (~2500 rpm), then a shorter time is needed to achieve high recoveries; in contrast, at low, stirring rates (~1500 rpm) longer contact time is necessary to get a good extraction yield. These results confirm the hypothesis that the high rotational speed provided by vortex agitation weakens the adhesive interactions (such as van der Waals forces, hydrogen bonding, and dipole attractions) between triacylglycerides molecules and active sites of the matrix, and increases the surface area for mass transfer. Consequently, the extractant diffuses easily into the matrix, and the extraction equilibrium is achieved within a relatively short time.10,22 Therefore, the time was fixed at its lowest level (1 min) whereas the stirring rate was kept at the highest level (2500 rpm).
The statistical significance of the number of extraction cycles suggests that the solvent must be renewed throughout the extraction process, i.e. the first extraction does not ensure a quantitative extraction of the oil present in Jatropha seeds.22 Since the sample:solvent ratio is not statistically significant, it was maintained at the lowest level (1:5 mg:μL) to save costs of acquisition of chemicals and avoid discarding a large amount of organic solvents to the environment. In summary, the optimum levels for the extraction time, stirring rate, and sample:solvent ratio were 1 min, 2500 rpm, and 1:5 mg:μL.
Being the unique statistically significant factor, the number of extraction cycles was optimized univariately, keeping the stirring rate, sample:solvent ratio, and time at the optimum levels, namely 2500 rpm, 1:5 mg:μL, and 1 min, respectively. Three extraction levels (1, 2, and 3) were tested, and the respective recoveries are displayed in Fig. 3.

The amount of oil varied from 51.5 ± 1.0 % to 58.9 ± 3.0 % in the single and three extractions, respectively. Assuming 58.9 % as the total amount of oil present in this Jatropha seed, over 87 % of seed oil content is recovered in the first cycle and the remainder is recovered in the other cycles. Notwithstanding this fact, the oil recovery in the single extraction differs significantly (p<0.05) from that of two and three extractions cycles. Further, the differences between the oil content from two and three extractions are not statistically significant (P > 0.05). Therefore, the number of extraction cycles was kept at 2, and the optimum conditions were two extractions with hexane at a sample:solvent ratio of 1:5 mg:μL, under vortex stirring at 2500 rpm for 1 min.
3.2. Comparison with Other Studies
The Soxhlet method is the most widespread analytical technique for the determination of oil content in a variety of oilseeds, including Jatropha. Hence, the performance of the proposed method was compared with the modified Soxhlet method described elsewhere.14 The oil content recovered by the Soxhlet method was 58.1 ± 1.7 %, which does not differ significantly (P > 0.05) from the content obtained using the vortex-assisted extraction method (57.9 ± 2.4 %).
The GC-MS chromatograms of the main components of the oils obtained from both extraction methods are shown in Fig. 4.
In the oils obtained from both methods, linoleic (18:2 Δ9,12), oleic (18:1 Δ9), palmitic (16:0) and stearic (18:0) acids were found to be the major components, whilst palmitoleic acid (16:1 Δ9) and myristic acid (16:0) were detected in trace amounts. These findings are in line with the previous reports,1,15,25 which have revealed that palmitic and stearic acids are the major saturated fatty acids, whilst oleic and linoleic acids are the most abundant unsaturated fatty acids in Jatropha oil.
The close agreement between vortex-assisted extraction and the modified Soxhlet methods, both in oil yields and composition, implies that the former is a suitable alternative to the modified Soxhlet method in Jatropha oil quantification. This method (vortex-assisted extraction method) has numerous advantages over the Soxhlet method, including the possibility of simultaneous analysis of various replicates, simplicity, short analysis time (37 min vs. 9 h), and requirement of a small amount of sample (300 mg vs. 4-5 g). Furthermore, the proposed method is relatively cheap and environmentally sustainable, since it does not require the use of rotavapor, and consumes a lower quantity of expensive organic solvents than Soxhlet (3 mL vs. 150 mL).
3.3. Analysis of Jatropha Samples
To assess the effectiveness of the method for the proposed application, Jatropha seeds samples from different accessions were analyzed, and the results are presented in Fig. 5.
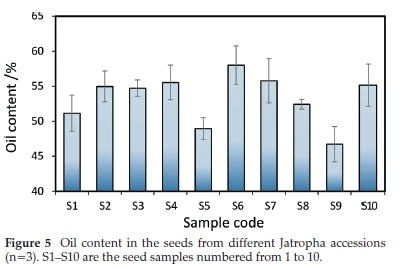
The oil content ranged from 46.7 % to 58.0 %, and the method showed good repeatability with RSDs varying from 0.4 % to 5.7 %. One-way ANOVA test showed that the differences between samples are statistically significant. Since Jatropha exhibits low genetic variability,23 these differences may be associated with epigenetic mechanisms24 whereby genetically identical cultivars may exhibit different phenotypic characteristics according to the agroclimatic conditions.
4. Conclusions
A vortex-assisted extraction method for the determination of Jatropha seed oil was optimized. Out of the studied effects, only the extraction cycles and the interaction stirring rate x extraction time were found to be statistically significant. The optimum conditions were two consecutive extractions with hexane at the sample:solvent ratio of 1:5 mg:μL under vortex stirring at 2500 rpm for 1 min. The method has demonstrated to be fast, simple, accurate, and requires a relatively low amount of both organic solvent and sample. The proposed method is a potential alternative to the modified Soxhlet method in the analysis of oil in Jatropha seeds.
Acknowledgements
Financial support from Japan International Cooperation Agency (JICA) through the Project on Sustainable Production of Biodiesel from Jatropha in Mozambique is gratefully acknowledged. Authors would like to express their sincere thanks to Mr. Silvestre Muiambo for the GC-MS peak integration.
ORCID iDs
H.E.Zimila: orcid.org/0000-0001-5183-6586
J.S. Mandlate: orcid.org/0000-0001-6790-5574
E.M. Artur: orcid.org/0000-0001-8822-652X
H.F. Muiambo: orcid.org/0000-0001-5812-0636
A.A. Uamusse: orcid.org/0000-0003-4492-4443
References
1 J. Martínez-Herrera, P. Siddhuraju, G. Francis, G. Dávila-Ortíz and K. Becker, Chemical composition, toxic/antimetabolic constituents, and effects of different treatments on their levels, in four provenances of Jatropha curcas L. from Mexico, Food Chem., 2006, 96, 80-89. [ Links ]
2 M. Takase, T. Zhao, M. Zhang, Y. Chen, H. Liu, L. Yang and X. Wu, An expatiate review of neem, jatropha, rubber and karanja as multipurpose non-edible biodiesel resources and comparison of their fuel, engine and emission properties, Renew. Sustain. Energy Rev., 2015,43, 495-520. [ Links ]
3 G. Hua, F. Sun and P. Liu, Status of molecular breeding for improving Jatropha curcas and biodiesel, Renew. Sustain. Energy Rev., 2013, 26, 332-343. [ Links ]
4 W. Xu, S. Mulpuri and A. Liu, Genetic diversity in the Jatropha genus and its potential application, CAB Rev, 2012, 7, 1-15. [ Links ]
5 M. Baldini, C. Ferfuia, R. Bortolomeazzi, G. Verardo, J. Pascali, E. Piasentier and L. Franceschi, Determination of phorbol esters in seeds and leaves of Jatropha curcas and in animal tissue by highperformance liquid chromatography tandem mass spectrometry, Ind. Crops Prod., 2014, 59, 268-276. [ Links ]
6 Q. Zhang, Z. Zhang, X. Yue, X. Fan, T. Li and S. Chen, Response surface optimisation of ultrasound-assisted oil extraction from autoclaved almond powder, Food Chem., 2009,116, 513-518. [ Links ]
7 A.E. Melchinger, S. Munder, F.J. Mauch, V Mirdita, J. Böhm and J. Müller, High-throughput platform for automated sorting and selection of single seeds based on time-domain nuclear magnetic resonance (TD-NMR) measurement of oil content, Biosyst. Eng., 2017,164, 213-220. [ Links ]
8 J.M. Montes, F. Technow, B. Bohlinger and K. Becker, Grain quality determination by means of near infrared spectroscopy in Jatropha, Ind. Crop. Prod, 2013,43, 301-305. [ Links ]
9 J. Min, S. Li, J. Hao and N. Liu, Supercritical CO2 extraction of Jatropha oil and solubility correlation, J. Chem. Eng. Data, 2010, 55, 3755-3758. [ Links ]
10 E. Psillakis, Vortex-assisted liquid-liquid microextraction revisited, Trends Anal. Chem., 2018, 113, 332-339. [ Links ]
11 R. Zhang, Z. Tan, K. Huang, Y. Wen, X. Li, J. Zhao and C. Liu, A vortex-assisted dispersive liquid-liquid microextraction followed by UPLC-MS/MS for simultaneous determination of pesticides and aflatoxins in herbal tea, Molecules, 2019, 24, 1029. [ Links ]
12 G.D.T.M. Jayasinghe, R. Domínguez-González, P. Bermejo-Barrera and A. Moreda-Pineiro, Combining ultrasound-assisted extraction and vortex-assisted liquid-liquid microextraction for the sensitive assessment of aflatoxins in aquaculture fish species, J. Sep. Sci., 2020, 43, 1331-1338. [ Links ]
13 Z. Ni, Z. Chen, J. Cheng and F. Tang, Simultaneous determination of arsenic and lead in vegetable oil by atomic fluorescence spectrometry after vortex-assisted extraction, Anal. Lett, 2017, 50, 2129-2138. [ Links ]
14 P. Shivani, P. Khushbu, N. Faldu, V. Thakkar and R. B. Shubramanian, Extraction and analysis of Jatropha curcas L. seed oil, African J. Biotechnol., 2011,10, 18210-18213. [ Links ]
15 H. Senou, C. X. Zheng, M.B. Traore, F. Folega and B.M. Traore, Quantification of seed oil content and fatty acid profile of Jatropha cucas L. from Guizhou, China, Int. J. Biol., 2016, 8, 92-97. [ Links ]
16 S.K. Amin, S. Hawash, G.E. Diwani and S.E. Rafei, Kinetics and thermodynamics of oil extraction from Jatropha curcas in aqueous acidic hexane solutions, J. Am. Sci., 2010, 6, 293-300. [ Links ]
17 S. Gaur, Development and Evaluation ofan Effective Process for the Recovery ofOil and Detoxification ofMeal from Jatropha curcas, MSc thesis, Missouri University of Science and Technology, USA, 2009. [ Links ]
18 M.D. Kostic, N.M. Jokovic, O.S. Stamenkovic, K.M. Rajkovic, P.S. Milic and V.B. Veljkovic, Optimisation of hempseed oil extraction by n-hexane, Ind. Crops Prod., 2013,48, 133-143. [ Links ]
19 R.F. Santos, C.H. Fornasari, D. Bassegio and S. Nelson, Optimisation of oil extraction from high energetic potential plants performed through drying and solvent extraction methods, African J. Biotechnol., 2013,12, 6761-6765. [ Links ]
20 S. Sayyar, Z.Z. Abidin, R. Yunus and A. Muhammad, Extraction of oil from Jatropha seeds - optimisation and kinetics, Am. J. Appl. Sci., 2009, 6, 1390-1395. [ Links ]
21 N. Salgueiro-González, I. Turnes-Carou, S. Muniategui-Lorenzo, P. López-Mahía and D. Prada-Rodríguez, Analysis of endocrine disruptor compounds in marine sediments by in cell clean up-pressurized liquid extraction-liquid chromatography tandem mass spectrometry determination, Anal. Chim. Acta, 2014, 852, 112-120. [ Links ]
22 M.D.L. De Castro and J.L.L. García, Acceleration and Automation of Solid Sample Treatment, vol. 24, Elsevier Science B.V., 2002, pp. 233-279. [ Links ]
23 S. Popluechai, D. Breviario, S. Mulpuri, H.P.S. Makkar, M. Raorane, A.R. Reddy, E. Palchetti, A.M.R. Gatehouse, J.K. Syers, A.G. O'Donnell and A. Kohli, Narrow genetic and apparent phenetic diversity in Jatropha curcas: initial success with generating low phorbol ester interspecific hybrids, Nat. Proc., 2009. [ Links ]
24 R. Brittaine and N. Lutaladio, Jatropha: a Smallholder Bioenergy Crop -The Potential for Pro-poor Development, vol. 8, FAO, 2010. [ Links ]
25 T. Issariyakul and A.K. Dalai, Biodiesel from vegetable oils, Renew. Sustain. Energy Rev, 2014, 31, 446-471. [ Links ]
Received 22 March 2020
Revised 21 October 2020
Accepted 10 November 2020
* To whom correspondence should be addressed. E-mail: herciliozimila@gmail.com














